PPIP5K2 Facilitates Proliferation and Metastasis of Non-Small Lung Cancer (NSCLC) through AKT Signaling Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
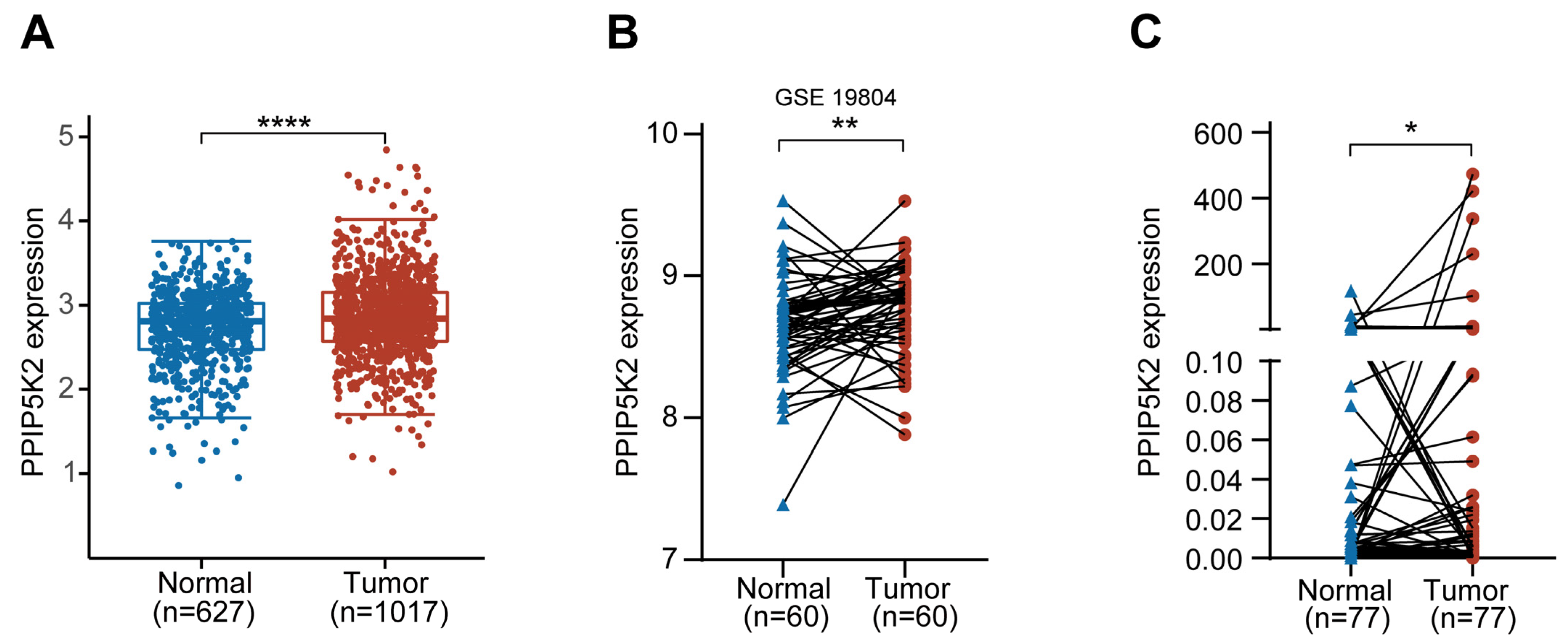
3. Results
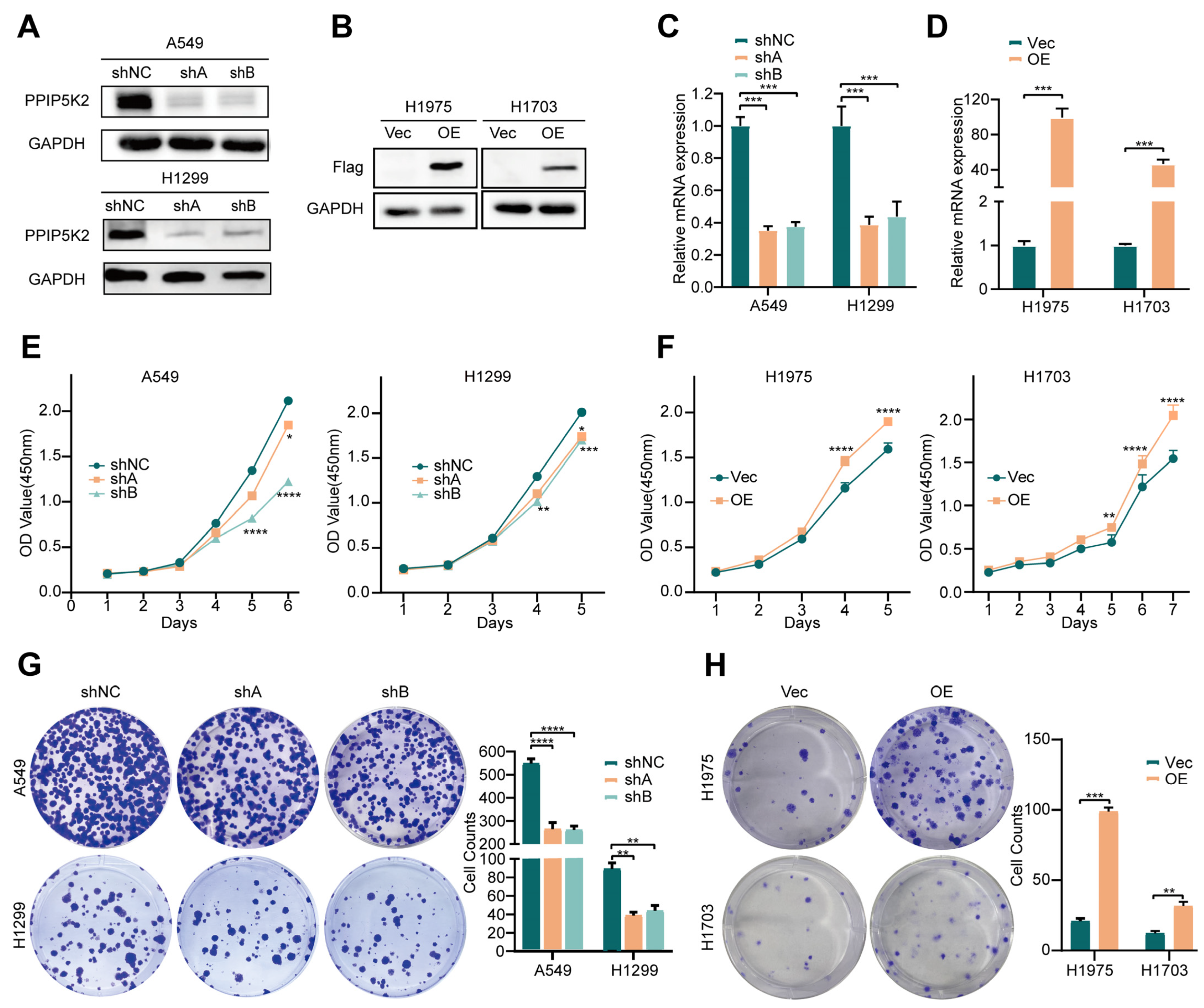
3.1. PPIP5K2 Promotes NSCLC Cell Proliferation In Vitro
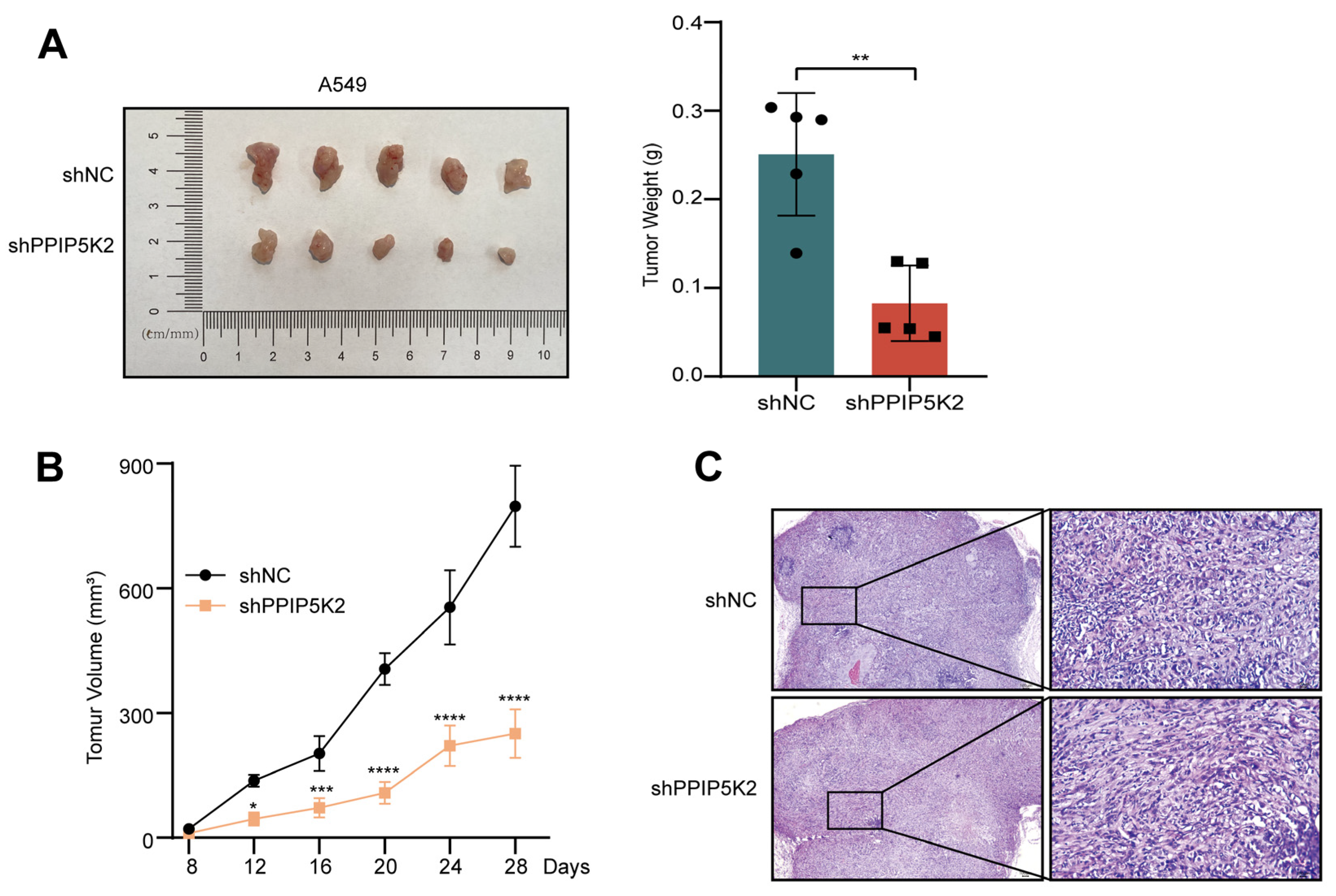
3.2. PPIP5K2 Possesses Oncogenic Capacities of NSCLC Cells In Vivo
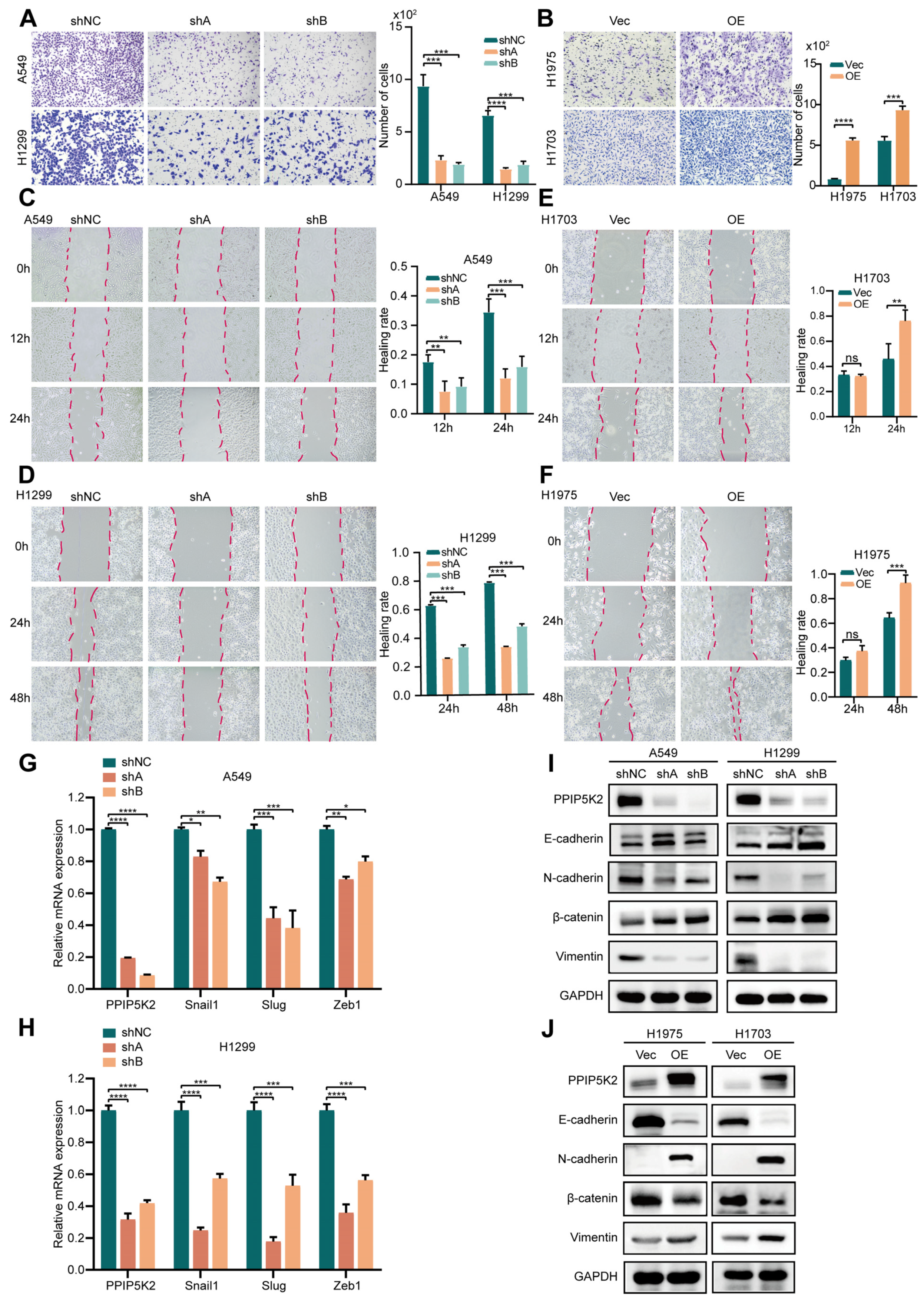
3.3. PPIP5K2 Is Essential for EMT-Dependent Cell Migration in NSCLC
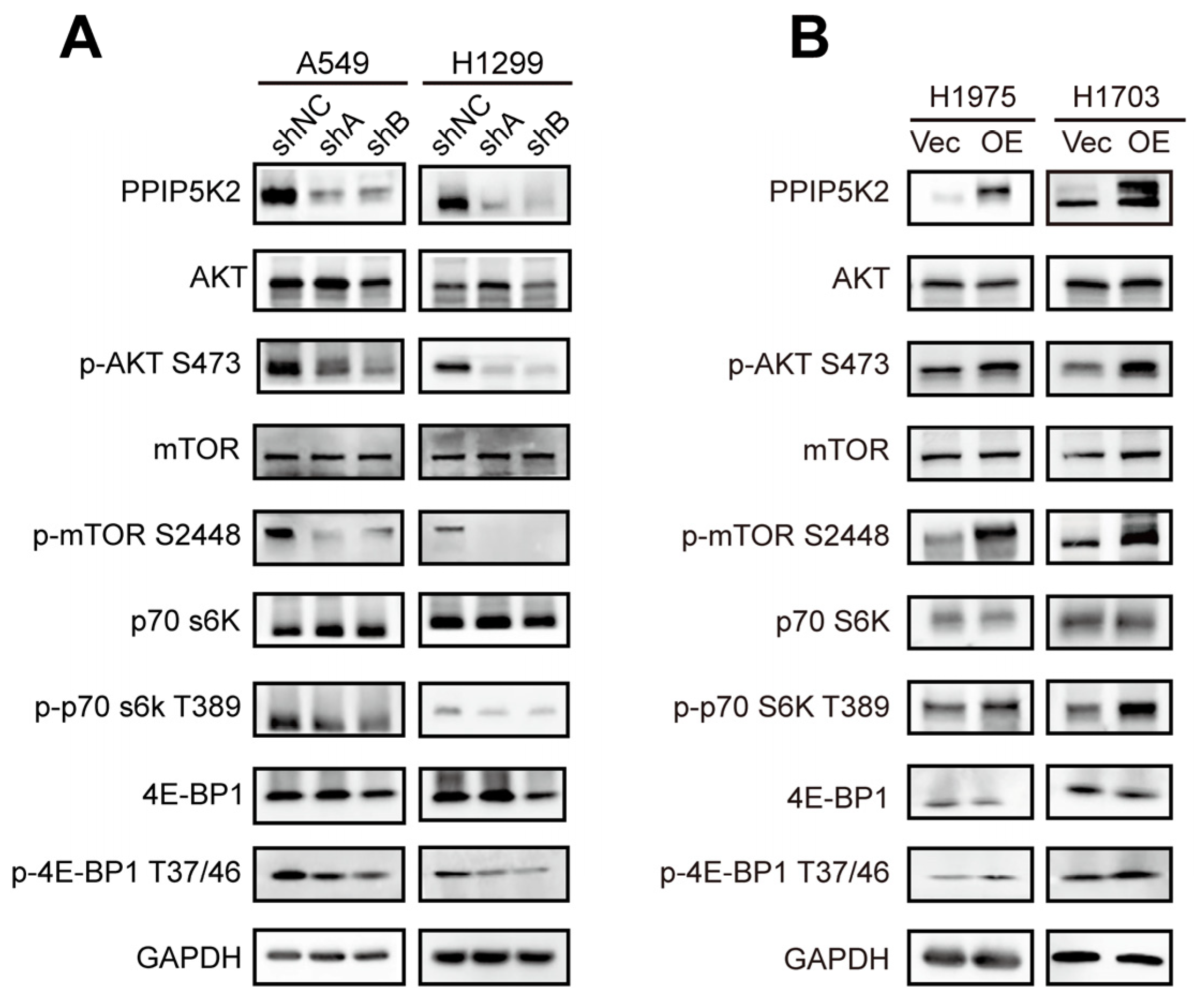
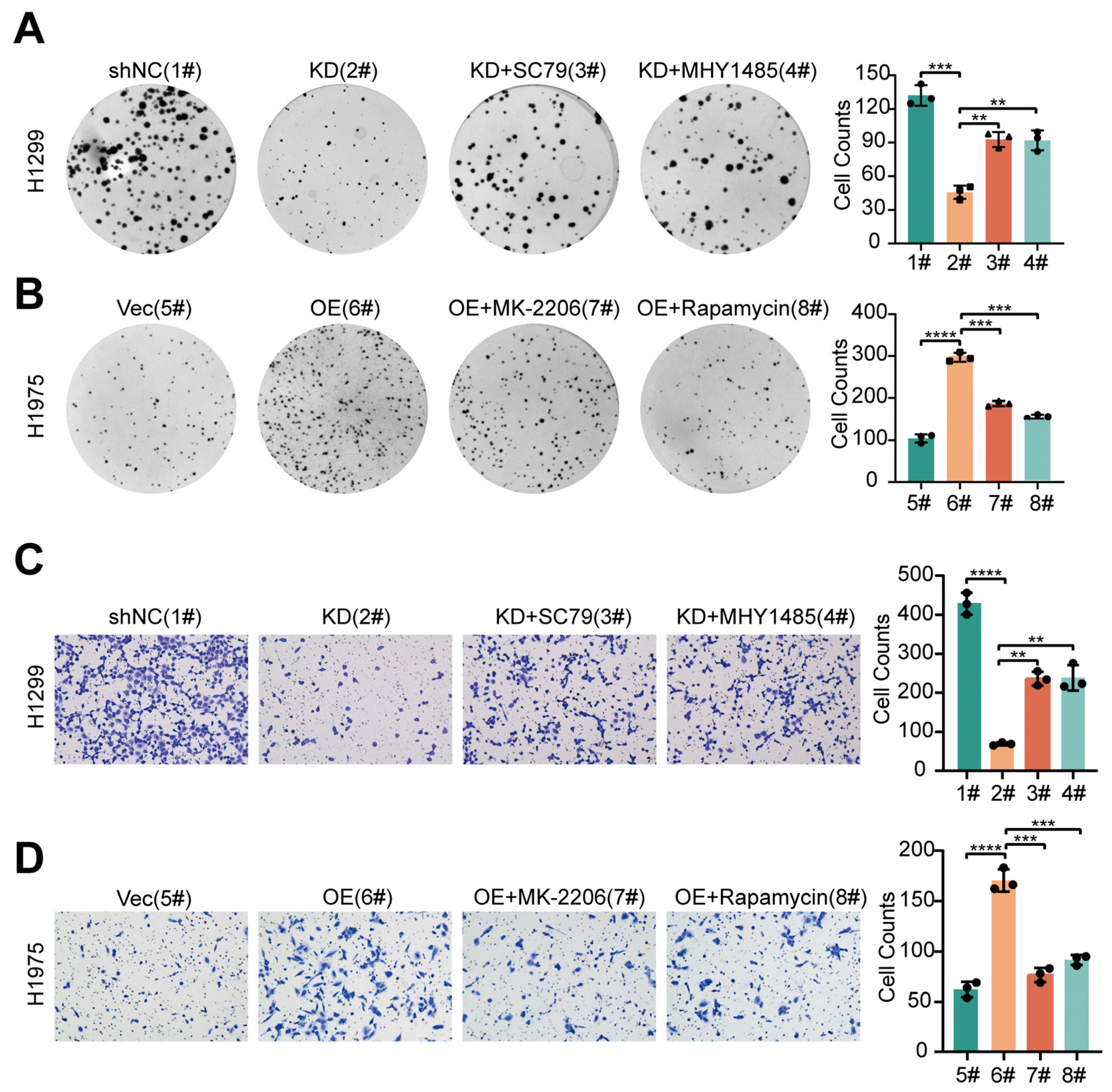
3.4. PPIP5K2 Promotes the Proliferation and Metastasis of NSCLC Cells through the AKT/mTOR Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| FBS | Fetal bovine serum |
| PPIP5K2 | Diphosphoinositol pentakisphosphate kinase 2 |
| EMT | Epithelial–mesenchymal transition |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| HE staining | Hematoxylin-eosin staining |
| IHC | Immunohistochemistry |
| CCK-8 | Cell counting kit-8 |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small cell lung cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar] [CrossRef]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Hernando, E.; Charytonowicz, E.; Dudas, M.E.; Menendez, S.; Matushansky, I.; Mills, J.; Socci, N.D.; Behrendt, N.; Ma, L.; Maki, R.G.; et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat. Med. