Simple Summary
Kaempferol, a natural compound commonly found in fruits, vegetables, and plants, has gained interest within the scientific community because of its anticancer properties against different types of tumors. The results of this review reveal that kaempferol exerts anticancer effects on many types of tumor cells by different mechanisms, providing evidence of its potential as a cancer drug.
Abstract
Given the heterogeneity of different malignant processes, planning cancer treatment is challenging. According to recent studies, natural products are likely to be effective in cancer prevention and treatment. Among bioactive flavonoids found in fruits and vegetables, kaempferol (KMP) is known for its anti-inflammatory, antioxidant, and anticancer properties. This systematic review aims to highlight the potential therapeutic effects of KMP on different types of solid malignant tumors. This review was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. Searches were performed in EMBASE, Medline/PubMed, Cochrane Collaboration Library, Science Direct, Scopus, and Google Scholar. After the application of study criteria, 64 studies were included. In vitro experiments demonstrated that KMP exerts antitumor effects by controlling tumor cell cycle progression, proliferation, apoptosis, migration, and invasion, as well as by inhibiting angiogenesis. KMP was also able to inhibit important markers that regulate epithelial–mesenchymal transition and enhanced the sensitivity of cancer cells to traditional drugs used in chemotherapy, including cisplatin and 5-fluorouracil. This flavonoid is a promising therapeutic compound and its combination with current anticancer agents, including targeted drugs, may potentially produce more effective and predictable results.
1. Introduction
Cancer comprises a group of malignant disorders that develop in different tissues and organs and are characterized by uncontrolled growth of altered cells and spread to distant sites. Cancer is a leading cause of death worldwide. According to the 2020 GLOBOCAN online database report, the annual number of cases is estimated to increase from 19.3 million in 2020 to 28.4 million in 2025 (an increase of 47% compared to 2020) [1]. The main cancer treatments are surgery, radiotherapy, and chemotherapy; however, additional strategies are applied to some tumors, such as hormonal therapy or photodynamic therapy [2]. Although biological treatments, including targeted therapy and immunotherapy, have changed the face of cancer therapy, they are constantly being improved and evaluated [2,3]. So far, all therapeutic options are associated with important side effects, in addition to the development of resistance to antineoplastic drugs [4]. In contrast, natural compounds are increasingly being used for the prevention and treatment of cancer, with a reduced risk of adverse reactions [5].
Natural compounds derived from plants (phytochemical compounds) are particularly interesting and have been widely used in the treatment of different pathological processes such as cancer because of their increased bioavailability and better tolerability compared to synthetic drugs [6]. Furthermore, recent studies indicate that a variety of phytochemicals can sensitize tumor cells to antitumor drugs, reversing tumor resistance and decreasing toxic effects in different malignant neoplastic processes [7,8]. Flavonoids are polyphenolic compounds synthesized as secondary bioactive metabolites that are responsible for the color, flavor, and pharmacological activities of plants [9]. Kaempferol (KMP) (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) (Figure 1), a flavonoid widely distributed in a variety of vegetables, fruits, and medicinal plants, has shown antioxidant and anti-inflammatory activity [10]. KMP has been suggested to reduce the risk of cardiovascular and neuroinflammatory diseases, and recent studies indicate it as a promising antineoplastic agent [11,12,13]. Within this context, KMP has been shown to regulate different mechanisms involved in oncogenesis and to sensitize neoplastic cells to chemotherapeutic agents [14,15,16]. Furthermore, KMP can induce mechanisms that disrupt the survival of tumor cells [14,17].
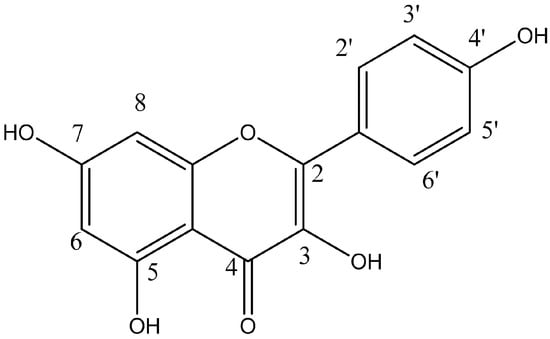
Figure 1.
Chemical structure of Kaempferol. Kaempferol is a tetrahydroxyflavone, with the hydroxy groups distributed at positions 3, 5, 7, and 4′.
The literature exploring KMP potential in cancer treatment is extensive, but no systematic synthesis of in vitro studies elucidating the molecular mechanisms and pathways targeted by KMP is available. In this systematic review, we sought to summarize the available literature on the in vitro anticancer effects of KMP, discussing the pathways and molecular mechanisms regulated by KMP and highlighting its potential as a natural anticancer drug. We also critically analyzed the gaps in knowledge that limit the use of KMP in cancer treatment.
2. Materials and Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Materials Table S1) [18]. The PICO criteria were used to develop the research questions: population—cell lines derived from malignant solid tumors; intervention—treatment with KMP; comparison—control group not treated with KMP; outcomes—analysis of the behavior of the neoplastic cell after treatment with KMP using functional assays (migration, invasion, viability, proliferation, sensitization to chemotherapy).
The research questions were: “Is KMP a potential anticancer agent?”, “What are the molecular mechanisms regulated by KMP in malignant neoplastic cell lines?” and “Does KMP exert a modulatory effect and act as a potential antineoplastic agent?”. The study protocol was registered in the Open Science Framework: https://osf.io/z5nsg/ (accessed on 27 January 2024).
2.1. Search Strategy
To identify primary research articles that evaluated the in vitro effects of KMP on cancer cells, we searched the EMBASE, Medline/PubMed, Cochrane Collaboration Library, Science Direct, Scopus, and Google Scholar databases. In addition, the references of pre-selected articles were hand-searched. Searches were performed in December 2022. However, a second literature search was performed at the beginning of September 2023, retrieving articles published between January and August 2023. The search strategy was based on combinations of the following keywords: (“cancer cell” [MeSH] AND “in vitro studies” [MeSH] AND “kaempferol” [MeSH] AND “biological behavior” [MeSH] AND (“humans” [MeSH]). The complete search strategy is given in Appendix A.
2.2. Study Selection and Selection Criteria
Initial screening based on titles and abstracts was performed by two independent reviewers who classified the studies as “yes”, “no”, or “maybe” based on inclusion criteria. Studies classified as “yes” or “maybe” were selected for full-text reading. The eligibility criteria were applied in both steps.
