High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material and Study Design
2.2. Assessment of TAMs
2.3. The Standard Histopathological Parameters
2.4. Statistical Methods
3. Results
3.1. Prognostic Value of TAMs in HER2+ Breast Cancer
3.2. Prognostic Value of TAMs in HER2+ Breast Cancer According to Hormone Receptor Status
3.3. Prognostic Value of TAMs in HER2− Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, L.M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early Breast Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023. [Google Scholar] [CrossRef]
- Baxevanis, C.; Sofopoulos, M.; Fortis, S.; Perez, S. The Role of Immune Infiltrates as Prognostic Biomarkers in Patients with Breast Cancer. Cancer Immunol. Immunother. 2019, 68, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The Tale of TILs in Breast Cancer: A Report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-Associated Macrophages in Breast Cancer: Innocent Bystander or Important Player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cao, J.; Zu, X. Tumor-Associated Macrophages: An Important Player in Breast Cancer Progression. Thorac. Cancer 2022, 13, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, T.; Li, M.; Jia, Q. Tumor-Associated Macrophages: Potential Therapeutic Targets and Diagnostic Markers in Cancer. Pathol. Res. Pract. 2023, 249, 154739. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Yang, L.; Xu, Q.; Yuan, H.; Wang, W.; Xia, W.; Gong, D.; Zhang, W.; Yu, K. CD68- and CD163-Positive Tumor Infiltrating Macrophages in Non-Metastatic Breast Cancer: A Retrospective Study and Meta-Analysis. J. Cancer 2019, 10, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic Significance of Tumor-Associated Macrophages in Breast Cancer: A Meta-Analysis of the Literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef] [PubMed]
- Allison, E.; Edirimanne, S.; Matthews, J.; Fuller, S.J. Breast Cancer Survival Outcomes and Tumor-Associated Macrophage Markers: A Systematic Review and Meta-Analysis. Oncol. Ther. 2023, 11, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Kreutzfeldt, J.; Rozeboom, B.; Dey, N.; De, P. The Trastuzumab Era: Current and Upcoming Targeted HER2+ Breast Cancer Therapies. Am. J. Cancer Res. 2020, 10, 1045–1067. [Google Scholar]
- Vivekanandhan, S.; Knutson, K.L. Resistance to Trastuzumab. Cancers 2022, 14, 5115. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.-M.; Oikari, S.; Auvinen, P. High Numbers of Macrophages, Especially M2-like (CD163-Positive), Correlate with Hyaluronan Accumulation and Poor Outcome in Breast Cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Honkanen, T.J.; Tikkanen, A.; Karihtala, P.; Mäkinen, M.; Väyrynen, J.P.; Koivunen, J.P. Prognostic and Predictive Role of Tumour-Associated Macrophages in HER2 Positive Breast Cancer. Sci. Rep. 2019, 9, 10961. [Google Scholar] [CrossRef]
- Zwager, M.C.; Bense, R.; Waaijer, S.; Qiu, S.Q.; Timmer-Bosscha, H.; de Vries, E.G.E.; Schröder, C.P.; van der Vegt, B. Assessing the Role of Tumour-Associated Macrophage Subsets in Breast Cancer Subtypes Using Digital Image Analysis. Breast Cancer Res. Treat. 2023, 198, 11–22. [Google Scholar] [CrossRef]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.B.A.G.; Van Vugt, M.A.T.M.; De Vries, E.G.E.; Schroder, C.P.; Fehrmann, R.S.N. Relevance of Tumor-Infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J. Natl. Cancer Inst. 2017, 109, djw192. [Google Scholar] [CrossRef]
- Andre, F.; Dieci, M.V.; Dubsky, P.; Sotiriou, C.; Curigliano, G.; Denkert, C.; Loi, S. Molecular Pathways: Involvement of Immune Pathways in the Therapeutic Response and Outcome in Breast Cancer. Clin. Cancer Res. 2013, 19, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Luque, M.; Sanz-Álvarez, M.; Morales-Gallego, M.; Madoz-Gúrpide, J.; Zazo, S.; Domínguez, C.; Cazorla, A.; Izarzugaza, Y.; Arranz, J.L.; Cristóbal, I.; et al. Tumor-Infiltrating Lymphocytes and Immune Response in HER2-Positive Breast Cancer. Cancers 2022, 14, 6034. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, M.; Du, X.; Li, S.; Li, H.; Li, X.; Li, Y.; Wang, Y.; Qin, Z.; Fu, Y.-X.; et al. Intratumoral Delivery of IL-21 Overcomes Anti-Her2/Neu Resistance through Shifting Tumor-Associated Macrophages from M2 to M1 Phenotype. J. Immunol. 2015, 194, 4997–5006. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Kosma, V.-M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased Hyaluronan Content and Stromal Cell CD44 Associate with HER2 Positivity and Poor Prognosis in Human Breast Cancer. Int. J. Cancer 2013, 132, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Histological Typing of Breast Tumors, 2nd ed.; WHO: Geneva, Switzerland, 1981. [Google Scholar]
- Tavassoli, F.D.P. (Ed.) Pathology and Genetics of Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours, 3rd ed.; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- AJCC American Joint Committee of Cancer (AJCC) Cancer Staging Manual, 5th ed.; 1997; Available online: https://www.facs.org/media/c35h2r0i/ajcc_5thed_cancer_staging_manual.pdf (accessed on 27 December 2023).