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A. The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1203–1227. [Google Scholar] [CrossRef]
- Cao, C.H.; Ling, H.; Han, K.; Lu, X.P.; Cai, M.Y.; Cao, J.H.; Zhou, J.; Xiang, Z.C.; Chen, J.W.; Li, S.; et al. PPIP5K2 promotes colorectal carcinoma pathogenesis through facilitating DNA homologous recombination repair. Oncogene 2021, 40, 6680–6691. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Wu, M.; Zhang, P.; Huang, L.; Ai, X.; Yang, Z.; Shen, Q.; Wang, Y.; Wang, P.; et al. Suppressing MDSC Infiltration in Tumor Microenvironment Serves as an Option for Treating Ovarian Cancer Metastasis. Int. J. Biol. Sci. 2022, 18, 3697–3713. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Liu, J.; Liu, X.; Zhang, H.; Luo, J.; Wang, H.; Locasale, J.W.; Shears, S.B. Metabolic supervision by PPIP5K, an inositol pyrophosphate kinase/phosphatase, controls proliferation of the HCT116 tumor cell line. Proc. Natl. Acad. Sci. USA 2021, 118, e2020187118. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Nguyen, H.N.; Ganini, D.; Chen, Z.; Jessen, H.J.; Gu, Z.; Wang, H.; Shears, S.B. KO of 5-InsP(7) kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 11968–11973. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Chen, J.; Wang, J.; Liu, J.; Jiang, Y. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Lin, X.; Wang, Y.; Liu, X.; Xiao, Y.; Liu, J.; Snijders, A.M.; Wei, G.; Mao, J.H.; Zhang, P. FAM83D promotes epithelial-mesenchymal transition, invasion and cisplatin resistance through regulating the AKT/mTOR pathway in non-small-cell lung cancer. Cell. Oncol. 2020, 43, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.R.; Huang, Y.E.; Chen, J.C.; Saiardi, A.; Iijima, M.; Ye, K.; Huang, Y.; Nagata, E.; Devreotes, P.; Snyder, S.H. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 2003, 114, 559–572. [Google Scholar] [CrossRef]
- Wang, X.; Hills, L.B.; Huang, Y.H. Lipid and Protein Co-Regulation of PI3K Effectors Akt and Itk in Lymphocytes. Front. Immunol. 2015, 6, 117. [Google Scholar] [CrossRef]
- Chakraborty, A.; Koldobskiy, M.A.; Bello, N.T.; Maxwell, M.; Potter, J.J.; Juluri, K.R.; Maag, D.; Kim, S.; Huang, A.S.; Dailey, M.J.; et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 2010, 143, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Jadav, R.S.; Kumar, D.; Buwa, N.; Ganguli, S.; Thampatty, S.R.; Balasubramanian, N.; Bhandari, R. Deletion of inositol hexakisphosphate kinase 1 (IP6K1) reduces cell migration and invasion, conferring protection from aerodigestive tract carcinoma in mice. Cell. Signal. 