Articles that evaluated the effects of KMP treatment on the biological behavior of malignant solid tumor cells were selected for the present systematic review. The search was performed without time or language restrictions. The following studies were excluded from this systematic review: (i) studies that used only non-cancer cells; (ii) in vivo studies only; (iii) studies that did not evaluate KMP or did not evaluate it alone; (iv) studies that used only in silico analysis or bioinformatics. Two reviewers independently selected the articles and any disagreement was resolved by consensus.
2.3. Data Extraction, Analysis, and Risk of Bias Assessment
The authors independently extracted the following data from the included studies using a pre-established form: authors, year of publication, country, tissue origin of cell lines, origin/extraction method of KMP, dosage of KMP, treatment time, effects on biological behavior, effects on expression of biomarkers, effects on cell sensitization to chemotherapy, and main findings of study. The results of the individual studies were then summarized, categorized, pooled, and analyzed. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria [19,20], which were adapted for in vitro studies as described by Pavan et al. [21]. The quality of evidence of the articles was also classified as high, moderate, low, or very low.
3. Results
3.1. Study Characteristics
A total of 1238 articles were retrieved; after the removal of duplicates, 231 articles remained for the first screening based on titles and abstracts. One hundred and twenty-six articles were selected for the next phase. After full-text reading, 64 articles were included in the qualitative synthesis [10,12,14,17,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. The list of excluded studies and reasons for exclusion are shown in Appendix B. Figure 2 depicts the flowchart of the study screening and selection process.

Figure 2.
Flowchart of literature search and selection criteria. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The selected studies were published between 2003 and 2022 and were all written in English. The studies were conducted in 14 different countries, 25 (38.5%) of them by Chinese groups. The KMP dosage, the duration of treatment, and the cell lines used in the selected studies varied. The main features and findings of the studies are presented in Appendix C.
3.2. KMP Inhibits Cell Proliferation, Invasion, and Migration and Promotes Cell Death
KMP exhibits notable properties in combating cancer. Our systematic review indicated that this compound exerts inhibitory effects on cell proliferation, disrupting the cell cycle at key stages and halting cell division. Additionally, KMP was associated with increased cytotoxicity in neoplastic cells, reinforcing its potential as a therapeutic agent against cancer development and progression. KMP significantly affected the migration and invasion of neoplastic cells, directly interfering with cancer dissemination. These properties highlight the potential of KMP as a promising option in the pursuit of effective therapeutic strategies to treat cancer (Figure 3). Table 1 shows the main findings according to type of tumor.
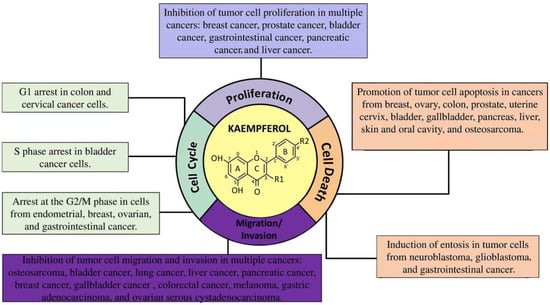
Figure 3.
Antineoplastic mechanisms regulated by kaempferol in different types of cancer.

Table 1.
Main findings, including cell lines, dosage of kaempferol, and time on treatment, distributed by type of tumor.
Most of the studies included in the present review explored the effects of KMP on tumor cell death [10,12,22,27,28,29,30,31,32,35,36,40,46,51,52,53,55,56,57,62,63,66,70,74,75,76,77,80,81]. These studies reported the apoptosis-inducing properties of KMP, which can be partially attributed to its impacts on the MAPK pathway. In human lung cancer cells (A549 cell line), activation of the mitogen-activated protein kinase (MAPK) pathway is a key factor in KMP-induced apoptosis [22]. Furthermore, KMP was able to reduce B-cell lymphoma 2 (BCL2) levels and increase the expression of tumor suppressor proteins such as p53 and BCL2-associated agonist of cell death (BAD) [40]. Protein p53 plays a key role in the repair of damaged DNA. According to Luo et al. [40], KMP prevents the phosphorylation of protein kinase B (PKB, also known as AKT) and upregulates p53 expression, inducing apoptosis of ovarian cancer cells. In addition to apoptotic effects, KMP also has antioxidant activity. Studies have shown that KMP reduces the production of free radicals and other products such as reactive oxygen species (ROS) in different neoplastic cell lines, increasing the expression of manganese superoxide dismutase, a mitochondrial antioxidant enzyme [10].
Twelve of the studies included in this systematic review explored the role of cellular toxicity of KMP in lung cancer cells using A549 [22,44,47,48,49,53,70,73,80] or H460 cells [27,60,61]. The findings were consistent, indicating a strong cytotoxicity of KMP. Another focus of studies on KMP was ovarian cancer [34,37,40,41,57,60,79]. The results demonstrated positive effects, with KMP reducing cell viability and angiogenesis and increasing apoptosis, with cell cycle arrest at G2/M. Luo et al. [34] suggested that KMP inhibits tumor angiogenesis in human ovarian cancer by suppressing vascular endothelial growth factor (VEGF) expression through a hypoxia-inducible factor (HIF)-dependent (AKT/HIF) pathway in ovarian cancer cells. In cervical cancer, KMP has been shown to reduce cell viability and increase apoptosis [55]. Two studies included in this systematic review evaluated the effects of KMP in bladder cancer, with both reporting consistent findings of increased apoptosis, increased cytotoxicity, induction of S-phase cell cycle arrest, and reduced cell proliferation, motility, and invasion [46,62].
Several studies have shown that KMP is strongly involved in the control of cell cycle arrest [29,43,45,49,51,56,57,62,63,65,77,81]. Gao et al. [57], investigating the effects of KMP on the cell cycle and extrinsic apoptosis of ovarian cancer cells, demonstrated that KMP induced G2/M cell cycle arrest via checkpoint kinase 2 (CHK2)/cell division cycle 25C (CDC25C)/cyclin-dependent kinase 2 (CDC2) and CHK2/p21/CDC2 in A2780/CP70 human ovarian cancer cells. The authors further showed that KMP stimulates apoptosis triggered by Fas-associated death domain protein (FADD) and caspase-8, suggesting that CHK2 and death receptors play important roles in the anticancer activity of KMP in A2780/CP70 cells [57]. Corroborating these findings, KMP treatment increased p53 levels in MDA-MB-453 breast cancer cells, which led to G2 cell cycle arrest [29]. Luo et al. [37] reported that the increased c-Myc levels after KMP treatment antagonized cyclin-dependent kinase inhibitor 1A (CDKN1A) mRNA expression, interrupting cell cycle progression by inhibiting the activity of cyclin-dependent kinases. KMP also yielded positive results in colon cancer, inhibiting cyclin D-dependent kinase 2 (CDK2) and cyclin D-dependent kinase 4 (CDK4) activities and inducing cell cycle arrest both at G1 (after 6 h of treatment) and G2/M (after 12 h) [43], as well as reducing glucose consumption [76], cell viability [24] (Liu et al., 2019), proliferation [14,45,70], and invasion and migration [59]. Zhang et al. [81], analyzing three different prostate cancer cell lines (22Rv1, PC-3, and DU145), showed that KMP exerts similar effects in terms of reducing cell proliferation but at different points depending on the cell line, arresting the cell cycle at G1 in 22Rv1 and at S/G2 in PC-3. Similar results were reported by Campbell et al. [25] and Yoshida et al. [32]. In the G1 phase, there is a crucial checkpoint where the cell assesses its environment and internal conditions before committing to DNA synthesis and cell division. By arresting the cell cycle at G1, KMP may be preventing the progression of these cells into the synthesis (S) phase, thereby inhibiting DNA replication and subsequent cell division. By affecting the cell cycle at the S/G2 transition, KMP may be interfering with DNA replication and the preparation for cell division. Understanding how a substance like KMP affects the cell cycle at different points in cancer cell lines is crucial for developing targeted and effective therapies for cancer. This tailored approach could potentially enhance therapeutic outcomes by exploiting the unique vulnerabilities of specific cancer cell types.
