NORAD-Regulated Signaling Pathways in Breast Cancer Progression
Abstract
Simple Summary
Abstract
1. Introduction
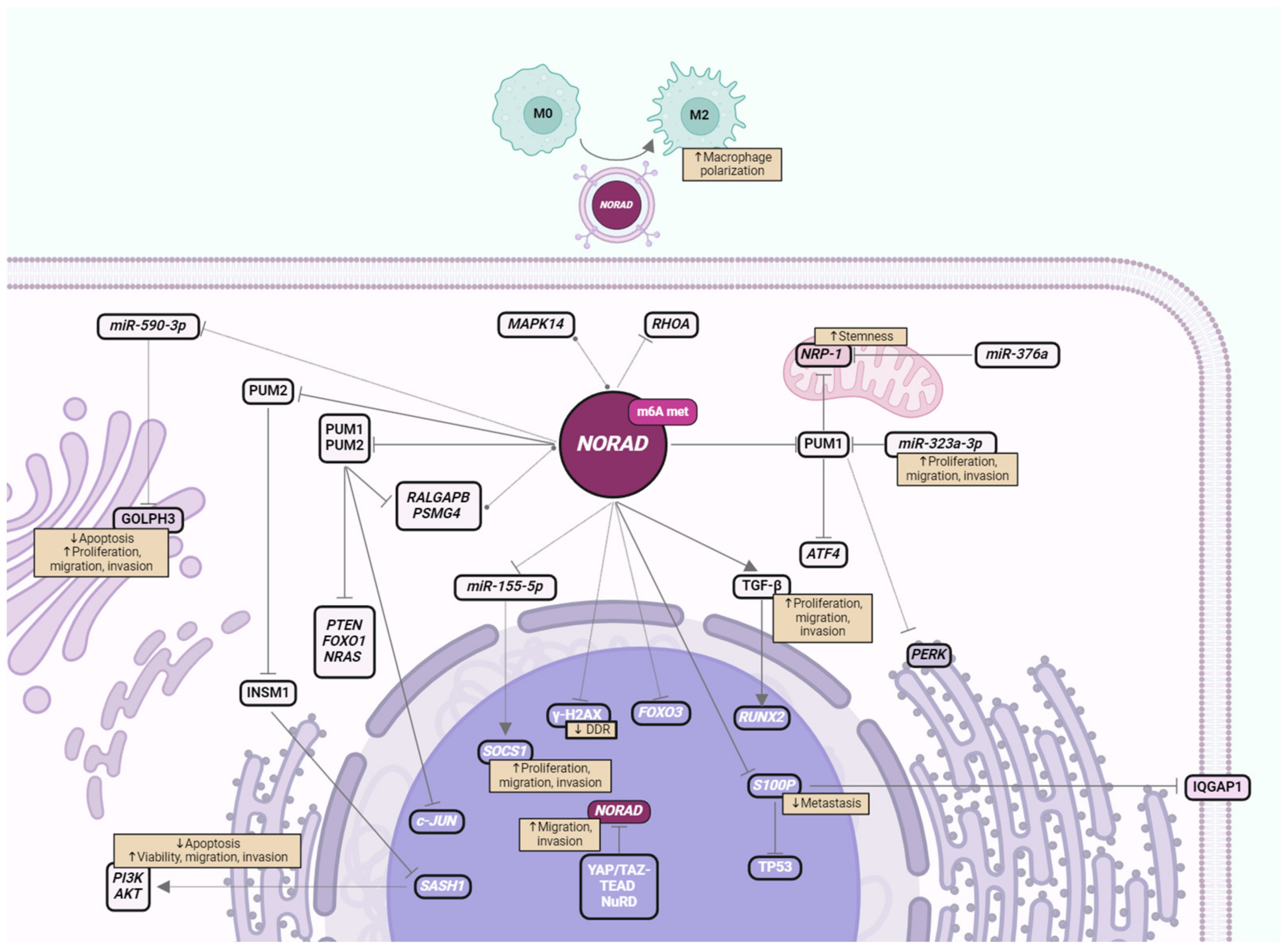
2. Impact of NORAD in BC Signaling Pathways
2.1. PUM Proteins and Target Genes
2.2. NORAD-Regulated Signaling Pathways via ncRNA Sponging
2.3. Protein- and mRNA-Mediated Regulation of Signaling Pathways by NORAD
2.4. NORAD-Regulated Cytokines and Immune Cells
3. Potential Implication of NORAD in BC Therapies
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Zhao, Y.; Lin, G.; Zhou, X.; Jiang, X.; Zhao, H. Noncoding RNA activated by DNA damage (NORAD): Biologic function and mechanisms in human cancers. Clin. Chim. Acta 2019, 489, 5–9. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann. Transl. Med. 2018, 6, 241. [Google Scholar] [CrossRef]
- Karakas, D.; Ozpolat, B. The Role of LncRNAs in Translation. Noncoding RNA 2021, 7, 16. [Google Scholar] [CrossRef]
- Guh, C.Y.; Hsieh, Y.H.; Chu, H.P. Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J. Biomed. Sci. 2020, 27, 44. [Google Scholar] [CrossRef]
- Sun, M.; Kraus, W.L. From Discovery to Function: The Expanding Roles of Long NonCoding RNAs in Physiology and Disease. Endocr. Rev. 2015, 36, 25–64. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 924–933. [Google Scholar] [CrossRef]
- Park, E.G.; Pyo, S.J.; Cui, Y.; Yoon, S.H.; Nam, J.W. Tumor immune microenvironment lncRNAs. Brief. Bioinform. 2022, 23, bbab504. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kopp, F.; Chang, T.C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Munschauer, M.; Nguyen, C.T.; Sirokman, K.; Hartigan, C.R.; Hogstrom, L.; Engreitz, J.M.; Ulirsch, J.C.; Fulco, C.P.; Subramanian, V.; Chen, J.; et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 2018, 561, 132–136. [Google Scholar] [CrossRef]
- Kopp, F.; Elguindy, M.M.; Yalvac, M.E.; Zhang, H.; Chen, B.; Gillett, F.A.; Lee, S.; Sivakumar, S.; Yu, H.; Xie, Y.; et al. PUMILIO hyperactivity drives premature aging of Norad-deficient mice. Elife 2019, 8, e42650. [Google Scholar] [CrossRef] [PubMed]