2016, 28, 1124–1136. [Google Scholar] [CrossRef]
- Pöhlmann, J.; Risse, C.; Seidel, C.; Pohlmann, T.; Jakopec, V.; Walla, E.; Ramrath, P.; Takeshita, N.; Baumann, S.; Feldbrügge, M.; et al. The Vip1 inositol polyphosphate kinase family regulates polarized growth and modulates the microtubule cytoskeleton in fungi. PLoS Genet. 2014, 10, e1004586. [Google Scholar] [CrossRef] [PubMed]
- Pulloor, N.K.; Nair, S.; McCaffrey, K.; Kostic, A.D.; Bist, P.; Weaver, J.D.; Riley, A.M.; Tyagi, R.; Uchil, P.D.; York, J.D.; et al. Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in Type-I interferon response. PLoS Pathog. 2014, 10, e1003981. [Google Scholar] [CrossRef] [PubMed]
- Thota, S.G.; Bhandari, R. The emerging roles of inositol pyrophosphates in eukaryotic cell physiology. J. Biosci. 2015, 40, 593–605. [Google Scholar] [CrossRef]
- Shears, S.B. Inositol pyrophosphates: Why so many phosphates? Adv. Biol. Regul. 2015, 57, 203–216. [Google Scholar] [CrossRef]