Zhu et al. [65] demonstrated that the inhibitory effects of KMP on proliferation are greater in estrogen receptor-positive breast cancer cells (BT474 cells) compared to estrogen receptor-negative cells (MDA-MB-231 cells). Furthermore, the authors observed that KMP contributed to the induction of G2/M arrest, apoptosis, and DNA damage in MDA-MB-231 cells. Oh et al. [26] showed that KMP blocks estradiol-induced tumor formation, confirming that this flavonoid can inhibit the malignant transformation caused by estrogens. The authors suggested that KMP may ensure an adequate level of estrogenic activity in the body, potentially exerting beneficial effects on diseases caused by estrogen imbalance such as breast cancer. The study by Abdullah et al. [64] evaluated the effects of KMP on neuroblastoma and reported an increase in neuroblastic differentiation and apoptosis and a reduction in cell viability and proliferation. Inden et al. [71] found that KMP provided significant protection against α-Syn-related neurotoxicity and induced autophagy through an increase in lysosome biogenesis by inducing transcription factor EB (TFEB) expression and reducing the formation and accumulation of α-Syn amyloid fibrils, thus preventing the death of neuronal cells. Complementary results were obtained by Siegelin et al. [31] and Chen et al. [66] who described higher rates of autophagy and apoptosis and lower cell proliferation in glioblastomas. Gastrointestinal cancers have also been studied and the results support and encourage the use of KMP to reduce cell proliferation [23,53] and colony formation [28] and to increase cell cycle arrest at G2/M, autophagy, and cell death [51,58]. Only the study conducted by Liu et al. [74] reported the effects of KMP on gallbladder cancer, with favorable results that included increases in apoptosis and DNA damage and a reduction in cell viability, invasion, and migration. For pancreatic cancer, five studies investigated the effects of KMP using in vitro functional assays. The results revealed reduced viability, proliferation, and migration of both MIA PaCa-2 and Panc-1 cells, as well as increased apoptosis [33,52,60,78].
KMP showed inhibitory effects against aryl hydrocarbon receptor (AHR)- and nuclear factor erythroid 2-related factor 2 (Nrf2)-induced expression of the drug-metabolizing enzyme in hepatocellular carcinoma [68]. KMP’s inhibitory effects on AHR- and Nrf2-induced expression of drug-metabolizing enzymes suggest that KMP interferes with the activation of these pathways, which is relevant in the sense that the dysregulation of AHR and Nrf2 pathways has been associated with the promotion of tumor growth and resistance to chemotherapy in certain cancers, including hepatocellular carcinoma. In addition, the compound increased autophagy and reduced cell viability [39], proliferation [54], and migration and invasion [72]. Li et al. [28], Nair et al. [69], Wang et al. [44], and Yang et al. [77] investigated the effects of KMP in liver cancer. The authors reported positive results, including reduced colony formation, cell proliferation, migration, and invasion and increased cell viability, apoptosis, and cell cycle arrest in the G1 phase. Huang et al. [35] studied the anticancer effects and molecular mechanisms of KMP in human osteosarcoma cells and demonstrated that KMP significantly reduced the viability of U-2 OS, HOB, and 143B cells in a dose-dependent manner, with low cytotoxicity on hFOB cells, a human fetal osteoblast progenitor cell line. In vitro assays confirmed the effects of DNA damage, apoptosis in U-2 OS cells, increased cytoplasmic levels of calcium ions, and decreased mitochondrial membrane potential [35]. In additional experiments, Chen et al. [42] later showed that KMP decreased the DNA-binding activity of activator protein-1 transcription factor (AP-1), suggesting a potential role of the compound in the treatment of osteosarcoma metastasis. Dysregulated AP-1 activity is related with the development and progression of several cancers, and a reduction in the AP-1 DNA-binding activity promoted by KMP implies an interference in the events controlled by AP-1. Skin and oral cavity squamous cell carcinoma cell lines were also studied in the articles included in this systematic review, with KMP inducing cell cytotoxicity [60] and apoptosis and reducing proliferation [36] and colony formation [28]. Similar results were found for the treatment of melanoma with KMP, characterized by a reduction in colony formation, viability, and migration and an increase in apoptosis and cell cycle arrest at G2/M [28,63]. Zheng et al. [17] also highlighted the reduction in aerobic glycolysis in melanoma cells after treatment with KMP.
Wang et al. [75] demonstrated that KMP induces ROS-dependent apoptosis in pancreatic cancer cells through transglutaminase 2-mediated AKT/mammalian target of rapamycin (mTOR) signaling. The study by Chen et al. [66] points out that KMP increases ROS and decreases mitochondrial membrane potential in glioma cells. High levels of ROS induce autophagy and ultimately trigger glioma cell pyroptosis. The paradoxical behavior of cancer cells is intriguing. These cells redirect metabolic processes towards utilizing glutamine in the tricarboxylic acid cycle to mitigate cell damage induced by ROS and to maintain a stable cellular redox balance. ROS exert a dual role, acting either as harmful agents or as beneficial molecules that regulate cellular functions or induce cytotoxic effects depending on their concentration and duration. It is, therefore, crucial to devise strategies that harness cellular redox signaling in cancer, aiming to develop effective anticancer therapies or to target regulators of ROS for improved cancer treatments.