- Elguindy, M.M.; Kopp, F.; Goodarzi, M.; Rehfeld, F.; Thomas, A.; Chang, T.C.; Mendell, J.T. PUMILIO, but not RBMX, binding is required for regulation of genomic stability by noncoding, RNA NORAD. Elife 2019, 8, e48625. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Azimi, T.; Hussen, B.M.; Abak, A.; Taheri, M.; Dilmaghani, N.A. Non-coding RNA Activated by DNA Damage: Review of Its Roles in the Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 714787. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Wu, Q.; Yin, R.; Tang, X.; Shi, H.; Wang, T.; Zhang, M.; Pan, C. LncRNA NORAD Promotes Vascular Endothelial Cell Injury and Atherosclerosis Through Suppressing VEGF Gene Transcription via Enhancing H3K9 Deacetylation by Recruiting HDAC6. Front. Cell Dev. Biol. 2021, 9, 701628. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, L.; Xu, J.; Luo, W.; Pan, L.; Niu, Q.; Wei, D. Upregulated lncRNA NORAD can diagnose acute cerebral ischemic stroke patients and predict poor prognosis. Folia Neuropathol. 2023, 61, 105–110. [Google Scholar] [CrossRef]
- Song, Q.; Geng, Y.; Li, Y.; Wang, L.; Qin, J. Long noncoding RNA NORAD regulates MPP+-induced Parkinson’s disease model cells. J. Chem. Neuroanat. 2019, 101, 101668. [Google Scholar] [CrossRef] [PubMed]
- Soghli, N.; Yousefi, T.; Abolghasemi, M.; Qujeq, D. NORAD, a critical long non-coding RNA in human cancers. Life Sci. 2021, 264, 118665. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, L.; Zhang, J.; Cheng, X.; Xu, Q.; Wang, J.; Mao, F. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed. Pharmacother. 2020, 129, 110268. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Li, J.; Gong, R.; Wang, Y.; Qin, Y.; Ping, Q.; Hu, L. Long noncoding RNA NORAD acts as a ceRNA mediates gemcitabine resistance in bladder cancer by sponging miR-155–5p to regulate WEE1 expression. Pathol. Res. Pract. 2021, 228, 153676. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhang, Y.; Xiao, X.; Chu, F.; Zhang, L. Induction of lncRNA NORAD accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the miR-495-3p/hypoxia-inducible factor-1α (HIF-1α). Bioengineered 2022, 13, 950–962. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Pietenpol, J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015, 24, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 288. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Treatment Options-National Breast Cancer Foundation [Internet]. 2020. Available online: https://www.nationalbreastcancer.org/breast-cancer-treatment/ (accessed on 6 September 2023).