- Khaled, M.L.; Bykhovskaya, Y.; Gu, C.; Liu, A.; Drewry, M.D.; Chen, Z.; Mysona, B.A.; Parker, E.; McNabb, R.P.; Yu, H.; et al. PPIP5K2 and PCSK1 are Candidate Genetic Contributors to Familial Keratoconus. Sci. Rep. 2019, 9, 19406. [Google Scholar] [CrossRef]
- Yousaf, R.; Gu, C.; Ahmed, Z.M.; Khan, S.N.; Friedman, T.B.; Riazuddin, S.; Shears, S.B.; Riazuddin, S. Mutations in Diphosphoinositol-Pentakisphosphate Kinase PPIP5K2 are associated with hearing loss in human and mouse. PLoS Genet. 2018, 14, e1007297. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Wu, Y.; Liao, W.; Xiong, J.; Zhu, Y.; Liu, C.; Han, W.; Wang, Y.; Han, S.; et al. Renal Klotho and inorganic phosphate are extrinsic factors that antagonistically regulate hematopoietic stem cell maintenance. Cell Rep. 2022, 38, 110392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e823. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Parker, V.E.; Darling, T.N.; Martinez-Agosto, J.A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. Part C Semin. Med. Genet. 2016, 172, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Khuri, F.R. Targeting the PI3K/AKT/mTOR pathway: Biomarkers of success and tribulation. Am. Soc. Clin. Oncol. Educ. Book. Am. Soc. Clin. Oncology. Annu. Meet. 2013, 40, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.C.; Kasamon, Y.; Pazdur, R.; Gormley, N. The saga of PI3K inhibitors in haematological malignancies: Survival is the ultimate safety endpoint. Lancet. Oncol. 2022, 23, 563–566. [Google Scholar] [CrossRef]
