Survival of Women with Advanced Stage Cervical Cancer: Neo-Adjuvant Chemotherapy Followed by Radiotherapy and Hyperthermia versus Chemoradiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Study Design
2.2. Chemoradiotherapy with or without Nodal Debulking
2.3. Triple Therapy
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Treatment Characteristics
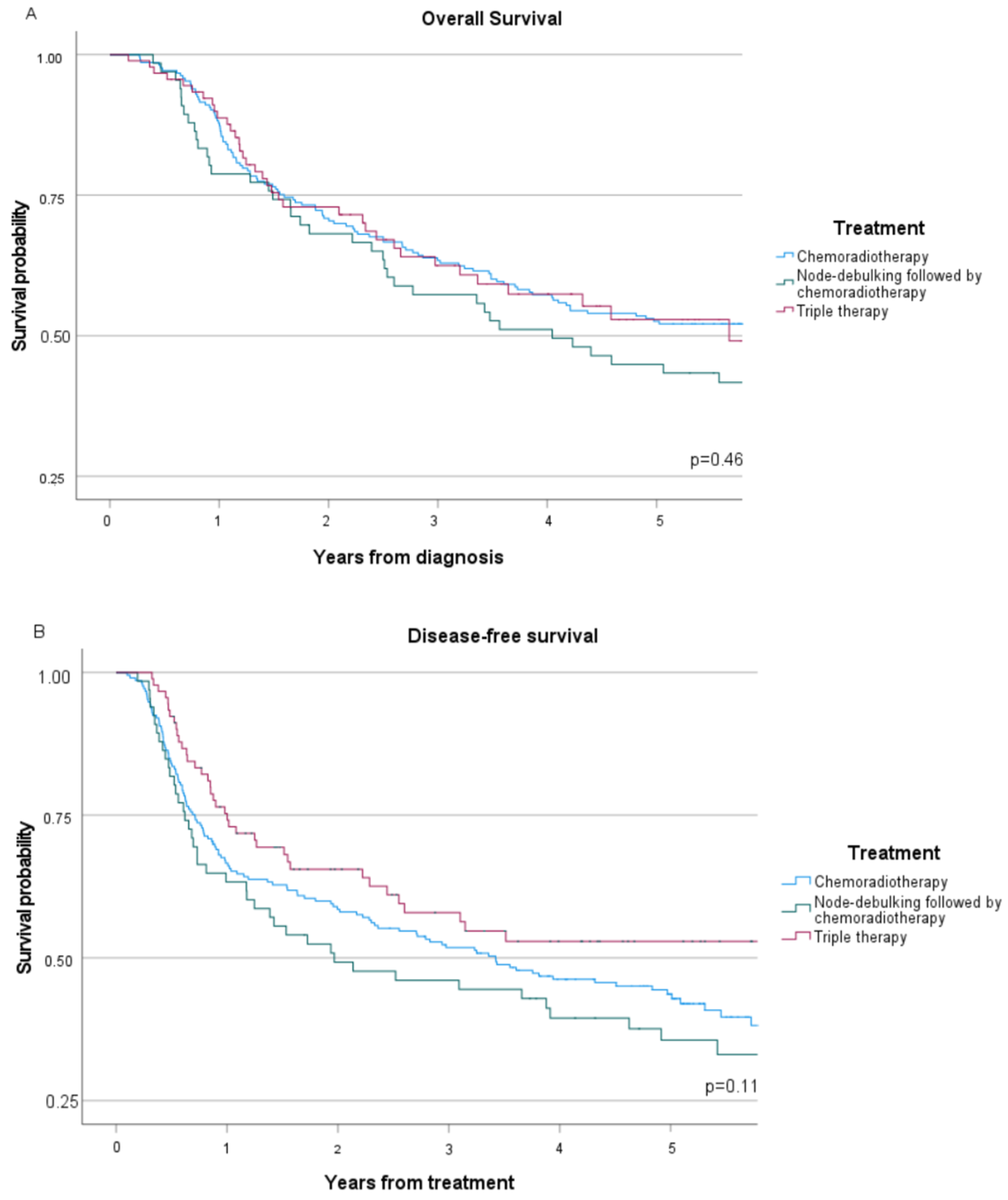
3.3. Oncological Outcome
3.4. Toxicity
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leath, C.A., 3rd; Monk, B.J. Twenty-first century cervical cancer management: A historical perspective of the gynecologic oncology group/NRG oncology over the past twenty years. Gynecol. Oncol. 2018, 150, 391–397. [Google Scholar] [CrossRef]
- Du, R.; Li, L.; Ma, S.; Tan, X.; Zhong, S.; Wu, M. Lymph nodes metastasis in cervical cancer: Incidences, risk factors, consequences and imaging evaluations. Asia Pac. J. Clin. Oncol. 2018, 14, e380–e385. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fang, J.; Zhang, L.; Huang, Y.; Shen, H.; Ma, X.; Zhang, S.; Zhang, B. Efficacy and safety of adjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2023, 184, 103953. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Gallardo Rincón, D.; Eminowicz, G.; Diez, P.; Farrelly, L.; Kent, C.; Hudson, E.; Panades, M.; Mathews, T.; Anand, A.; et al. LBA8 A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer: The GCIG INTERLACE trial. Ann. Oncol. 2023, 34, S1276. [Google Scholar] [CrossRef]
- Kenter, G.G.; Greggi, S.; Vergote, I.; Katsaros, D.; Kobierski, J.; van Doorn, H.; Landoni, F.; van der Velden, J.; Reed, N.; Coens, C.; et al. Randomized Phase III Study Comparing Neoadjuvant Chemotherapy Followed by Surgery Versus Chemoradiation in Stage IB2-IIB Cervical Cancer: EORTC-55994. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 5035–5043. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri Chopra, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Olthof, E.P.; Wenzel, H.; van der Velden, J.; Spijkerboer, A.M.; Bekkers, R.; Beltman, J.J.; Nijman, H.W.; Slangen, B.; Smolders, R.; van Trommel, N.; et al. Treatment of bulky lymph nodes in locally advanced cervical cancer: Boosting versus debulking. Int. J. Gynecol. Cancer 2022, 32, 861–868. [Google Scholar] [CrossRef] [PubMed]
- IJff, M.; Crezee, J.; Oei, A.L.; Stalpers, L.J.A.; Westerveld, H. The role of hyperthermia in the treatment of locally advanced cervical cancer: A comprehensive review. Int. J. Gynecol. Cancer 2022, 32, 288–296. [Google Scholar] [CrossRef]
- Heijkoop, S.T.; Franckena, M.; Thomeer, M.G.; Boere, I.A.; Van Montfort, C.; Van Doorn, H.C. Neoadjuvant chemotherapy followed by radiotherapy and concurrent hyperthermia in patients with advanced-stage cervical cancer: A retrospective study. Int. J. Hyperth. 2012, 28, 554–561. [Google Scholar] [CrossRef]
- Gao, X.S.; Boere, I.A.; van Beekhuizen, H.J.; Franckena, M.; Nout, R.; Kruip, M.; Kulawska, M.D.; van Doorn, H.C. Acute and long-term toxicity in patients undergoing induction chemotherapy followed by thermoradiotherapy for advanced cervical cancer. Int. J. Hyperth. 2022, 39, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.; Gonzalez Gonzalez, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, L.C.; Koper, P.C.; Jobsen, J.J.; van der Steen-Banasik, E.M.; Creutzberg, C.L.; van den Berg, H.A.; Ottevanger, P.B.; van Rhoon, G.C.; van Doorn, H.C.; Houben, R.; et al. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: Results of the randomized RADCHOC trial. Radiother. Oncol. 2016, 120, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Yea, J.W.; Park, J.W.; Oh, S.A.; Park, J. Chemoradiotherapy with hyperthermia versus chemoradiotherapy alone in locally advanced cervical cancer: A systematic review and meta-analysis. Int. J. Hyperth. 2021, 38, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) v4.0; National Institutes of Health: Bethesda, MD, USA, 2010.