3.3. KMP Modulates the Expression of Cancer Biomarkers
Although KMP has been extensively studied as a natural flavonoid with cytotoxic effects against tumor cells, its effects on other carcinogenic processes have also been demonstrated. One step necessary for tumor cell migration and invasion is the acquisition of cell plasticity promoted by epithelial–mesenchymal transition (EMT). Within this context, many of the studies included in this review demonstrated the effects of KMP on EMT markers (Figure 4) [47,48,79]. KMP was able to reverse the EMT process in gastric, ovarian, and breast cancer cells by increasing E-cadherin expression and reducing the expression of mothers against decapentaplegic homolog 2 (Smad2), mothers against decapentaplegic homolog 4 (Smad4), transforming growth factor-β1 (TGF-β), N-cadherin, vimentin, and Snail [79]. EMT also depends on the expression of several proteases, including matrix metalloproteinases (MMPs). Ju et al. [72] evaluated the effect of KMP on hepatocellular carcinoma cells and the results showed that MMP-9 was dramatically decreased after KMP treatment. KMP also suppressed the phosphorylation of AKT, a key component of cell growth and survival, and has been associated with cell migration and adhesion through regulation of MMP-9.

Figure 4.
Schematic representation of the pathways and targets affected by kaempferol identified in in vitro cancer studies.
KMP is considered to be a potent anti-inflammatory and antiangiogenic agent. The anti-inflammatory effects of KMP are primarily facilitated by the downregulation of numerous sequence-specific DNA binding factors, such as signal transducer and activator of transcription (STAT) [24], which induce the activation of inflammatory cytokines. Neoplastic cells also need nutrients and oxygen to survive. The main mediator of angiogenesis is VEGF. Evaluating the effect of KMP on ovarian cancer cell lines, Luo et al. [34] observed a significant reduction in VEGF and HIF-1, which are critical for adaptive vascular responses to ischemia/hypoxia. By targeting these key mediators, KMP may disrupt the angiogenic process, thereby limiting the blood supply to the tumor and impeding its growth.
3.4. KMP Sensitizes Cancer Cells to Chemotherapy
The studies included in this review also investigated the effects of the combination of KMP with antineoplastic drugs, including cisplatin [40], 5-fluorouracil (5-FU) [12,16,33], sorafenib [69], doxorubicin [77], and erlotinib [78].
Cisplatin is a commonly used alkylating chemotherapeutic agent that is known to induce cell death in different neoplastic processes. In the study conducted by Luo et al. [40], the combined administration of KMP and cisplatin reduced c-Myc mRNA concentration and increased CDKN1A mRNA levels in ovarian cancer cell lines, potentiating cell death. 5-FU is an antimetabolite chemotherapeutic agent widely used in the treatment of different types of cancers. Two studies included in this review evaluated its effect in combination with KMP [12,33]. In the study by Li et al. [12], the combination decreased the viability of colorectal cancer cells, with 5-FU inducing apoptosis potentiated by KMP. The combination of KMP and 5-FU was found to be more effective in promoting cell viability than either agent alone. The authors also observed the upregulation of proteins associated with apoptosis, such as BCL2-associated X (BAX), BCL2, and thymidylate synthase [12]. Combining low doses of KMP and 5-FU exerted an additive effect on the inhibition of MIA PaCa-2 pancreatic cancer cells [33]. In colorectal cancer cells, KMP overcame 5-FU resistance by regulating the microRNA-326–heterogeneous nuclear ribonucleoprotein A1/A2/polypyrimidine tract-binding protein 1–pyruvate kinase M2 (miR-326–hnRNPA1/A2/PTBP1–PKM2) axis [16].
Sorafenib, a multikinase inhibitor used in the treatment of hepatocellular carcinoma and other types of tumors, is associated with severe toxicity, which limits its utilization [69]. Nair et al. [69] combined sorafenib and KMP and found higher cytotoxicity at lower concentrations of sorafenib when combined with KMP than when used alone. KMP exhibited inhibitory effects on different liver cancer cell lines in a time- and dose-dependent manner and was not toxic to normal hepatocytes. Furthermore, KMP acted synergistically with the antitumor antibiotic doxorubicin, increasing its antineoplastic capacity [77]. Finally, Zhang et al. [78] observed that KMP potentiates the sensitivity of pancreatic cancer cells to the epidermal growth factor receptor (EGFR) tyrosine kinase activity inhibitor erlotinib, inhibiting cell proliferation and inducing apoptosis, and, thus, suggested KMP to be a valuable candidate for potentiating the effects of erlotinib.
3.5. Risk of Bias
Based on the GRADE assessment, 20 articles were classified as carrying a high overall quality of evidence, 43 articles were classified with moderate quality of evidence, and 1 article was classified as low quality (Appendix D). The main issues that drove to downgrading were related to indirectness, since 5 articles did not evaluate the effects of KMP in all cell lines of the study, and imprecision, as 41 articles did not calculate and adopt IC50 for treatments with KMP. IC50 is the most widely used and informative measure of a compound/drug’s efficacy, and its proper determination facilitates the development of useful methods for drug discovery and clinical tests [82]. Moreover, it allows a clear view of drug effects, and IC50 at a low concentration is prone to cause lower systemic toxicity when given to patients for treatment [83].
4. Discussion
Emerging data highlight the health-promoting properties of plant-derived flavonoids such as KMP. This tetrahydroxyflavonoid, in which four hydroxyl groups are located at positions 3, 5, 7, and 4′ [84], has received much attention over recent years as a major active flavonoid found in several medical plants, especially because of its anticarcinogenesis effects [85]. Our systematic review indicates that KMP exerts in vitro inhibitory effects on several carcinogenic pathways in different tumors, such as breast, prostate, liver, head, and neck cancers (Appendix C). KMP not only promotes the death of neoplastic cells but also inhibits proliferation, migration, invasion, angiogenesis, and EMT. Furthermore, KMP sensitizes tumor cells to chemotherapy with different drugs.