- Meng, D.; He, W.; Zhang, Y.; Liang, Z.; Zheng, J.; Zhang, X.; Zheng, X.; Zhan, P.; Chen, H.; Li, W.; et al. Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review). Pharmacol. Res. 2021, 173, 105900. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- McLeod, R.; Kumar, R.; Papadatos-Pastos, D.; Mateo, J.; Brown, J.S.; Garces, A.H.I.; Ruddle, R.; Decordova, S.; Jueliger, S.; Ferraldeschi, R.; et al. First-in-Human Study of AT13148, a Dual ROCK-AKT Inhibitor in Patients with Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4777–4784. [Google Scholar] [CrossRef]
- Gu, C.; Nguyen, H.N.; Hofer, A.; Jessen, H.J.; Dai, X.; Wang, H.; Shears, S.B. The Significance of the Bifunctional Kinase/Phosphatase Activities of Diphosphoinositol Pentakisphosphate Kinases (PPIP5Ks) for Coupling Inositol Pyrophosphate Cell Signaling to Cellular Phosphate Homeostasis. J. Biol. Chem. 2017, 292, 4544–4555. [Google Scholar] [CrossRef]
- Wang, H.; Falck, J.R.; Hall, T.M.; Shears, S.B. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nat. Chem. Biol. 2011, 8, 111–116. [Google Scholar] [CrossRef]