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, J.; Li, X.; Li, W.; Li, H.; Fu, P. Long non-coding RNA NORAD inhibition upregulates microRNA-323a-3p to suppress tumorigenesis and development of breast cancer through the PUM1/eIF2 axis. Cell Cycle 2021, 20, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, X.; Hong, J.; Yang, E.; Xuan, C.; Fang, H.; Ding, X. Diagnostic implications of lncRNA NORAD in breast cancer. Sci. Rep. 2023, 13, 20426. [Google Scholar] [CrossRef]
- Shan, Q.; Qu, F.; Yang, W.; Chen, N. Effect of LINC00657 on Apoptosis of Breast Cancer Cells by Regulating miR-590-3p. Cancer Manag. Res. 2020, 12, 4561–4571. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Akbariqomi, M.; Asgari, Y.; Ebrahimi, D.; Alinejad-Rokny, H. A systematic review of long non-coding RNAs with a potential role in breast cancer. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108375. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.A.; Guan, J.; Mo, H.; He, J.L.; Zhan, X.L. lncRNA LSINCT5 Regulates miR-20a-5p/XIAP to Inhibit the Growth and Metastasis of Osteosarcoma Cells. Onco Targets Ther. 2020, 13, 8209–8221. [Google Scholar] [CrossRef]
- Zhou, K.; Ou, Q.; Wang, G.; Zhang, W.; Hao, Y.; Li, W. High long non-coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF-β pathway. Cancer Cell Int. 2019, 19, 63. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wu, M.; Yuan, Y.; Wu, W. m6A Modification Mediates Exosomal LINC00657 to Trigger Breast Cancer Progression Via Inducing Macrophage M2 Polarization. Clin. Breast Cancer 2023, 23, 546–560. [Google Scholar] [CrossRef]
- Yi, D.; Xu, F.; Wang, R.; Jiang, C.; Qin, J.; Lee, Y.H.; Shi, X.; Sang, J. Deciphering the map of METTL14-mediated lncRNA m6A modification at the transcriptome-wide level in breast cancer. J. Clin. Lab. Anal. 2022, 36, e24754. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, Y.; Jiang, H.; Chen, A. Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/SOCS1 Axis. J. Breast Cancer 2021, 24, 330. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.; Yang, M.C.; Singh, S.; Chou, Y.C.; Chen, H.Y.; Wang, M.Y.; Wang, Y.C.; Chen, R.H. LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene 2019, 38, 5612–5626. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Lin, Y.C.; Liu, C.H.; Chung, H.C.; Wang, Y.T.; Lin, Y.W.; Ma, H.I.; Tu, P.H.; Lawler, S.E.; Chen, R.H. USP11 regulates PML stability to control Notch-induced malignancy in brain tumours. Nat. Commun. 2014, 5, 3214. [Google Scholar] [CrossRef]
- Muller, C.S.M.; Giner, I.S.; Zambalde, É.P.; Carvalho, T.M.; Ribeiro, E.M.d.S.F.; Carvalho de Oliveira, J.; Mathias, C.; Gradia, D.F. The Potential of NORAD–PUMILIO–RALGAPB Regulatory Axis as a Biomarker in Breast Cancer. Noncoding RNA 2022, 8, 76. [Google Scholar] [CrossRef]
- Mathias, C.; Pedroso, G.A.; Pabst, F.R.; de Lima, R.S.; Kuroda, F.; Cavalli, I.J.; de Oliveira, J.C.; Ribeiro, E.M.d.S.F.; Gradia, D.F. So alike yet so different. Differential expression of the long non-coding RNAs NORAD and HCG11 in breast cancer subtypes. Genet. Mol. Biol. 2021, 44, e20200153. [Google Scholar] [CrossRef]
- Alves-Vale, C.; Capela, A.M.; Tavares-Marcos, C.; Domingues-Silva, B.; Pereira, B.; Santos, F.; Gomes, C.P.; Espadas, G.; Vitorino, R.; Sabidó, E.; et al. Expression of NORAD correlates with breast cancer aggressiveness and protects breast cancer cells from chemotherapy. Mol. Ther. Nucleic Acids 2023, 33, 910–924. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar]
- PSMG4 Gene-GeneCards|PSMG4 Protein|PSMG4 Antibody [Internet]. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PSMG4 (accessed on 18 December 2023).
- Zhang, L.; Chen, Y.; Li, C.; Liu, J.; Ren, H.; Li, L.; Zheng, X.; Wang, H.; Han, Z. RNA binding protein PUM2 promotes the stemness of breast cancer cells via competitively binding to neuropilin-1 (NRP-1) mRNA with miR-376a. Biomed. Pharmacother. 2019, 114, 108772. [Google Scholar] [CrossRef]
- Miles, W.O.; Lembo, A.; Volorio, A.; Brachtel, E.; Tian, B.; Sgroi, D.; Provero, P.; Dyson, N. Alternative Polyadenylation in Triple-Negative Breast Tumors Allows NRAS and c-JUN to Bypass PUMILIO Posttranscriptional Regulation. Cancer Res. 2016, 76, 7231–7241. [Google Scholar] [CrossRef]
- Tao, W.; Ma, J.; Zheng, J.; Liu, X.; Liu, Y.; Ruan, X.; Shen, S.; Shao, L.; Chen, J.; Xue, Y. Silencing SCAMP1-TV2 Inhibited the Malignant Biological Behaviors of Breast Cancer Cells by Interaction with PUM2 to Facilitate INSM1 mRNA Degradation. Front. Oncol. 2020, 10, 613. [Google Scholar] [CrossRef]