- AJCC American Joint Committee of Cancer (AJCC) Cancer Staging Manual, 6th ed.; 2002; Available online: https://www.facs.org/media/tauiudl3/ajcc_6thed_cancer_staging_manual_part1.pdf (accessed on 27 December 2023).
- Onkar, S.S.; Carleton, N.M.; Lucas, P.C.; Bruno, T.C.; Lee, A.V.; Vignali, D.A.A.; Oesterreich, S. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023, 13, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Griguolo, G.; Pascual, T.; Dieci, M.V.; Guarneri, V.; Prat, A. Interaction of Host Immunity with HER2-Targeted Treatment and Tumor Heterogeneity in HER2-Positive Breast Cancer. J. Immunother. Cancer 2019, 7, 90. [Google Scholar] [CrossRef]
- You, D.; Kim, H.; Jeong, Y.; Yoon, S.Y.; Lo, E.; Kim, S.; Lee, J.E. Tumorigenicity of EGFR- and/or HER2-Positive Breast Cancers Is Mediated by Recruitment of Tumor-Associated Macrophages. Int. J. Mol. Sci. 2023, 24, 1443. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, S.Y.; Jeong, H.; Han, J.; Chae, H.Y.; Yang, H.; Sung, C.O.; Choi, Y.L.; Shin, Y.K.; Kwon, M.J. Matrix Metalloproteinase 11 (MMP11) in Macrophages Promotes the Migration of HER2-Positive Breast Cancer Cells and Monocyte Recruitment through CCL2–CCR2 Signaling. Lab. Investig. 2022, 102, 376–390. [Google Scholar] [CrossRef]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef]
- Kondov, B.; Milenkovikj, Z.; Kondov, G.; Petrushevska, G.; Basheska, N.; Bogdanovska-Todorovska, M.; Tolevska, N.; Ivkovski, L. Presentation of the Molecular Subtypes of Breast Cancer Detected by Immunohistochemistry in Surgically Treated Patients. Open Access Maced. J. Med. Sci. 2018, 6, 961–967. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.T.; Stein, U.; El Tayebi, H.M. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers 2022, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Pegram, M.D.; Lee, K.-W.; Sharma, M.; Lee, J.; Spira, A.I.; Hanna, G.J.; Kang, Y.-K.; Rasco, D.W.; Moore, K.N.; et al. A Phase 1/2 Study of a First-in-Human Immune-Stimulating Antibody Conjugate (ISAC) BDC-1001 in Patients with Advanced HER2-Expressing Solid Tumors. J. Clin. Oncol. 2023, 41, 2358. [Google Scholar] [CrossRef]
- Anna Reiss, K.; Yuan, Y.; Ueno, N.T.; Lynne Johnson, M.; Gill, S.; Claire Dees, E.; Chao, J.; Angelos, M.; Shestova, O.; Stuart Serody, J.; et al. A Phase 1, First-in-Human. (FIH) Study of the Anti-HER2 CAR Macrophage CT-0508 in Subjects with HER2 Overexpressing Solid. Tumors. J. Clin. Oncol. 2022, 40, 2533. [Google Scholar] [CrossRef]
- Jääskeläinen, M.M.; Tiainen, S.; Siiskonen, H.; Ahtiainen, M.; Kuopio, T.; Rönkä, A.; Kettunen, T.; Hämäläinen, K.; Rilla, K.; Harvima, I.; et al. The Prognostic and Predictive Role of Tumor-Infiltrating Lymphocytes (FoxP3+ and CD8+) and Tumor-Associated Macrophages in Early HER2+ Breast Cancer. Breast Cancer Res. Treat. 2023, 201, 183–192. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Pascual, T.; Singh, B.; Nuciforo, P.; Rashid, N.U.; Ballman, K.V.; Campbell, J.D.; Hoadley, K.A.; Spears, P.A.; Pare, L.; et al. Prognostic and Predictive Value of Immune-Related Gene Expression Signatures vs Tumor-Infiltrating Lymphocytes in Early-Stage ERBB2/HER2-Positive Breast Cancer. JAMA Oncol. 2023, 9, 490–499. [Google Scholar] [CrossRef]