The preventive role of KMP in the development of cancer may be due, among other factors, to the elimination of ROS species. Elevated ROS production is generally considered a pro-tumoral factor, leading to DNA, protein, and lipid damage, improving adaptations to hypoxia, and promoting genetic instability [86,87]. However, in pancreatic cancers, ROS have been shown to act as a double-edged sword, either facilitating cancer progression or dramatically enhancing cell death [88,89]. ROS-mediated anticancer properties, including the inhibition of cell proliferation, cell cycle arrest, induction of apoptosis, and suppression of cell invasion and migration, have been reported for KMP in several types of cancer such as colorectal cancer, breast cancer, melanoma, and hepatocarcinoma [29,53,90]. For example, KMP triggers ROS-induced apoptosis in human breast cancer via the c-Jun N-terminal kinase (JNK) signaling pathway [91] and potentiates apoptosis in human hepatoma cells through the mitochondrial apoptotic pathway regulated by p53-induced gene 3 (PIG3) [90]. In vitro experiments using cells from squamous cell carcinomas of the oral tongue (SCC-25, SCC-4, and SCC-1483), esophagus (Eca-109), oral cavity (PCI-13), and hypopharynx (FaDu) demonstrated the antiproliferative effects of KMP [36,92,93]. The expression of MMP-2, Bcl-2, c-Jun (AP-1 transcription factor subunit), and hexokinase-2 was reduced by KMP, which contributed to cell cycle arrest in the G0/G1 phase. In a mouse xenograft model, the ability of KMP to successfully inhibit tumor growth through loss of hexokinase-2 expression and EGFR activity in cancerous tissues provided further evidence of the anticancer efficacy of the drug. KMP has also been shown to suppress the invasion and migration of SCC-4 cells, decreasing enzyme activity and MMP-2 gene expression. Furthermore, KMP inhibits MAPK1/2 phosphorylation, efficiently downregulating MMP-2 [92,93].
EMT is believed to be the main factor promoting cell migration and invasion in cancer [94] and is also associated with the acquisition of resistance to chemotherapy. TGF-β1 acts as a metastasis inducer, promoting EMT in advanced stages of tumor progression. Jo et al. [48], analyzing human non-small lung cancer cells, demonstrated that KMP strongly inhibited TGF-β1-induced EMT, elevating E-cadherin expression and suppressing the induction of mesenchymal markers. Interestingly, KMP reversed TGF-β1-mediated Snail1 induction. Resistance to chemotherapy is one of the main causes of antineoplastic treatment failure. Combination therapy with sensitizing agents represents a successful approach to suppressing cancer cells and inhibiting the emergence of drug resistance. Within this context, Riahi-Chebbi et al. [95] evaluated the anticancer properties of some polyphenols, particularly KMP, alone or together with 5-FU, in a 5-FU-resistant colorectal cancer cell line (LS174-R). The authors showed that KMP can overturn 5-FU resistance of LS174-R cells through the induction of apoptosis and cell cycle arrest [95]. Furthermore, this agent prevented the production of ROS. Thus, KMP may be a promising chemotherapeutic agent to be used alone or in conjunction with 5-FU to reverse resistance to chemotherapy drugs [95].
Despite the limitations of in vitro models in fully mimicking the native tumor microenvironment and, consequently, in testing effective antineoplastic therapies, significant results have been obtained with the use of KMP in different neoplastic processes. Future studies using 3D in vitro models, which are more representative of tumor complexity, and animal models may strengthen the understanding of the use of KMP in cancer and its different mechanisms of action. In addition, as a dietary flavonoid, KMP has the limitations of rapid metabolization, low solubility, and low bioavailability, which may represent major obstacles to its anticancer effects in vivo. In view of the findings of the in vitro studies, we believe that there is a strong enough justification to explore the potential applications of KMP in in vivo models. Epidemiological studies evaluating the preventive role of KMP in the development of carcinogenesis are also recommended.
Given the in vitro potential of KMP to act deeply at the molecular level, affecting the DNA structure in the inhibition of cancer and the different mechanisms involved in tumorigenesis, further research is needed to establish the exact signaling pathway mediated by tumor-specific KMP. First, it is necessary to study and identify potential biomarkers that can predict the sensitivity of different tumors to KMP. Second, ex vivo experiments are needed to further clarify the antitumor effects of KMP for cancer prevention and treatment, in addition to studies exploring the effects of KMP combined with other drugs. Finally, the preventive role, efficacy, and tolerability of KMP should be evaluated in longitudinal clinical studies to support the clinical application of KMP in the future. Therefore, further research into the development of KMP as a new molecularly targeted agent against cancer is needed.
5. Conclusions
Previous reviews have evaluated the effects of KMP on different types of cancer and other pathological processes [96,97]. Nonetheless, this is the first systematic review that evaluates the in vitro effects of KMP on different types of cancer. The results of this systematic review indicate that KMP exerts in vitro inhibitory effects on several carcinogenesis pathways in different solid cancers, particularly the promotion of cell death, inhibition of proliferation, angiogenesis, and EMT, and sensitization of neoplastic cells to chemotherapy with different drugs. Therefore, KMP as an adjuvant drug, combined with other chemotherapeutics, even targeted drugs, may be a more effective approach to improving therapeutic outcomes and the quality of life of patients with malignant neoplasms.
Research into cancer therapies has resulted in the development of natural medications that demonstrate a milder negative impact compared to traditional cancer drugs. The different functions exerted by KMP allow this molecule to act on various targeted proteins to downregulate disease progression. However, their translation from bench to bedside is still an area that needs to be explored. Regarding future directions, investigations should be carried out in animal models into the effects of KMP in neoplastic treatment, evaluating its mechanisms, possible adverse effects, and appropriate dosages for treatment in different types of cancer. As KMP targets multiple pathways, its use in a multi-targeted therapy that provides additive or synergistic chemotherapy effects could be a good option for cancer treatment. When it comes to clinical application, the use of KMP in clinical studies faces several challenges. The bioavailability of KMP, selection of a dosage range for clinical/therapeutic application, possible restrictions on pharmacokinetics, and the development of delivery systems should be the focus of future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16030585/s1, Table S1: PRISMA 2020 Checklist.
Author Contributions
Conceptualization, E.F.d.M., L.Q.R.d.O. and R.D.C.; methodology, E.F.d.M., L.Q.R.d.O., H.G.d.F.M., M.R.d.S.M., R.d.A.F., C.O.R. and R.D.C.; formal analysis, E.F.d.M., L.Q.R.d.O., H.G.d.F.M., M.R.d.S.M., R.d.A.F., C.O.R. and R.D.C.; investigation, E.F.d.M., L.Q.R.d.O., H.G.d.F.M. and M.R.d.S.M.; resources, E.F.d.M., L.Q.R.d.O., H.G.d.F.M., M.R.d.S.M., R.d.A.F., C.O.R. and R.D.C.; writing—original draft preparation, E.F.d.M., L.Q.R.d.O., H.G.d.F.M., M.R.d.S.M., R.d.A.F., C.O.R. and R.D.C.; writing—review and editing, all authors; visualization, R.d.A.F., C.O.R. and R.D.C.; supervision, R.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2022/08908-0 to R. D. Coletta). E.F. de Morais is a research fellow supported by FAPESP (grant 2022/00994-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 5-FU | 5-Fluorouracil |
| AHR | Aryl hydrocarbon receptor |
| AP-1 | Activator protein-1 transcription factor |
| BAD | BCL2-associated agonist of cell death |
| BAX | BCL2-associated X |
| BCL2 | B-cell lymphoma 2 |
| CDC2 | Cyclin-dependent kinase 2 |
| CDC25C | Cell Division Cycle 25C |
| CDK2 | Cyclin D-dependent kinase 2 |
| CDK4 | Cyclin D-dependent kinase 4 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A |
| Chk2 | Checkpoint kinase 2 |
| DNA | Deoxyribonucleic Acid |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| FADD | Fas-associated death domain |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| HIF | Hypoxia-inducible factor |
| JNK | c-Jun N-terminal kinase |
| KMP | Kaempferol |
| MAPK | Mitogen-activated protein kinase |
| MMPs | Matrix metalloproteinases |
| mRNA | Messenger ribonucleic acid |
| mTOR | Mammalian target of rapamycin |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PKB | Protein kinase B, also known as AKT |
| ROS | Reactive oxygen species |
| Smad2 | Mothers against decapentaplegic homolog 2 |
| Smad4 | Mothers against decapentaplegic homolog 4 |
| STAT | Signal transducer and activator of transcription |
| TFEB | Transcription factor EB |
| TGF-β1 | Transforming growth factor-β1 |
| VEGF | Vascular endothelial growth factor |
Appendix A

Table A1.