- Dashti, S.; Taherian-Esfahani, Z.; Kholghi-Oskooei, V.; Noroozi, R.; Arsang-Jang, S.; Ghafouri-Fard, S.; Taheri, M. In silico identification of MAPK14-related lncRNAs and assessment of their expression in breast cancer samples. Sci. Rep. 2020, 10, 8316. [Google Scholar] [CrossRef]
- Hassani, B.; Mollanoori, H.; Pouresmaeili, F.; Asgari, Y.; Ghafouri-Fard, S. Constructing mRNA, miRNA, circRNA and lncRNA regulatory network by Analysis of microarray data in breast cancer. Gene Rep. 2022, 26, 101510. [Google Scholar] [CrossRef]
- Nicknam, A.; Khojasteh Pour, S.; Hashemnejad, M.A.; Hussen, B.M.; Safarzadeh, A.; Eslami, S.; Taheri, M.; Ghafouri-Fard, S.; Jamali, E. Expression analysis of Rho GTPase-related lncRNAs in breast cancer. Pathol. Res. Pract. 2023, 244, 154429. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1-or BRCA2 -Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, R.; Kahlert, U.D.; Chen, Z.; Torres-dela Roche, L.A.; Soliman, A.; Miao, C.; De Wilde, R.L.; Shi, W. Construction of ceRNA Networks Associated with CD8 T Cells in Breast Cancer. Front. Oncol. 2022, 12, 883197. [Google Scholar] [CrossRef]
- Arvola, R.M.; Chang, C.T.; Buytendorp, J.P.; Levdansky, Y.; Valkov, E.; Freddolino, P.L.; Goldstrohm, A.C. Unique repression domains of Pumilio utilize deadenylation and decapping factors to accelerate destruction of target mRNAs. Nucleic Acids Res. 2020, 48, 1843–1871. [Google Scholar] [CrossRef]
- Goldstrohm, A.C.; Hall, T.M.T.; McKenney, K.M. Post-transcriptional Regulatory Functions of Mammalian Pumilio Proteins. Trends Genet. 2018, 34, 972–990. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wu, C.C.; Chou, C.K.; Huang, C.Y.F. A Translational Regulator, PUM2, Promotes Both Protein Stability and Kinase Activity of Aurora-A. Meurs EF, editor. PLoS ONE 2011, 6, e19718. [Google Scholar]
- Elguindy, M.M.; Mendell, J.T. NORAD-induced Pumilio phase separation is required for genome stability. Nature 2021, 595, 303–308. [Google Scholar] [CrossRef]
- Sindi, S.; Hamdi, N.; Hassan, S.; Ganash, M.; Alharbi, M.; Alburae, N.; Azhari, S.; Alkhayyat, S.; Linjawi, A.; Alkhatabi, H.; et al. Promoter Methylation-Regulated Differentially Expressed Genes in Breast Cancer. Breast Cancer Targets Ther. 2023, 15, 435–450. [Google Scholar] [CrossRef]
- Personnic, N.; Lakisic, G.; Gouin, E.; Rousseau, A.; Gautreau, A.; Cossart, P.; Bierne, H. A role for Ral GTPase-activating protein subunit β in mitotic regulation. FEBS J. 2014, 281, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, X.; Trinh, D.A.; Yoshimachi, S.; Goto, K.; Sakata, N.; Ishida, M.; Ohtsuka, H.; Unno, M.; Wang, Y.; et al. Ral GTPase promotes metastasis of pancreatic ductal adenocarcinoma via elevation of TGF-β1 production. J. Biol. Chem. 2023, 299, 104754. [Google Scholar] [CrossRef] [PubMed]
- Yoshimachi, S.; Shirakawa, R.; Cao, M.; Trinh, D.A.; Gao, P.; Sakata, N.; Miyazaki, K.; Goto, K.; Miura, T.; Ariake, K.; et al. Ral GTPase–activating protein regulates the malignancy of pancreatic ductal adenocarcinoma. Cancer Sci. 2021, 112, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.D.; Chen, X.W.; Kaplan, R.E.W.; Saltiel, A.R.; Walker, C.L.; Reiner, D.J.; Der, C.J. Ral and Rheb GTPase Activating Proteins Integrate mTOR and GTPase Signaling in Aging, Autophagy, and Tumor Cell Invasion. Mol. Cell 2014, 53, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, S.; Yoshida, R.; Shi, C.Y.; Yoshimachi, S.; Sakata, N.; Goto, K.; Kimura, T.; Shirakawa, R.; Nakayama, H.; et al. Ral GTPase Activation by Downregulation of RalGAP Enhances Oral Squamous Cell Carcinoma Progression. J. Dent. Res. 2019, 98, 1011–1019. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, G.; Zhang, Y.; Xu, J.; Chen, C.; Zhang, L.; Xu, Z.; Du, X. Effects of RNA interference-mediated NRP-1 silencing on the proliferation and apoptosis of breast cancer cells. Mol. Med. Rep. 2015, 12, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Al-Zeheimi, N.; Bakheit, C.S.; Al Riyami, M.; Al Jarrah, A.; Al Moundhri, M.S.; Al Habsi, Z.; Basheer, M.; Adham, S.A. Neuropilin-1 Associated Molecules in the Blood Distinguish Poor Prognosis Breast Cancer: A Cross-Sectional Study. Sci. Rep. 2017, 7, 3301. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Kasimir-Bauer, S.; Goebel, A.; Erdmann, K.; Hoffmann, O.; Rauner, M.; Hofbauer, L.C.; Kimmig, R.; Bittner, A.K. Soluble Neuropilin-1 is an independent marker of poor prognosis in early breast cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeheimi, N.; Adham, S.A. Modeling Neoadjuvant chemotherapy resistance in vitro increased NRP-1 and HER2 expression and converted MCF7 breast cancer subtype. Br. J. Pharmacol. 2020, 177, 2024–2041. [Google Scholar] [CrossRef] [PubMed]