- Jamiyan, T.; Kuroda, H.; Yamaguchi, R.; Abe, A.; Hayashi, M. CD68- and CD163-Positive Tumor-Associated Macrophages in Triple Negative Cancer of the Breast. Virchows Arch. 2020, 477, 767–775. [Google Scholar] [CrossRef]
- López-Janeiro, Á.; Padilla-Ansala, C.; de Andrea, C.E.; Hardisson, D.; Melero, I. Prognostic Value of Macrophage Polarization Markers in Epithelial Neoplasms and Melanoma. A Systematic Review and Meta-Analysis. Mod. Pathol. 2020, 33, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
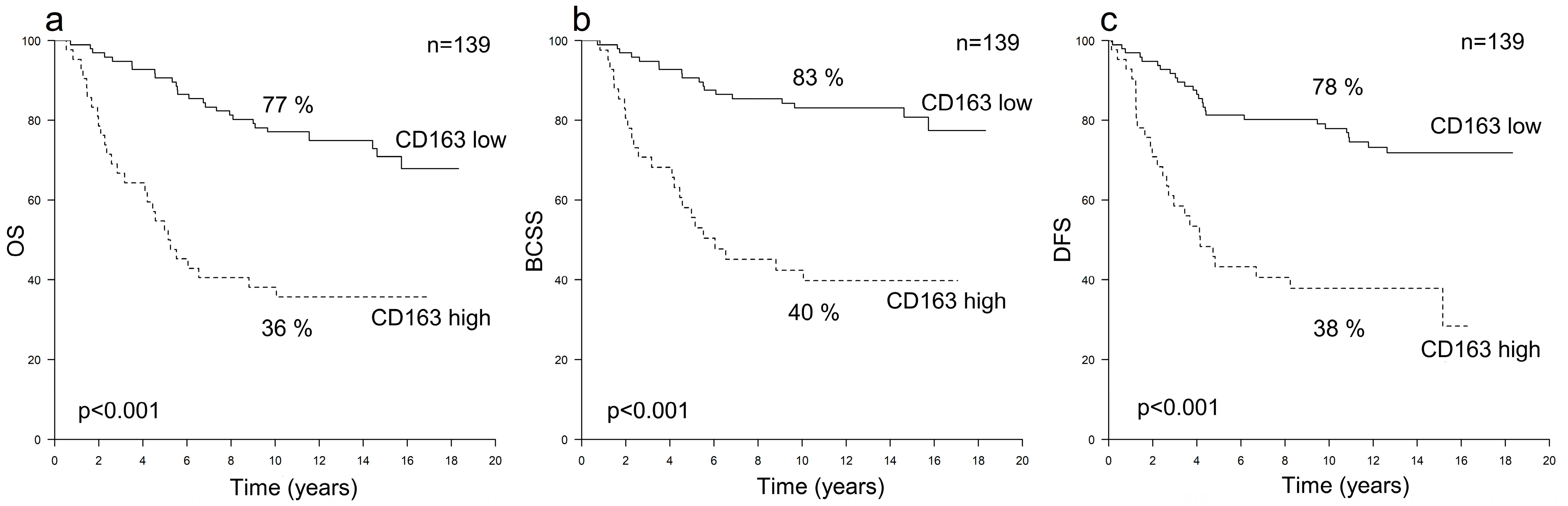
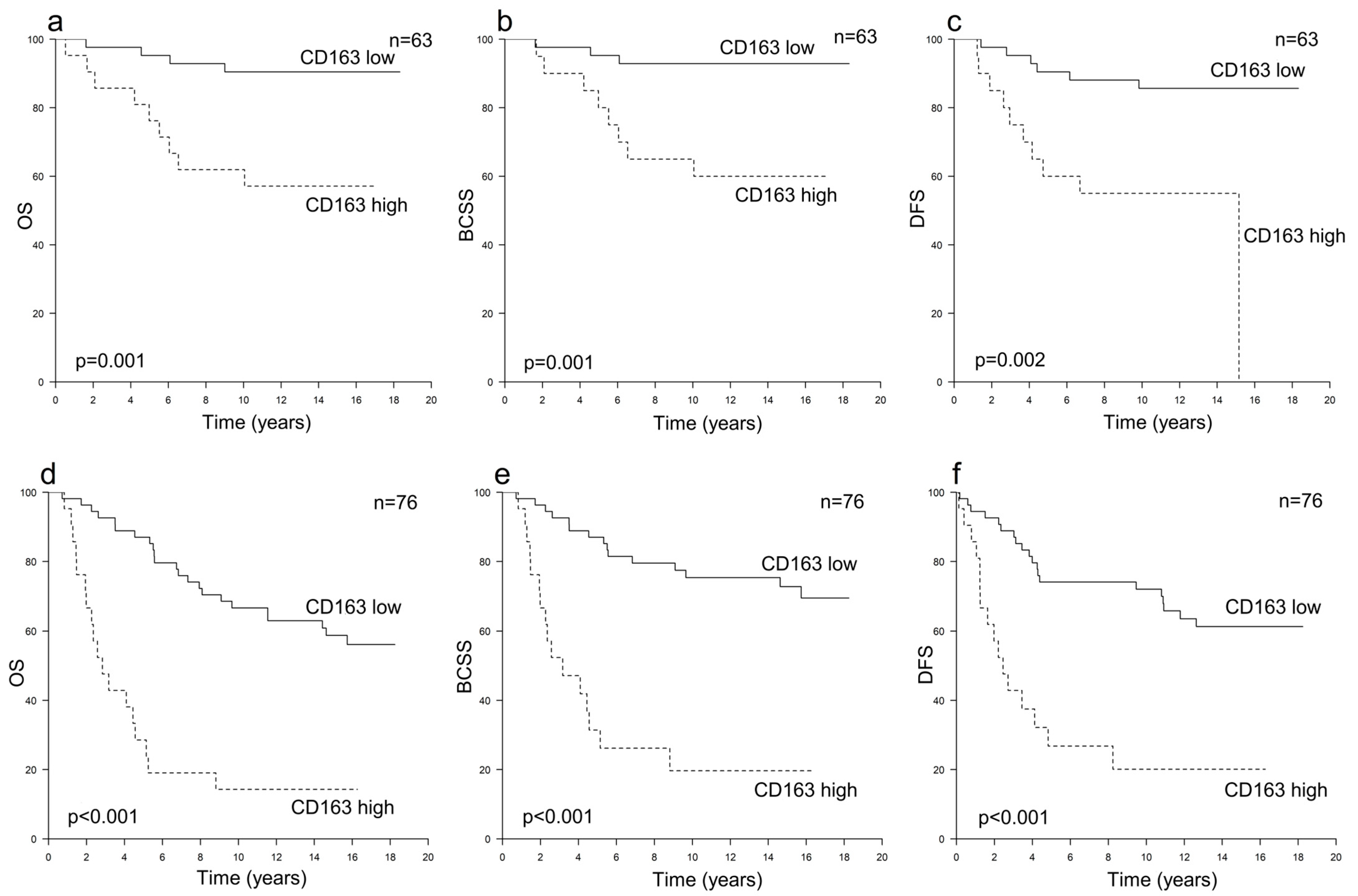
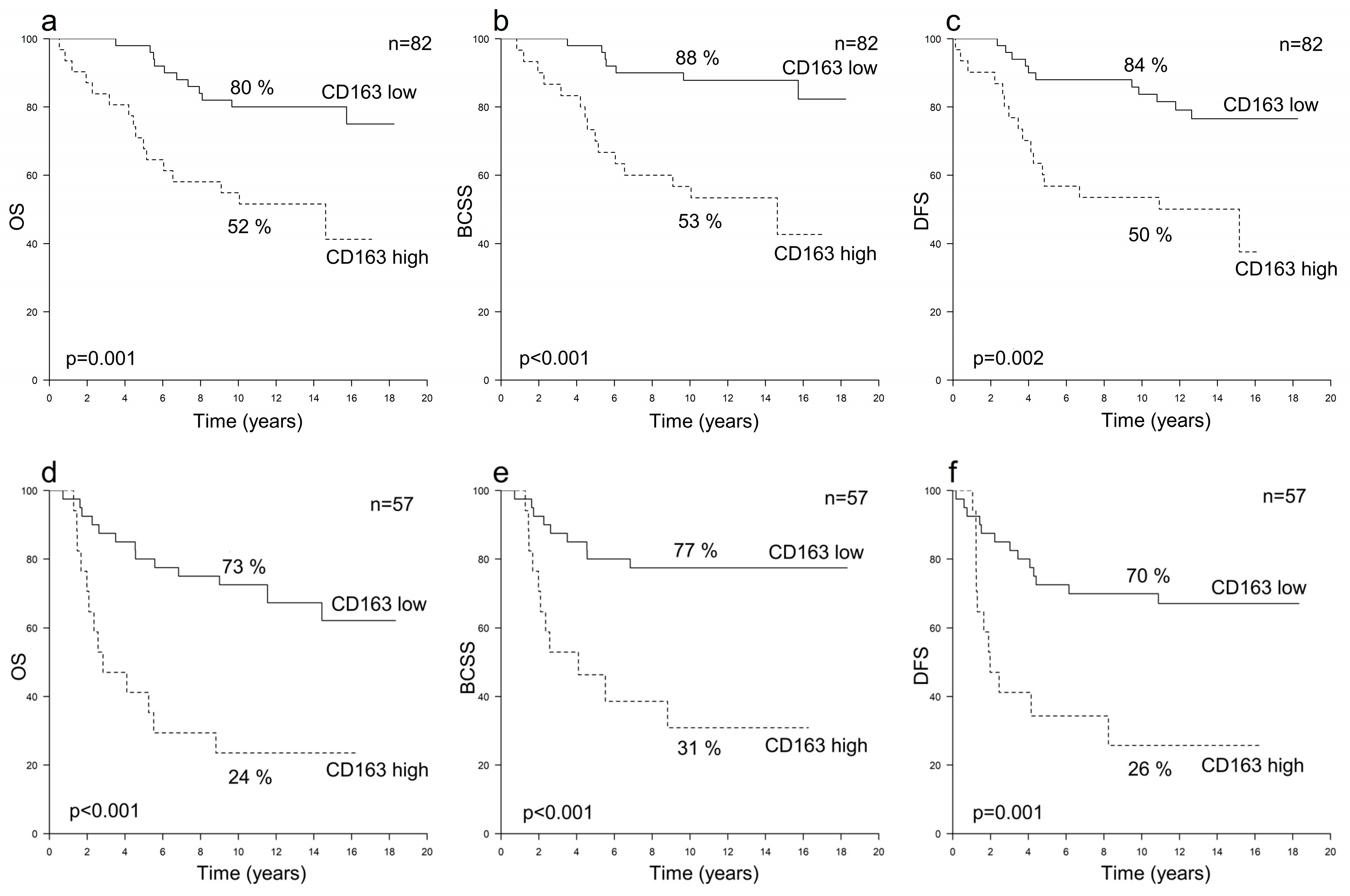
| All Patients n = 278 | All HER2+ n = 139 | HER2+/HR+ n = 82 | HER2+/HR− n = 57 | All HER2− n = 139 | |
|---|---|---|---|---|---|
| Age, median (range) | 58.6 (32–86) | 58.7 (32–86) | 59.3 (32–86) | 58.2 (40–82) | 58.6 (33–84) |
| Adjuvant treatment, n (%) | |||||
| Chemotherapy | 204 (73) | 110 (79) | 62 (76) | 48 (84) | 94 (68) |
| Radiation therapy | 248 (89) | 124 (89) | 72 (88) | 52 (91) | 124 (89) |
| Hormonal therapy | 174 (63) | 73 (53) | 71 (87) | 2 (4) | 101 (73) |
| Trastuzumab | 63 (45 *) | 63 (45) | 36 (44) | 27 (47) | 0 (0) |
| Tumor classification, n (%) | |||||
| pT1 | 156 (56) | 67 (48) | 45 (55) | 22 (39) | 89 (64) |
| pT2 | 99 (36) | 60 (43) | 32 (39) | 28 (49) | 39 (28) |
| pT3 | 10 (4) | 4 (3) | 2 (2) | 2 (4) | 6 (4) |
| pT4 | 13 (5) | 8 (6) | 3 (4) | 5 (9) | 5 (4) |
| Nodal classification, n (%) | |||||
| pN0 | 103 (37) | 47 (34) | 34 (41) | 13 (23) | 56 (40) |
| pN1 | 121 (44) | 55 (40) | 29 (35) | 26 (46) | 66 (48) |
| pN2 | 35 (13) | 22 (16) | 12 (15) | 10 (18) | 13 (9) |
| pN3 | 19 (7) | 15 (11) | 7 (9) | 8 (14) | 4 (3) |