- Wang, H.; Godage, H.Y.; Riley, A.M.; Weaver, J.D.; Shears, S.B.; Potter, B.V. Synthetic inositol phosphate analogs reveal that PPIP5K2 has a surface-mounted substrate capture site that is a target for drug discovery. Chem. Biol. 2014, 21, 689–699. [Google Scholar] [CrossRef]
| Gene Name | Primers (5′-3′) | |
|---|---|---|
| PPIP5K2 | Forward | AAGCAATGTACGAAAAACAGGC |
| Reverse | AAGGCAGACTTTCCAAGCAAT | |
| Snail1 | Forward | TCGGAAGCCTAACTACAGCGA |
| Reverse | AGATGAGCATTGGCAGCGAG | |
| Slug | Forward | CGAACTGGACACACATACAGTG |
| Reverse | CTGAGGATCTCTGGTTGTGGT | |
| Zeb1 | Forward | TTACACCTTTGCATACAGAACCC |
| Reverse | TTTACGATTACACCCAGACTGC | |
| GAPDH | Forward | CTGGGCTACACTGAGCACC |
| Reverse | AAGTGGTCGTTGAGGGCAATG | |
| Antibody Name | Brand | Catalog Number |
|---|---|---|
| Rabbit anti-PPIP5K2 | Sigma | Cat#PA5-29340 |
| Rabbit anti-E-cadherin | CST | Cat#3195S |
| Rabbit anti-N-cadherin | CST | Cat#13116S |
| Rabbit anti-β-Catenin | CST | Cat#8480S |
| Rabbit anti-Vimentin | CST | Cat#5741 |
| Rabbit anti-mTOR | CST | Cat#2983 |
| Rabbit anti-Phospho-mTOR (Ser2448) | CST | Cat#5536 |
| Rabbit anti-4E-BP1 | CST | Cat#9644 |
| Rabbit anti-Phospho-4E-BP1 (Thr37/46) | CST | Cat#2855 |
| Rabbit anti-p70 S6 Kinase | CST | Cat#34475 |
| Rabbit anti-Phospho-p70 S6 Kinase | CST | Cat#9234 |
| Rabbit anti-Akt | CST | Cat#4691 |