- Cuk, K.; Zucknick, M.; Madhavan, D.; Schott, S.; Golatta, M.; Heil, J.; Marmé, F.; Turchinovich, A.; Sinn, P.; Sohn, C.; et al. Plasma MicroRNA Panel for Minimally Invasive Detection of Breast Cancer. PLoS ONE 2013, 8, e76729. [Google Scholar] [CrossRef] [PubMed]
- Holý, P.; Brynychová, V.; Šeborová, K.; Haničinec, V.; Koževnikovová, R.; Trnková, M.; Vrána, D.; Gatěk, J.; Kopečková, K.; Mrhalová, M.; et al. Integrative analysis of mRNA and miRNA expression profiles and somatic variants in oxysterol signaling in early-stage luminal breast cancer. Mol. Oncol. 2023, 17, 2074–2089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Wang, H.; Zheng, X.; Li, C.; Han, Z. miR-376a inhibits breast cancer cell progression by targeting neuropilin-1, N.R. Onco Targets Ther. 2018, 11, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Di Cunto, F.; Provero, P. Shortening of 3′UTRs Correlates with Poor Prognosis in Breast and Lung Cancer. PLoS ONE 2012, 7, e31129. [Google Scholar] [CrossRef]
- Bhavsar, S.P.; Olsen, L.; Løkke, C.; Koster, J.; Flægstad, T.; Einvik, C. Hsa-miR-323a-3p functions as a tumor suppressor and targets STAT3 in neuroblastoma cells. Front. Pediatr. 2023, 11, 1098999. [Google Scholar] [CrossRef]
- Men, Y.; Zhai, Y.; Wu, L.; Liu, L.; Zhang, W.; Jiang, W.; Bi, N.; Song, Y.; Hui, Z.; Wang, L. MiR-323a-3p acts as a tumor suppressor by suppressing FMR1 and predicts better esophageal squamous cell carcinoma outcome. Cancer Cell Int. 2022, 22, 140. [Google Scholar] [CrossRef]
- Xuan, D.T.M.; Yeh, I.J.; Su, C.Y.; Liu, H.L.; Ta, H.D.K.; Anuraga, G.; Chiao, C.C.; Wang, C.Y.; Yen, M.C. Prognostic and Immune Infiltration Value of Proteasome Assembly Chaperone (PSMG) Family Genes in Lung Adenocarcinoma. Int. J. Med. Sci. 2023, 20, 87–101. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Zhou, Y.; Maimaitirexiati, G.; Yan, Q.; Li, Y.; Maimaitiyimin, A.; Zhou, C.; Ren, J.; Liu, C.; et al. LncRNA SCAMP1 disrupts the balance between miR-26a-5p and ZEB2 to promote osteosarcoma cell viability and invasion. Front. Oncol. 2022, 12, 967000. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, Y.; Liu, Z.; Zhang, Y.; Cao, M.; Wang, W.; Tao, J.; Yao, Y. INSM1 promotes breast carcinogenesis by regulating, C.-M.Y.C. Am. J. Cancer Res. 2023, 13, 3500–3516. [Google Scholar]
- Razvi, H.; Tsang, J.Y.; Poon, I.K.; Chan, S.K.; Cheung, S.Y.; Shea, K.H.; Tse, G.M. INSM1 is a novel prognostic neuroendocrine marker for luminal B breast cancer. Pathology 2021, 53, 170–178. [Google Scholar] [CrossRef]
- Zhong, E.; Pareja, F.; Hanna, M.G.; Jungbluth, A.A.; Rekhtman, N.; Brogi, E. Expression of novel neuroendocrine markers in breast carcinomas: A study of INSM1, ASCL1, and POU2F3. Hum. Pathol. 2022, 127, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Castellano, I.; Marinelli, E.; Osella-Abate, S.; Sapino, A.; Cassoni, P.; Papotti, M. INSM1 Expression in Breast Neoplasms with Neuroedocrine Features. Endocr. Pathol. 2021, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liu, P.; Xu, H.; Liang, D.; Fang, K.; Du, S.; Cheng, W.; Ye, L.; Liu, T.; Zhang, X.; et al. SASH1 suppresses triple-negative breast cancer cell invasion through YAP-ARHGAP42-actin axis. Oncogene 2020, 39, 5015–5030. [Google Scholar] [CrossRef] [PubMed]
- Zeller, C.; Hinzmann, B.; Seitz, S.; Prokoph, H.; Burkhard-Goettges, E.; Fischer, J.; Jandrig, B.; Schwarz, L.E.; Rosenthal, A.; Scherneck, S. SASH1: A candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 2003, 22, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Gnann, A.; Scheikl, D.; Holzmann, B.; Janssen, K.P. The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell–matrix adhesion. Int. J. Biochem. Cell Biol. 2011, 43, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Dauphinee, S.M.; Clayton, A.; Hussainkhel, A.; Yang, C.; Park, Y.J.; Fuller, M.E.; Blonder, J.; Veenstra, T.D.; Karsan, A. SASH1 Is a Scaffold Molecule in Endothelial TLR4 Signaling. J. Immunol. 2013, 191, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Grillone, K.; Riillo, C.; Scionti, F.; Rocca, R.; Tradigo, G.; Guzzi, P.H.; Alcaro, S.