| Histological grade, n (%) | |||||
| 1 | 23 (8) | 4 (3) | 4 (5) | 0 (0) | 19 (14) |
| 2 | 123 (44) | 47 (34) | 33 (40) | 14 (25) | 76 (55) |
| 3 | 132 (47) | 88 (63) | 45 (55) | 43 (75) | 44 (32) |
| Histological type, n (%) | |||||
| Ductal | 228 (82) | 119 (86) | 72 (88) | 47 (82) | 109 (78) |
| Lobular | 27 (10) | 9 (6) | 4 (5) | 5 (9) | 18 (13) |
| Other | 23 (8) | 11 (8) | 6 (7) | 5 (9) | 12 (9) |
| HR+, n (%) | 139 (50) | 82 (59) | 82 (100) | 0 (0) | 122 (88) |
| CD163+ TAMs, median (range) | 26 (5–65) | 28 (8–64) | 26 (9–64) | 30 (8–60) | 24 (5–65) |
| CD68+ TAMs, median (range) | 32 (7–73) | 38.5 (13–73) | 35.5 (13–60) | 41.5 (13–73) | 29 (7–60) |
| Any relapse, n (%) | 91 (33) | 53 (38) | 28 (34) | 25 (44) | 38 (27) |
| Distant metastases, n (%) | 73 (26) | 45 (32) | 23 (28) | 22 (39) | 28 (20) |
| Death, n (%) | 98 (35) | 55 (40) | 28 (34) | 27 (47) | 43 (31) |
| Breast cancer death, n (%) | 68 (24) | 43 (31) | 23 (28) | 20 (35) | 25 (18) |
| OS HR (95% CI), p | BCSS HR (95% CI), p | DFS HR (95% CI), p | ||
|---|---|---|---|---|
| All (n = 278) | CD163+ TAMs | 1.55 (1.33–1.80), <0.001 * | 1.64 (1.37–1.96), <0.001 * | 1.45 (1.24–1.70), <0.001 * |
| CD68+ TAMs | 1.20 (1.03–1.40), 0.022 * | 1.30 (1.08–1.57), 0.005 * | 1.20 (1.03–1.40), 0.023 * | |
| All HER2+ (n = 139) | CD163+ TAMs | 1.67 (1.37–2.02), <0.001 * | 1.79 (1.43–2.23), <0.001 * | 1.55 (1.28–1.89), <0.001 * |
| CD68+ TAMs | 1.23 (1.00–1.51), 0.057 | 1.28 (1.01–1.63), 0.041 * | 1.12 (0.91–1.38), 0.296 | |
| HER2+/HR+ (n = 82) | CD163+ TAMs | 1.64 (1.26–2.14), <0.001 * | 1.85 (1.37–2.51), <0.001 * | 1.67 (1.27–2.19), <0.001 * |