| Rabbit anti-Phospho-Akt (Ser473) | CST | Cat#4060 |
| Rabbit anti-GAPDH | Proteintech | Cat#10494-1-AP |
| Rabbit anti-Flag | Proteintech | Cat#20543-1-AP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Cao, C.; Wu, B.; Yang, H.; Tan, T.; Shang, D.; Xu, C.; Huang, X. PPIP5K2 Facilitates Proliferation and Metastasis of Non-Small Lung Cancer (NSCLC) through AKT Signaling Pathway. Cancers 2024, 16, 590. https://doi.org/10.3390/cancers16030590
Yang Q, Cao C, Wu B, Yang H, Tan T, Shang D, Xu C, Huang X. PPIP5K2 Facilitates Proliferation and Metastasis of Non-Small Lung Cancer (NSCLC) through AKT Signaling Pathway. Cancers. 2024; 16(3):590. https://doi.org/10.3390/cancers16030590
Chicago/Turabian StyleYang, Qi, Chenhui Cao, Binghuo Wu, Haochi Yang, Tian Tan, Dan Shang, Chuan Xu, and Xiaoyi Huang. 2024. "PPIP5K2 Facilitates Proliferation and Metastasis of Non-Small Lung Cancer (NSCLC) through AKT Signaling Pathway" Cancers 16, no. 3: 590. https://doi.org/10.3390/cancers16030590
APA StyleYang, Q., Cao, C., Wu, B., Yang, H., Tan, T., Shang, D., Xu, C., & Huang, X. (2024). PPIP5K2 Facilitates Proliferation and Metastasis of Non-Small Lung Cancer (NSCLC) through AKT Signaling Pathway. Cancers, 16(3), 590. https://doi.org/10.3390/cancers16030590