- Pötter, R.; Tanderup, K.; Schmid, M.; Jürgenliemk-Schulz, I.; Haie Meder, C.; Fokdal, L.; Sturdza, A.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- van der Zee, J.; Gonzalez, G.D. The Dutch Deep Hyperthermia Trial: Results in cervical cancer. Int. J. Hyperth. 2002, 18, 1–12. [Google Scholar] [CrossRef]
- Shrivastava, S.; Mahantshetty, U.; Engineer, R.; Chopra, S.; Hawaldar, R.; Hande, V.; Kerkar, R.A.; Maheshwari, A.; Shylasree, T.S.; Ghosh, J.; et al. Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Harima, Y.; Ohguri, T.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int. J. Hyperth. 2016, 32, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, W.; Che, S.; Zhang, Y.; Meng, D.; Shi, F.; Su, J.; Yang, Y.; Ma, H.; Liu, R.; et al. Outcomes for Hyperthermia Combined with Concurrent Radiochemotherapy for Patients with Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 499–511. [Google Scholar] [CrossRef]
- Matsushita, S.; Reynolds, R.; Urano, M. Synergism between alkylating agent and cis-platin with moderate local hyperthermia: The effect of multidrug chemotherapy in an animal system. Int. J. Hyperth. 1993, 9, 285–296. [Google Scholar] [CrossRef]
- Liao, S.; Hu, X.; Liu, Z.; Lin, Y.; Liang, R.; Zhang, Y.; Li, Q.; Li, Y.; Liao, X. Synergistic action of microwave-induced mild hyperthermia and paclitaxel in inducing apoptosis in the human breast cancer cell line MCF-7. Oncol. Lett. 2019, 17, 603–615. [Google Scholar] [CrossRef]
- Marnitz, S.; Kohler, C.; Roth, C.; Fuller, J.; Hinkelbein, W.; Schneider, A. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol. Oncol. 2005, 99, 536–544. [Google Scholar] [CrossRef]
- Leblanc, E.; Narducci, F.; Frumovitz, M.; Lesoin, A.; Castelain, B.; Baranzelli, M.C.; Taieb, S.; Fournier, C.; Querleu, D. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol. Oncol. 2007, 105, 304–311. [Google Scholar] [CrossRef]
- Diaz-Feijoo, B.; Acosta, U.; Torne, A.; Gil-Ibanez, B.; Hernandez, A.; Domingo, S.; Bradbury, M.; Gil-Moreno, A. Surgical Outcomes of Laparoscopic Pelvic Lymph Node Debulking during Staging Aortic Lymphadenectomy in Locally Advanced Cervical Cancer: A Multicenter Study. Cancers 2022, 14, 1974. [Google Scholar] [CrossRef]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Monk, B.J.; Toita, T.; Wu, X.; Vazquez Limon, J.C.; Tarnawski, R.; Mandai, M.; Shapira-Frommer, R.; Mahantshetty, U.; Del Pilar Estevez-Diz, M.; Zhou, Q.; et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva Galves, M.H.; Ramos Elias, P.; Acevedo, A.; Vizkeleti, J.; Gomes, A.J.P.D.S.; Contreras Mejia, F.; et al. LBA38 Pembrolizumab plus chemoradiotherapy for high-risk locally advanced cervical cancer: A randomized, double-blind, phase III ENGOT-cx11/GOG-3047/KEYNOTE-A18 study. Ann. Oncol. 2023, 34, 1279–1280. [Google Scholar] [CrossRef]