| CD68+ TAMs | 1.14 (0.85–1.53), 0.367 | 1.29 (0.93–1.78), 0.133 | 1.18 (0.89–1.58), 0.255 | |
| HER2+/HR− (n = 57) | CD163+ TAMs | 1.80 (1.33–2.45), <0.001 * | 1.79 (1.28–2.53), <0.001 * | 1.48 (1.09–2.02), 0.012 * |
| CD68+ TAMs | 1.24 (0.91–1.69), 0.165 | 1.22 (0.86–1.75), 0.271 | 1.00 (0.73–1.36), 0.987 | |
| All HER2− (n = 139) | CD163+ TAMs | 1.28 (1.00–1.65), 0.052 | 1.22 (0.88–1.70), 0.235 | 1.22 (0.94–1.58), 0.139 |
| CD68+ TAMs | 1.06 (0.83–1.36), 0.642 | 1.15 (0.84–1.58), 0.372 | 1.23 (0.96–1.58), 0.102 | |
| HER2-/HR+ (n = 122) | CD163+ TAMs | 1.25 (0.92–1.69), 0.150 | 1.17 (0.78–1.74), 0.453 | 1.27 (0.94–1.72), 0.117 |
| CD68+ TAMs | 0.99 (0.74–1.33), 0.951 | 1.02 (0.70–1.48), 0.926 | 1.25 (0.93–1.66), 0.138 | |
| HER2-/HR− (n = 17) | CD163+ TAMs | 1.30 (0.69–2.44), 0.418 | 1.16 (0.51–2.63), 0.721 | 1.16 (0.51–2.63), 0.721 |
| CD68+ TAMs | 1.17 (0.62–2.20), 0.621 | 1.75 (0.66–4.63), 0.262 | 1.75 (0.66–4.63), 0.262 |
| OS | BCSS | DFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | |
| All patients (n = 278) | |||||||||
| CD163+ TAMs | <0.001 * | 2.71 | 1.77–4.14 | <0.001 * | 3.36 | 2.04–5.55 | <0.001 * | 2.37 | 1.53–3.66 |
| CD68+ TAMs | 0.469 | 1.19 | 0.74–1.91 | 0.264 | 1.35 | 0.80–2.30 | 0.476 | 1.19 | 0.74–1.91 |
| Tumor size | 0.002 * | 1.98 | 1.27–3.08 | 0.001 * | 2.50 | 1.42–4.39 | 0.070 | 1.52 | 0.97–2.37 |
| Nodal status | 0.015 * | 1.81 | 1.12–2.94 | 0.001 * | 3.09 | 1.56–6.13 | 0.001 * | 2.34 | 1.40–3.93 |
| Grade | 0.711 | 1.09 | 0.69–1.73 | 0.096 | 1.62 | 0.92–2.86 | 0.045 * | 1.64 | 1.01–2.65 |
| Hormone receptor- status | 0.254 | 0.76 | 0.48–1.21 | 0.701 | 0.90 | 0.53–1.54 | 0.928 | 0.98 | 0.61–1.58 |