Search strategies with appropriate key words and MeSH terms.
Table A1.
Search strategies with appropriate key words and MeSH terms.
| Database | Strategy |
|---|---|
| PubMed/Medline | (“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” [Title/Abstract]) (“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”[Title/Abstract]) AND “Cancer”[Title/Abstract]) (“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”[Title/Abstract]) AND (“cell lines”[Title/Abstract] OR “cancer cell lines”[Title/Abstract] OR “neoplastic cell lines”[Title/Abstract] OR “malignancy”[Title/Abstract]) (“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”[Title/Abstract]) AND (“cell lines”[Title/Abstract] OR “cancer cell lines”[Title/Abstract] OR “neoplastic cell lines”[Title/Abstract] OR “malignancy”[Title/Abstract]) AND “Treatment”[Title/Abstract]) |
| Science Direct | “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” AND “Treatment” |
| Scopus | TITLE-ABS-KEY((“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”)) TITLE-ABS-KEY((“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”) AND (“cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy”)) TITLE-ABS-KEY((“Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one”) AND (“cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy”) AND (“Treatment”)) |
| EMBASE | (‘Kaempferol’ OR ‘3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one’) AND [embase]/lim NOT ([embase]/lim AND [medline]/lim) (‘Kaempferol’ OR ‘3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one’) AND (‘cell lines’ OR ‘cancer cell lines’ OR ‘neoplastic cell lines’ OR ‘malignancy’) AND [embase]/lim NOT ([embase]/lim AND [medline]/lim) (‘Kaempferol’ OR ‘3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one’) AND (‘cell lines’ OR ‘cancer cell lines’ OR ‘neoplastic cell lines’ OR ‘malignancy’) AND (‘Treatment’) AND [embase]/lim NOT ([embase]/lim AND [medline]/lim) |
| Cochrane Collaboration Library | “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” AND “Treatment” |
| Google Scholar | “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” “Kaempferol” OR “3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one” AND “cell lines” OR “cancer cell lines” OR “neoplastic cell lines” OR “malignancy” AND “Treatment”. |
Appendix B

Table A2.
List of excluded studies along with reasons for exclusion (n = 62).
Table A2.
List of excluded studies along with reasons for exclusion (n = 62).
| Author (Year) | Title and DOI/PMID | Reason for Exclusion |
|---|---|---|
| Alkandahri et al. [98] | Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. DOI: 10.1155/2023/1387665. | Study did not evaluate cancer cells |
| Ayob et al. [99] | Cytotoxicity Activities in Local Justicia gendarussa Crude Extracts against Human Cancer Cell Lines. DOI: 10.11113/jt.v64.2043 | Study did not evaluate cancer cells |
| Bandyopadhyay et al. [100] | Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. DOI: 10.1016/j.mce.2008.01.015 | No information about kaempferol source |
| Bezdieniezhnykh et al. [101] | Establishment And Characterization of New Breast And Ovarian Cancer Cell Lines As A Model For Studying Cellular Plasticity In Vitro. PMID: 27356577 | Kaempferol was not assessed |
| Boadi et al. [102] | Effect of Quercetin, Genistein and Kaempferol on Glutathione and Glutathione-Redox Cycle Enzymes in 3T3-L1 Preadipocytes. DOI: 10.3109/01480545.2015.1082135 | Study did not evaluate cancer cells |
| Boadi et al. [103] | Flavonoids Reduce Lipid Peroxides and Increase Glutathione Levels in Pooled Human Liver Microsomes (HLMs). DOI: 10.4236/abc.2021.116019 | Study did not evaluate cancer cells |
| Budisan et al. [104] | Inhibitory effect of CAPE and kaempferol in colon cancer cell lines-possible implications in new therapeutic strategies. DOI: 0.3390/ijms20051199 | No information about kaempferol source and/or dilution |
| Catalán et al. [105] | Kaempferol Induces Cell Death and Sensitizes Human Head and Neck Squamous Cell Carcinoma Cell Lines to Cisplatin. DOI: 10.1007/5584_2020_603 | No information about kaempferol source and/or dilution |
| Chen et al. [106] | Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. DOI: 0.1016/j.bcp.2005.02.022 | Study did not evaluate cells from solid tumor |
| Chen et al. [107] | Biological Evaluation of Selected Flavonoids as Inhibitors of MNKs Targeting Acute Myeloid Leukemia. DOI: 10.1021/acs.jnatprod.0c00516 | Study did not evaluate cells from solid tumor |
| Chien et al. [108] | Kaempferol suppresses cell migration through the activation of the ERK signaling pathways in ARPE-19 cells. DOI: 10.1002/tox.22686 | Study did not evaluate cancer cells |
| Da et al. [109] | Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. DOI: 10.1155/2019/1907698 | Study did not evaluate cancer cells |
| De Sousa et al. [110] | Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. DOI: 10.1021/np030513u | Study did not evaluate cancer cells |
| Dimas et al. [111] | Cytotoxic activity of kaempferol glycosides against human leukaemic cell lines in vitro. DOI: 10.1006/phrs.1999.0562 | No information about kaempferol source and no application of solid cancer cells |
| Halimah et al. [112] | Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. DOI: 10.3892/br.2014.385 | No information about kaempferol source and/or dilution |
| Han et al. [113] | Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. DOI: 10.1016/j.biopha.2018.09.087 | Retracted article |
| Huang et al. [114] | Cytotoxicity of Kaempferol-3-O-rhamnoside against nasopharyngeal cancer via inhibition of EGFR-TK | No information about kaempferol source and/or dilution |
| Jeong et al. [115] | Kaempferol Induces Cell Death Through ERK and Akt-Dependent Down-Regulation of XIAP and Survivin in Human Glioma Cells. DOI: 10.1007/s11064-008-9868-5 | No information about kaempferol source and/or dilution |
| Jin et al. [116] | Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. DOI: 10.1039/D1FO00402F | Study did not evaluate cancer cells |
| Jin et al. [117] | Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. DOI: 10.1016/j.biopha.2023.115215. | Study did not evaluate cancer cells |
| Jo et al. [48] | Analysis of Inhibitory Effects of Kaempferol on Migration and Epithelial-mesenchymal Transition in Human Lung Cancer. DOI: 10.1016/j.neo.2015.06.004 | No information about kaempferol source and/or dilution |
| Joe et al. [118] | Engineering of flavonoid O-methyltransferase for a novel regioselectivity. DOI: 10.1007/s10059-010-0098-8 | Study did not evaluate cancer cells |
| Jokar et al. [119] | A comparative study of anti-leukemic effects of kaempferol and epigallocatechin-3-gallate (EGCG) on human leukemia HL-60 cells. DOI: 10.22038/AJP.2021.17604 | Study did not evaluate cells from solid tumor |
| Kang et al. [120] | Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. DOI: 10.1016/j.ejphar.2009.03.068 | No information about kaempferol source and no application of cancer cells |