- de Azevedo, C.R.; Thuler, L.C.; de Mello, M.J.; Ferreira, C.G. Neoadjuvant Chemotherapy Followed by Chemoradiation in Cervical Carcinoma: A Review. Int. J. Gynecol. Cancer 2016, 26, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, H.; Shen, L.; Wang, Q.; Shao, S.; Shen, Y.; Xu, H.; Liu, H.; Cai, R.; et al. Neoadjuvant chemotherapy with weekly cisplatin and paclitaxel followed by chemoradiation for locally advanced cervical cancer. BMC Cancer 2023, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Allahqoli, L.; Hakimi, S.; Lagana, A.S.; Momenimovahed, Z.; Mazidimoradi, A.; Rahmani, A.; Fallahi, A.; Salehiniya, H.; Ghiasvand, M.M.; Alkatout, I. 18F-FDG PET/MRI and 18F-FDG PET/CT for the Management of Gynecological Malignancies: A Comprehensive Review of the Literature. J. Imaging 2023, 9, 223. [Google Scholar] [CrossRef]
- Knoth, J.; Potter, R.; Jurgenliemk-Schulz, I.M.; Haie-Meder, C.; Fokdal, L.; Sturdza, A.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; Bruheim, K.; et al. Clinical and imaging findings in cervical cancer and their impact on FIGO and TNM staging—An analysis from the EMBRACE study. Gynecol. Oncol. 2020, 159, 136–141. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Blecharz, P.; Skotnicki, P.; Reinfuss, M.; Walasek, T.; Luczynska, E. Toxicity of concurrent chemoradiotherapy for locally advanced cervical cancer. Eur. J. Gynaecol. Oncol. 2014, 35, 393–399. [Google Scholar] [PubMed]
| Patient Characteristics | CRT (n = 213) | LND-CRT (n = 66) | TT (n = 91) | Missing (%) | p-Value |
|---|---|---|---|---|---|
| Median age, years (range) | 50 (22–86) | 50 (25–77) | 45 (22–79) | - | 0.01 * |
| BMI, kg/m2 (range) | 25 (15–51) | 25 (18–39) | 24 (16–42) | 23 (6) | 1.00 |
| Smoking, yes | 74 (35) | 25 (38) | 41 (45) | 49 (13) | 0.02 * |
| FIGO 2009 stage | 1 (0.3) | 0.02 * | |||
| IB2 | 32 (15) | 15 (23) | 9 (10) | ||
| IIA2 | 14 (7) | 4 (6) | 4 (4) | ||
| IIB | 90 (42) | 28 (42) | 46 (51) | ||
| III | 60 (28) | 16 (24) | 17 (19) | ||
| IVA | 17 (8) | 3 (5) | 14 (15) | ||
| Histology | 2 (1) | 0.95 | |||
| SCC | 196 (92) | 60 (91) | 82 (90) | ||
| Non-SCC | 17 (8) | 6 (9) | 7 (8) | ||
| Mean tumour size, mm (IQR) | 64 (60–70) | 60 (45–70) | 59 (50–66) | 9 (2) | 0.01 * |
| Pelvic lymph node status on imaging | - | <0.01 * | |||
| Negative | 3 (1) | 1 (2) | 13 (14) | ||
| Lymph nodes 0.5–2.0 cm deemed positive | 172 (81) | 17 (26) | 62 (68) | ||
| Bulky ≥ 2 cm | 38 (18) | 48 (73) | 16 (18) | ||
| Para-aortic lymph node status on imaging | 8 (2) | 0.01 * | |||
| Negative | 151 (71) | 37 (56) | 54 (59) | ||
| Lymph nodes 0.5–1.0 cm deemed positive | 19 (9) | 13 (20) | 21 (23) | ||
| Bulky ≥ 1 cm | 37 (17) | 16 (24) | 14 (15) | ||