| HER2 status | 0.300 | 1.26 | 0.82–1.94 | 0.181 | 1.42 | 0.85–2.39 | 0.337 | 1.24 | 0.80–1.93 |
| All HER2+ (n = 139) | |||||||||
| CD163+ TAMs | <0.001 * | 4.41 | 2.51–7.77 | <0.001 * | 5.67 | 2.94–10.95 | <0.001 * | 4.13 | 2.32–7.36 |
| CD68+ TAMs | 0.104 | 1.77 | 0.89–3.52 | 0.089 | 2.05 | 0.90–4.70 | 0.344 | 1.38 | 0.71–2.69 |
| Tumor size | 0.201 | 1.52 | 0.80–2.89 | 0.433 | 1.37 | 0.63–2.98 | 0.665 | 1.15 | 0.61–2.19 |
| Nodal status | 0.072 | 1.86 | 0.95–3.64 | 0.005 * | 4.09 | 1.54–10.89 | <0.001 * | 4.07 | 1.79–9.25 |
| Grade | 0.402 | 1.34 | 0.68–2.67 | 0.158 | 1.82 | 0.79–4.15 | 0.079 | 1.88 | 0.93–3.82 |
| Hormone receptor- status | 0.091 | 0.61 | 0.35–1.08 | 0.242 | 0.68 | 0.36–1.30 | 0.126 | 0.64 | 0.35–1.14 |
| Adjuvant trastuzumab | <0.001 * | 0.23 | 0.12–0.45 | <0.001 * | 0.27 | 0.13–0.57 | 0.001* | 0.36 | 0.20–0.67 |
| HER2+/HR+ (n = 82) | |||||||||
| CD163+ TAMs | 0.003 * | 3.62 | 1.55–8.45 | <0.001 * | 5.44 | 2.05–14.44 | 0.002 * | 3.71 | 1.60–8.62 |
| CD68+ TAMs | 0.592 | 1.28 | 0.52–3.20 | 0.485 | 1.46 | 0.51–4.22 | 0.573 | 1.29 | 0.53–3.11 |
| Tumor size | 0.425 | 1.51 | 0.55–4.11 | 0.950 | 1.04 | 0.32–3.33 | 0.639 | 0.79 | 0.30–2.09 |
| Nodal status | 0.238 | 1.85 | 0.67–5.11 | 0.055 | 3.79 | 0.97–14.77 | 0.034 * | 3.20 | 1.09–9.36 |
| Grade | 0.210 | 1.87 | 0.70–4.95 | 0.112 | 2.56 | 0.80–8.15 | 0.045 * | 2.73 | 1.02–7.28 |
| Adjuvant trastuzumab | 0.007 * | 0.27 | 0.11–0.71 | 0.015 * | 0.28 | 0.10–0.78 | 0.013 * | 0.32 | 0.13–0.79 |
| HER2+/HR− (n = 57) | |||||||||
| CD163+ TAMs | <0.001 * | 4.20 | 1.81–9.74 | <0.001 * | 5.46 | 2.02–14.79 | <0.001 * | 4.98 | 1.95–12.75 |