| Kang et al. [121] | Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals. DOI: 10.3346/jkms.2011.26.7.893 | Kaempferol was not assessed |
| Kim et al. [122] | Isolation of flavonol rhamnosides fromloranthus tanakae and cytotoxic effect of them on human tumor cell lines. DOI: 10.1007/BF02980044 | Kaempferol was not assessed |
| Kim et al. [123] | Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. DOI: 10.1016/j.jnutbio.2015.09.027 | No information about kaempferol source |
| Kluska et al. [124] | Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. DOI: 10.1039/c9tx00176j | Study did not evaluate cancer cells |
| Kuntz et al. [125] | Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. DOI: 10.1007/s003940050054 | No information about kaempferol source and/or dilution |
| Lee et al. [126] | Kaempferol protects HIT-T15 Pancreatic Beta Cells from 2-deoxy-D-ribose-induced Oxidative Damage. DOI: 10.1002/ptr.2983 | Study did not evaluate cancer cells |
| Lee et al. [127] | Kaempferol Isolated from Nelumbo nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes. DOI: 10.1089/jmf.2015.3457 | Study did not evaluate cancer cells |
| Li et al. [128] | Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. DOI: 10.1016/j.cbi.2008.10.048 | Study did not evaluate cancer cells |
| Lin et al. [129] | Kaempferol enhances the suppressive function of Treg cells by inhibiting FOXP3 phosphorylation. DOI: 10.1016/j.intimp.2015.03.044 | Study did not evaluate cancer cells |
| Lin et al. [130] | Isolation and identification of antiproliferative compounds from the roots of Tetrastigma hemsleyanum against MDA-MB-435S cell lines. PMID: 27393430 | No information about kaempferol source and/or dilution |
| Luo et al. [131] | Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. DOI: 10.1016/j.foodchem.2011.07.045 | No information about kaempferol source and/or dilution |
| Luo et al. [132] | Kaempferol attenuates streptozotocin-induced diabetic nephropathy by downregulating TRAF6 expression: The role of TRAF6 in diabetic nephropathy. DOI: 10.1016/j.jep.2020.113553 | Study did not evaluate cancer cells |
| Meng et al. [133] | A kaempferol-3-O-β-d-glucoside, intervention effect of astragalin on estradiol metabolism. DOI: 10.1016/j.steroids.2019.05.005 | Study did not evaluate cancer cells |
| Nejabati et al. [134] | Kaempferol: A potential agent in the prevention of colorectal cancer. DOI: 10.14814/phy2.15488 | Review |
| Park et al. [135] | Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. DOI: 10.1021/jf052900a | Study did not evaluate cancer cells |
| Raghavan et al. [136] | Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. DOI: 10.1016/j.procbio.2015.08.003 | Study did not evaluate cancer cells |
| Rahul et al. [137] | Effect of kaempferol on the transgenic Drosophila model of Parkinson’s disease. DOI: 10.1038/s41598-020-70236-2 | Study did not evaluate cancer cells |
| Riahi-Chebbi et al. [95] | The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. DOI: 10.1038/s41598-018-36808-z | No information about kaempferol source and/or dilution |
| Roth et al. [138] | Phytoestrogen kaempferol (3,4′,5,7-tetrahydroxyflavone) protects PC12 and T47D cells from β-amyloid–induced toxicity. PMID: 10412031 | Study did not evaluate cancer cells |
| Saraei et al. [139] | Kaempferol sensitizes tumor necrosis factor-related apoptosis-inducing ligand-resistance chronic myelogenous leukemia cells to apoptosis. DOI: 10.1007/s11033-021-06778-z | Study did not evaluate cells from solid tumor |
| Sengupta et al. [15] | Anticancer Properties of Kaempferol on Cellular Signaling Pathways. DOI: 10.2174/1568026622666220907112822 | Review |
| Sharma et al. [140] | Kaempferol induces apoptosis in glioblastoma acells through oxidative stress. DOI: 10.1158/1535-7163.MCT-06-0788 | Study did not evaluate cancer cells |
| Sharma et al. [141] | Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. DOI: 10.1016/j.biopha.2018.10.195 | Study did not evaluate cancer cells |
| Stapel et al. [142] | Polyphenol compounds with anti-carcinogenic qualities: Effects of quercetin (flavonol), chrysin (flavon), kaempferol (flavanol), naringenin (flavanon) and hesperidin (flavanoid) on in vitro breast cancer. DOI: 10.5897/JMPR12.5126 | No information about kaempferol source and/or dilution |
| Swanson et al. [143] | Impact of apigenin and kaempferol on human head and neck squamous cell carcinoma. DOI: 10.1016/j.oooo.2013.10.012 | Study did not evaluate cancer cells |
| Tu et al. [144] | The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. DOI: 10.1002/sca.21312 | No information about kaempferol source and/or dilution |
| Tu et al. [145] | Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. DOI: 10.1016/j.bmcl.2016.03.091 | No information about kaempferol source |
| Wang et al. [146] | The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. DOI: 10.1016/j.biopha.2019.109086 | Review |
| Wang et al. [147] | Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. DOI: 10.1016/j.biopha.2018.12.105 | Study did not evaluate cancer cells |
| Wang et al. [148] | Kaempferol-Driven Inhibition of Listeriolysin O Pore Formation and Inflammation Suppresses Listeria monocytogenes Infection. DOI: 10.1128/spectrum.01810-22 | Study did not evaluate cancer cells |
| Wu et al. [149] | Kaempferol protects mitochondria and alleviates damages against endotheliotoxicity induced by doxorubicin. DOI: 10.1016/j.biopha.2020.110040 | Study did not evaluate cancer cells |
| Xiao et al. [150] | Old wine in new bottles: Kaempferol is a promising agent for treating the trilogy of liver diseases. DOI: 10.1016/j.phrs.2021.106005 | Study did not evaluate cancer cells |
| Xie et al. [151] | Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. DOI: 10.3390/ijms141121215 | Study did not evaluate cancer cells |
| Yang et al. [152] | Exploration in the Mechanism of Kaempferol for the Treatment of Gastric Cancer Based on Network Pharmacology. DOI: 10.1155/2020/5891016 | Study based in bioinformatics only |
| Yusof et al. [153] | Hypolipidemic effects of quercetin and kaempferol in human hepatocellular carcinoma (HepG2) cells. * | No information about kaempferol source and/or dilution |
| Zeng et al. [154] | Kaempferol ameliorates in-vitro and in-vivo postovulatory oocyte ageing in mice. DOI: 10.1016/j.rbmo.2022.07.005 | Animal model study |
| Zhang et al. [155] | Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. DOI: 10.1590/1414-431x20187843 | Retracted article |
| Zheng et al. [156] | Molecular Mechanism Investigation on Monomer Kaempferol of the Traditional Medicine Dingqing Tablet in Promoting Apoptosis of Acute Myeloid Leukemia HL-60 Cells. DOI: 10.1155/2022/8383315. | Study did not evaluate cells from solid tumor |
* This article does not have DOI or PMID.