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. Non-coding RNAs in cancer: Platforms and strategies for investigating the genomic “dark matter”. J. Exp. Clin. Cancer Res. 2020, 39, 117. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Imai-Sumida, M.; Tanaka, Y.; Dahiya, R. Interaction and cross-talk between non-coding RNAs. Cell. Mol. Life Sci. 2018, 75, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Qiu, X.; Lu, Y.; Zhang, M. SOCS1 and its Potential Clinical Role in Tumor. Pathol. Oncol. Res. 2019, 25, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Rohini, M.; Gokulnath, M.; Miranda, P.J.; Selvamurugan, N. miR-590–3p inhibits proliferation and promotes apoptosis by targeting activating transcription factor 3 in human breast cancer cells. Biochimie 2018, 154, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.I.; Khaled, G.M.; Amleh, A. Functional role and epithelial to mesenchymal transition of the miR-590-3p/MDM2 axis in hepatocellular carcinoma. BMC Cancer 2023, 23, 396. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Shan, Y.; Bernaudo, S.; Peng, C. miR-590-3p Targets Cyclin G2 and FOXO3 to Promote Ovarian Cancer Cell Proliferation, Invasion, and Spheroid Formation. Int. J. Mol. Sci. 2019, 20, 1810. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Pan, H.; Wei, W.; Yang, H.; Liu, J.; Yang, R. GOLPH3: A novel biomarker that correlates with poor survival and resistance to chemotherapy in breast cancer. Oncotarget 2017, 8, 105155–105169. [Google Scholar] [CrossRef]
- Kalpana, G.; Figy, C.; Yeung, M.; Yeung, K.C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 2019, 9, 16351. [Google Scholar] [CrossRef]
- Pellegrino, M.; Rizza, P.; Donà, A.; Nigro, A.; Ricci, E.; Fiorillo, M.; Perrotta, I.; Lanzino, M.; Giordano, C.; Bonofiglio, D.; et al. FoxO3a as a Positive Prognostic Marker and a Therapeutic Target in Tamoxifen-Resistant Breast Cancer. Cancers 2019, 11, 1858. [Google Scholar] [CrossRef]
- Zou, Y.; Tsai, W.B.; Cheng, C.J.; Hsu, C.; Chung, Y.M.; Li, P.C.; Lin, S.H.; Hu, M.C.T. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008, 10, R21. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liu, C.; Li, W.; Zhou, F.; Wang, X.; Zheng, J. The Apolipoprotein C1 is involved in breast cancer progression via EMT and MAPK/JNK pathway. Pathol. Res. Pract. 2022, 229, 153746. [Google Scholar] [CrossRef]
- Xie, S.Y.; Shi, D.B.; Ouyang, Y.; Lin, F.; Chen, X.Y.; Jiang, T.C.; Xia, W.; Guo, L.; Lin, H.X. SHMT2 promotes tumor growth through VEGF and MAPK signaling pathway in breast cancer. Am. J. Cancer Res. 2022, 12, 3405–3421. [Google Scholar]
- Jiang, W.; Wang, X.; Zhang, C.; Xue, L.; Yang, L. Expression and clinical significance of MAPK and EGFR in triple-negative breast cancer. Oncol. Lett. 2020, 19, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Hidayat, H.J.; Abdullah, S.R.; Mohamadtahr, S.; Rasul, M.F.; Samsami, M.; Taheri, M. Role of long non-coding RNAs and TGF-β signaling in the regulation of breast cancer pathogenesis and therapeutic targets. Cytokine 2023, 170, 156351. [Google Scholar] [CrossRef]
- Kawasaki, N.; Miwa, T.; Hokari, S.; Sakurai, T.; Ohmori, K.; Miyauchi, K.; Miyazono, K.; Koinuma, D. Long noncoding RNA NORAD regulates transforming growth factor-β signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 2018, 109, 2211–2220. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Á.; Vera, I.; Diaz, M.; Navarro, C.; Rojas, M.; Torres, W.; Parra, H.; Salazar, J.; De Sanctis, J.; Bermúdez, V. The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises. Int. J. Mol. Sci. 2021, 23, 430. [Google Scholar] [CrossRef]
- Hillmer, R.E.; Link, B.A. The Roles of Hippo Signaling Transducers Yap and Taz in Chromatin Remodeling. Cells 2019, 8, 502. [Google Scholar] [CrossRef]
- Das, A.; Fischer, R.S.; Pan, D.; Waterman, C.M. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J. Biol. Chem. 2016, 291, 6096–6110. [Google Scholar] [CrossRef]