| CD68+ TAMs | 0.331 | 1.58 | 0.63–4.00 | 0.168 | 2.46 | 0.68–8.84 | 0.216 | 1.93 | 0.68–5.47 |
| Tumor size | 0.504 | 1.35 | 0.56–3.29 | 0.633 | 1.33 | 0.41–4.35 | 0.475 | 1.41 | 0.55–3.59 |
| Nodal status | 0.107 | 2.25 | 0.84–6.04 | 0.018 * | 6.50 | 1.37–30.82 | 0.004 * | 9.75 | 2.07–45.94 |
| Grade | 0.677 | 0.81 | 0.30–2.17 | 0.697 | 1.28 | 0.37–4.38 | 0.259 | 1.88 | 0.63–5.60 |
| Adjuvant trastuzumab | 0.001 * | 0.20 | 0.08–0.53 | 0.018 * | 0.26 | 0.09–0.80 | 0.037 * | 0.39 | 0.16–0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jääskeläinen, M.M.; Tumelius, R.; Hämäläinen, K.; Rilla, K.; Oikari, S.; Rönkä, A.; Selander, T.; Mannermaa, A.; Tiainen, S.; Auvinen, P. High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer. Cancers 2024, 16, 634. https://doi.org/10.3390/cancers16030634
Jääskeläinen MM, Tumelius R, Hämäläinen K, Rilla K, Oikari S, Rönkä A, Selander T, Mannermaa A, Tiainen S, Auvinen P. High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer. Cancers. 2024; 16(3):634. https://doi.org/10.3390/cancers16030634
Chicago/Turabian StyleJääskeläinen, Minna M., Ritva Tumelius, Kirsi Hämäläinen, Kirsi Rilla, Sanna Oikari, Aino Rönkä, Tuomas Selander, Arto Mannermaa, Satu Tiainen, and Päivi Auvinen. 2024. "High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer" Cancers 16, no. 3: 634. https://doi.org/10.3390/cancers16030634
APA StyleJääskeläinen, M. M., Tumelius, R., Hämäläinen, K., Rilla, K., Oikari, S., Rönkä, A., Selander, T., Mannermaa, A., Tiainen, S., & Auvinen, P. (2024). High Numbers of CD163+ Tumor-Associated Macrophages Predict Poor Prognosis in HER2+ Breast Cancer. Cancers, 16(3), 634. https://doi.org/10.3390/cancers16030634