| Treatment characteristics | |||||
| Nodal boost | 26 (7) | <0.01 * | |||
| Yes | 155 (73) | 27 (41) | 28 (31) | ||
| No | 48 (23) | 35 (53) | 51 (56) | ||
| Extended-field radiotherapy | 13 (4) | 0.01 * | |||
| Yes | 67 (32) | 32 (49) | 47 (52) | ||
| No | 137 (64) | 31 (47) | 42 (46) | ||
| Brachytherapy, yes | 191 (90) | 61 (92) | 82 (90) | 2 (1) | 0.70 |
| Time between diagnosis and start of chemo- or radiotherapy, median days § (IQR) | 47 (38–58) | 61 (49–77) | 35 (20–49) | 1 (0.3) | <0.01 * |
| Survival Data | CRT (n = 213) | LND-CRT (n = 66) | TT (n = 91) | Missing (%) | p-Value |
|---|---|---|---|---|---|
| Interval between start of chemo- or radiotherapy and death or recurrence, months | 36 (1–146) | 22 (2–129) | 26 (4–198) | 3 (1) | 0.77 |
| Recurrence, yes | 99 (47) | 37 (56) | 30 (33) | 1 (0.3) | 0.02 * |
| Location of recurrence | 7 (2) | 0.11 | |||
| No recurrence | 114 (54) | 29 (44) | 60 (66) | ||
| Central pelvic | 15 (7) | 6 (9) | 13 (14) | ||
| Lateral pelvic | 22 (10) | 10 (15) | 16 (1) | ||
| Para-aortic | 23 (11) | 12 (18) | 12 (13) | ||
| Distant metastases | 72 (34) | 27 (41) | 16 (18) | ||
| Infield recurrence, yes | 33 (16) | 15 (23) | 20 (22) | 9 (2) | 0.17 |
| Median time follow-up regarding overall survival, months | 66 (3–169) | 46 (5–171) | 30 (2–208) | - | <0.01 * |
| Vital status, deaths | 116 (55) | 41 (62) | 37 (48) | - | 0.02 * |
| Overall Survival | Disease-Free Survival | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Therapy group | ||||||
| CRT | 1.00 | Reference | 1.00 | Reference | ||
| LND-CRT | 1.40 | 0.95–2.06 | 0.09 | 1.17 | 0.80–1.71 | 0.43 |
| TT | 1.18 | 0.78–1.78 | 0.40 | 0.80 | 0.54–1.19 | 0.27 |
| Age | 1.03 | 1.02–1.04 | <0.01 * | 1.03 | 1.02–1.04 | <0.01 * |
| FIGO 2009 stage | ||||||
| <III | 1.00 | Reference | 1.00 | Reference | ||
| ≥III | 1.63 | 1.19–2.24 | <0.01 * | 1.51 | 1.11–2.06 | <0.01 * |
| Histology | ||||||
| Squamous | 1.00 | Reference | 1.00 | Reference | ||
| Non-squamous | 1.33 | 0.81–2.18 | 0.26 | 1.24 | 0.75–2.04 | 0.40 |
| Tumour size | 1.00 | 1.00–1.01 | 0.50 | 1.00 | 1.00–1.01 | 0.52 |
| Bulky nodes ‡ | ||||||
| Absent | 1.00 | Reference | 1.00 | Reference | ||
| Present | 1.04 | 0.73–1.48 | 0.84 | 1.16 | 0.82–1.64 | 0.41 |
| Location suspicious node | ||||||
| Absent | 1.00 | Reference | 1.00 | Reference | ||
| Pelvic | 1.54 | 0.55–4.31 | 0.41 | 1.45 | 0.52–4.03 | 0.48 |
| Para-aortic | 2.30 | 0.80–6.61 | 0.12 | 2.28 | 0.80–6.51 | 0.12 |
| CRT-O (n = 213) | LND-CRT (n = 66) | TT (n = 91) | p-Value | |
|---|---|---|---|---|
| Chemotherapy-related toxicity | N (%) | N (%) | N (%) | |
| Nephrotoxicity | 8 (4) | 1 (2) | 7 (8) | 0.14 |
| Ototoxicity | 1 (1) | 0 | 1 (1) | 0.64 |