- Von der Lippe Gythfeldt, H.; Lien, T.; Tekpli, X.; Silwal-Pandit, L.; Borgen, E.; Garred, Ø.; Skjerven, H.; Schlichting, E.; Lundgren, S.; Wist, E.; et al. Immune phenotype of tumor microenvironment predicts response to bevacizumab in neoadjuvant treatment of ER-positive breast cancer. Int. J. Cancer 2020, 147, 2515–2525. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; De Wilde, R.L.; Feng, R.; Su, M.; Torres-de la Roche, L.A.; Shi, W. A Machine Learning Model to Predict the Triple Negative Breast Cancer Immune Subtype. Front. Immunol. 2021, 12, 749459. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.N.; Zhou, X.B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.S.; Zhang, H.; et al. The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Lyu, X.; Yuan, S.; Wang, S.; Li, W.; Chen, Z.; Yu, H.; Li, F.; Jiang, Q. Oxidative stress: A critical hint in ionizing radiation induced pyroptosis. Radiat. Med. Prot. 2020, 1, 179–185. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Pan, S.; Yang, T.; Sun, X.; Wang, Y.; Shi, X.; Zhao, X.; Guo, J.; Zhang, X. LINC00657 played oncogenic roles in esophageal squamous cell carcinoma by targeting miR-615-3p and JunB. Biomed. Pharmacother. 2018, 108, 316–324. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Ma, Y.; Li, J.; Sun, X.; Zhao, X.; Shi, X.; Hu, Y.; Qu, F.; Zhang, X. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J. Exp. Clin. Cancer Res. 2021, 40, 306. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, J.; Yuan, J.; Li, J.; Yang, C.; Du, G.; Zhou, C.; Qiu, P.; Gao, J.; Zhang, Y.; et al. DNA damage chemotherapeutic drugs suppress basal-like breast cancer growth by down-regulating the transcription of the FOXO1-KLF5 axis. Genes Dis. 2024, 11, 91–94. [Google Scholar] [CrossRef]
- Flores, D.; Lopez, A.; Udawant, S.; Gunn, B.; Keniry, M. The FOXO1 inhibitor AS1842856 triggers apoptosis in glioblastoma multiforme and basal-like breast cancer cells. FEBS Open Bio 2023, 13, 352–362. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Chen, M.J.; Lin, J.C.; Lin, C.H.; Huang, W.C.; Cheng, S.P.; Chen, S.N.; Chang, Y.C. Doxorubicin Promotes Migration and Invasion of Breast Cancer Cells through the Upregulation of the RhoA/MLC Pathway. J. Breast Cancer 2019, 22, 185. [Google Scholar] [CrossRef]
- Barzaman, K.; Moradi-Kalbolandi, S.; Hosseinzadeh, A.; Kazemi, M.H.; Khorramdelazad, H.; Safari, E.; Farahmand, L. Breast cancer immunotherapy: Current and novel approaches. Int. Immunopharmacol. 2021, 98, 107886. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Kubo, M.; Umebayashi, M.; Yew, P.Y.; Yoshimura, S.; Park, J.H.; Kiyotani, K.; Kai, M.; Yamada, M.; Oda, Y.; et al. Neoantigens elicit T cell responses in breast cancer. Sci. Rep. 2021, 11, 13590. [Google Scholar] [CrossRef]
- Barros, E.M.; McIntosh, S.A.; Savage, K.I. The DNA damage induced immune response: Implications for cancer therapy. DNA Repair. 2022, 120, 103409. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Sahin, I.; Carneiro, B.A.; Dizon, D.S.; Safran, H.P.; El-Deiry, W.S. Strategies to sensitize cancer cells to immunotherapy. Hum. Vaccin. Immunother. 2021, 17, 2595–2601. [Google Scholar] [CrossRef]
| Targets | In Vitro | In Vivo | Mechanism of ACTION | BC Impact | References |
|---|---|---|---|---|---|
| PUM/PSMG4 | N/A | Human bioinformatic database (TCGA) and tumors | NORAD targets PUM, PUM targets PSMG4, and NORAD co-expresses with PSMG4 | Lower DFS in BL | [45] |
| PUM2/ miR-376a/ NRP-1 | Human cell lines (MCF-7, T47D, MDA-MB-231, MDA-MB-453, HMEpC) | Human tumors | NORAD targets PUM; PUM2 and miR-376a competitive-bind to NRP-1 | Higher cell stemness | [46] |
| PUM/ RALGAPB | N/A | Human bioinformatic databases (TCGA, cBioPortal) | NORAD co-expresses with RALGAPB and PUM targets RALGAPB | Worse prognosis and poor OS in LumA; subtype biomarker | [41] |
| PUM/ c-JUN, FOXO1, NRAS, PTEN | Human cell lines (SK-BR-3, MDA-MB-231, CAL51, BT-20, BT-549) | Human bioinformatic database (GEO) and tumors | NORAD targets PUM; PUM targets c-JUN, FOXO1, NRAS and PTEN | Lower cell proliferation and invasion | [47] |
| PUM1/ eIF2/ PERK/ ATF4 | Human cell lines (MCF10A, MCF-7, MDA-MB-231, MDA-MB-468, MDA-MB-453, T47D) | Human tumors and cancer xenograft mouse models | NORAD targets PUM; PUM1 targets eIF2/PERK/ATF4 | Suppression of tumor growth; lower cell viability, proliferation, migration and invasion | [30] |
| S100P | Human cell lines (293FT, MDA-MB-231, Hs578T, T47D, ZR75) | Human bioinformatic databases (TCGA, GEO, PROGgeneV2) and tumors, cancer mouse models | NORAD binds S100P, preventing its binding to TP53 and IQGAP1 | Suppression of migration, invasion and metastasis | [39] |
| PUM2/ INSM1/ SASH1/ PI3K/AKT | Human cell lines (MCF-10A, MCF-7, MDA-MB-231) | Human tumors and cancer xenograft mouse models | NORAD targets PUM; PUM2 targets INSM1, decreasing SASH1 repression and inhibiting PI3K/AKT | Lower cell viability, migration, invasion and tumor growth and reduced apoptosis | [48] |
| MAPK14 | N/A | Human bioinformatic databases (HGNC, lncBase v2, Expression Atlas, Co-lncRNA) and tumors | NORAD co-expresses with MAPK14 | Biomarker | [49] |
| miR-155-5p and SOCS1 | Human cell lines (HCC70, MCF-7, SKBR-3 and T-47D) | N/A | NORAD targets miR-155-5p, preventing its binding to SOCS1 | Lower cell proliferation and invasion | [38] |
| miR-590-3p and GOLPH3 | Human cell lines (MCF-7, MDA-MB-231, T47D, BT-549) | Human tumors | NORAD targets miR-590-3p, preventing the degradation of GOLPH3 | Higher cell proliferation, invasion and migration and lower apoptosis | [32] |
| miRNAs/FOXO3 and RHOA | N/A | Human bioinformatic database (GEO) | NORAD interacts with miR-183, miR-182, miR-7, miR-149, miR-200c, miR-101 and miR-342, regulates FOXO3 and RHOA | Biomarker | [50,51] |
| γ-H2AX | Human cell lines (MCF-7, MDA-MB-231, MDA-MB-436, MDA-MB-468) | N/A | NORAD recruits DDR proteins that repair damage through the phosphorylation of H2AX | Lower cell proliferation and invasion | [52] |
| TGF-β/ RUNX2 | Human cell lines (MDA-MB-231 and MCF-7) | Human tumors and cancer mouse models | NORAD depletion decreases TGF-β protein expression | Higher cell proliferation, invasion and migration and worse prognosis | [35] |
| Immune cells | Human cell lines (MCF-10A, MDA-MB-231) | Human bioinformatic database (TCGA) and tumors | NORAD higher in low CD8 T-cell count; the promotion of malignant M2 macrophage polarization by exosome internalization | Poorer prognosis, higher tumor progression | [36,53] |
| Therapy | BC Application | Mechanism of Action | Barriers to Therapy Response | Potential Impact of NORAD Depletion | Impact of NORAD Depletion in Therapy Response | References |
|---|---|---|---|---|---|---|
| PARP inhibitors | BRCA mutations | Impairment of SSB repair | Restoration of HR | Improved PARP downregulation and impairment of DDR | Inhibition of tumor cell growth and proliferation | [52] |
| DNA damage-inducing chemotherapy | First-line therapy | DNA damage leads to apoptosis and inhibition of proliferation | DNA damage repair and resistance to therapy | Potential synergistic effect on FOXO1 downregulation | Reinforcement of apoptosis and inhibition of proliferation | [113] |
| FOXO1 inhibitor (AS1842856) | BL tumors | FOXO1 pathway inhibition | Inhibitor does not bind to the phosphorylated form of FOXO1 | Potential synergistic effect on downregulating FOXO1 and its phosphorylated form | Reinforcement of apoptosis and inhibition of proliferation | [114] |
| PAM inhibitors combined with CDK4/6 inhibitors | ER+ tumors | PAM downregulation leads to the diminished capability of BC to acquire resistance to endocrine therapy | Acquired resistance to endocrine therapy | mTOR inhibition | Improved sensitization of tumor cells to endocrine therapy | [115] |
| PAM inhibitors combined with anti-HER2 antibodies | HER2+ tumors | PAM downregulation sensitizes to anti-HER2 antibodies | Acquired resistance to HER2 antibodies | Synergistic effect on downregulating PAM | Improved sensitization of tumor cells to HER2 antibodies | [115] |
| Doxorubicin | First-line therapy | DNA DSB and activation of RhoA/MLC pathway | Promotes migration and invasion via RhoA/MLC pathway | Impairment of DNA damage repair machinery | Decreased tumor cell survival and inhibition of migration and invasion | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capela, A.M.; Tavares-Marcos, C.; Estima-Arede, H.F.; Nóbrega-Pereira, S.; Bernardes de Jesus, B. NORAD-Regulated Signaling Pathways in Breast Cancer Progression. Cancers 2024, 16, 636. https://doi.org/10.3390/cancers16030636
Capela AM, Tavares-Marcos C, Estima-Arede HF, Nóbrega-Pereira S, Bernardes de Jesus B. NORAD-Regulated Signaling Pathways in Breast Cancer Progression. Cancers. 2024; 16(3):636. https://doi.org/10.3390/cancers16030636
Chicago/Turabian StyleCapela, Ana Maria, Carlota Tavares-Marcos, Hugo F. Estima-Arede, Sandrina Nóbrega-Pereira, and Bruno Bernardes de Jesus. 2024. "NORAD-Regulated Signaling Pathways in Breast Cancer Progression" Cancers 16, no. 3: 636. https://doi.org/10.3390/cancers16030636
APA StyleCapela, A. M., Tavares-Marcos, C., Estima-Arede, H. F., Nóbrega-Pereira, S., & Bernardes de Jesus, B. (2024). NORAD-Regulated Signaling Pathways in Breast Cancer Progression. Cancers, 16(3), 636. https://doi.org/10.3390/cancers16030636