| Mucositis/stomatitis | 0 | 0 | 2 (2) | 0.05 |
| Neurotoxicity | 1 (1) | 0 | 3 (3) | 0.06 |
| Anaphylactic shock | 0 | 0 | 0 | N/A |
| Total | 10 | 1 | 13 | |
| Total patients † | 7 | 1 | 8 | 0.38 |
| Postoperative complications | N/A | N/A | ||
| Intraoperative injury | 2 (3) | |||
| Infection | 3 (5) | |||
| Thromboembolism | 1 (2) | |||
| IC-admission | 1 (2) | |||
| Blood transfusion | 1 (2) | |||
| Bladder dysfunction | 0 | |||
| Total | 8 | |||
| Total patients † | 6 | |||
| Total | 22 | 17 | 17 | |
| Total patients † | 19 (9%) | 11 (17%) | 13 (14%) | 0.15 |
| Toxicity | |||
|---|---|---|---|
| Variables | OR | 95% CI | p-Value |
| Therapy group | |||
| CRT-O | 1.00 | Reference | |
| LND-CRT | 2.04 | 0.92–4.55 | 0.08 |
| TT | 1.70 | 0.80–3.61 | 0.17 |
| Age | 1.02 | 0.99–1.04 | 0.14 |
| Tumour size | 1.01 | 0.99–1.03 | 0.23 |
| FIGO 2009 | |||
| <III | 1.00 | Reference | |
| ≥III | 0.91 | 0.46–1.80 | 0.79 |
| Location suspicious node | |||
| Absent | 1.00 | Reference | |
| Pelvic | 2.09 | 0.27–16.37 | 0.48 |
| Para-aortic | 2.31 | 0.29–18.69 | 0.43 |
| Bulky node ‡ | |||
| Absent | 1.00 | Reference | |
| Present | 1.84 | 0.97–3.52 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servayge, J.; Olthof, E.P.; Mom, C.H.; van der Aa, M.A.; Wenzel, H.H.B.; van der Velden, J.; Nout, R.A.; Boere, I.A.; van Doorn, H.C.; van Beekhuizen, H.J. Survival of Women with Advanced Stage Cervical Cancer: Neo-Adjuvant Chemotherapy Followed by Radiotherapy and Hyperthermia versus Chemoradiotherapy. Cancers 2024, 16, 635. https://doi.org/10.3390/cancers16030635
Servayge J, Olthof EP, Mom CH, van der Aa MA, Wenzel HHB, van der Velden J, Nout RA, Boere IA, van Doorn HC, van Beekhuizen HJ. Survival of Women with Advanced Stage Cervical Cancer: Neo-Adjuvant Chemotherapy Followed by Radiotherapy and Hyperthermia versus Chemoradiotherapy. Cancers. 2024; 16(3):635. https://doi.org/10.3390/cancers16030635
Chicago/Turabian StyleServayge, Jonathan, Ester P. Olthof, Constantijne H. Mom, Maaike A. van der Aa, Hans H. B. Wenzel, Jacobus van der Velden, Remi A. Nout, Ingrid A. Boere, Helena C. van Doorn, and Heleen J. van Beekhuizen. 2024. "Survival of Women with Advanced Stage Cervical Cancer: Neo-Adjuvant Chemotherapy Followed by Radiotherapy and Hyperthermia versus Chemoradiotherapy" Cancers 16, no. 3: 635. https://doi.org/10.3390/cancers16030635
APA StyleServayge, J., Olthof, E. P., Mom, C. H., van der Aa, M. A., Wenzel, H. H. B., van der Velden, J., Nout, R. A., Boere, I. A., van Doorn, H. C., & van Beekhuizen, H. J. (2024). Survival of Women with Advanced Stage Cervical Cancer: Neo-Adjuvant Chemotherapy Followed by Radiotherapy and Hyperthermia versus Chemoradiotherapy. Cancers, 16(3), 635. https://doi.org/10.3390/cancers16030635