Appendix C

Table A3.
Main characteristics of the selected papers.
Table A3.
Main characteristics of the selected papers.
| Author | Country | Cell Lines | Origin of Cell Lines | Dosages of KMP | IC50 | Main Results |
|---|---|---|---|---|---|---|
| Wang et al. [10] | China | HepG2, CT26, B16F1 | Human hepatocellular carcinoma, mouse colon cancer, mouse melanoma | 10–100 µM | 30.92–88.02 µM | Inhibited proliferation and AKT phosphorylation and promoted the cleavage of caspase-9, caspase-7, caspase-3, and PARP. |
| Li et al. [12] | The United States | HcT-8, HcT-116 | Human colorectal cancer | 0–200 μM | 350 μM | Synergistic effect with 5-FU by inhibiting cell proliferation and inducing apoptosis. |
| Park et al. [14] | Republic of Korea | HT29, HCT116 | Human colon cancer | 20–40 µM | NI | Inhibited AP-1 in oxaliplatin-resistant cells. |
| Zheng et al. [17] | China | A375, B16F10 | Human and mouse melanoma | 2–64 μM | NI | Inhibited migration and invasion. |
| Nguyen et al. [22] | Republic of Singapore | A549 | Human lung cancer | 17.5–70 µM | NI | Induced apoptosis via activation of AKT-1, Bcl-2 family of proteins, and MAPK signaling. |
| Ackland et al. [23] | Australia | PMC42, HuTu-80, Caco-2 | Human breast cancer, duodenal adenocarcinoma, colon adenocarcinoma | 1–10 µM | NI | Reduced proliferation. |
| Nakamura et al. [24] | Japan | HCT116, KNC | Human colon cancer | 2.5–20 µM | NI | Promoted expression and phosphorylation of connexin 43 via a STAT3-dependent mechanism. |
| Campbell et al. [25] | The United States | LNCaP | Human prostate cancer | 50 mM | NI | Inhibited proliferation in a dose-dependent manner, without cytotoxicity. |
| Oh et al. [26] | Republic of Korea | MCF-7 | Human breast cancer | 10−7–10−4 M | NI | Antiproliferative effects are dependent of estrogen receptor activation. |
| Leung et al. [27] | Taiwan | H460 | Human lung carcinoma | 50–80 μM | NI | Induced apoptosis and MnSOD levels. |
| Li et al. [28] | China | BEL-7402, HeLa, 95-D, A375, MKN-45, A431 | Human liver cancer, cervical carcinoma, melanoma, gastric cancer, skin cancer | 100–400 μg/mL | 43–110 g/mL | Induced cell cycle arrest at G1 and apoptosis. |
| Choi et al. [29] | Republic of Korea | MDA-MB-453 | Human breast cancer | 1–200 μM | NI | Inhibited proliferation, induced cell cycle arrest at G2/M phase, downregulated CDK1, cyclin A, and cyclin B, and induced apoptosis which was associated with p53 upregulation. |
| Kim et al. [30] | Republic of Korea | MCF-7, T47D, MDA-MB-231, HC-11 | Human breast cancer | 20 μM | NI | Induced apoptosis via activation of MAPK, MEK1, and ELK1. |
| Siegelin et al. [31] | Germany | U87, U251, U373 | Human glioblastoma | 5–200 µM | NI | Promoted apoptosis after suppression of survivin. |
| Yoshida et al. [32] | Japan | SW480, DLD-1, PC3 | Human colon cancer, prostate cancer | 2.5–40 µM | NI | Upregulated TRAIL receptors (DR5 and DR4). |
| Zhang et al. [33] | The United States | MIA PaCa-2, Panc-1 | Human pancreatic cancer | 17.5–35 μM | NI | Inhibited proliferation and induced apoptosis. |
| Luo et al. [34] | The United States | OVCAR-3, A2780/CP70 | Human ovarian cancer | 5–80 µM | NI | Inhibited angiogenesis and VEGF expression via HIF-dependent (AKT/HIF) and HIF-independent pathways. |
| Huang et al. [35] | Taiwan | U-2 OS, HOB, 143B | Human osteosarcoma | 25–200 mM | NI | Reduced cell viability and regulated the expression of proteins involved in the endoplasmic reticulum stress pathway and the mitochondrial signaling pathway. |
| Kang et al. [36] | Republic of Korea | SCC-1483, SCC-QLL1, SCC-25 | Human oral squamous cell carcinoma | 20–80 μM | NI | Inhibited proliferation and induced apoptosis, which was dependent on caspase-3. |
| Luo et al. [37] | The United States | OVCAR-3 | Human ovarian cancer | 20 μM | NI | Enhanced cisplatin effects on promoting cell death. |
| Macpherson et al. [38] | Canada | T-47D, BT-549, MDA-MB-231 | Human breast cancer | 0.1–10 μM | NI | Induced cell death. |
| Mylonis et al. [39] | Greece | Huh7 | Human hepatocellular carcinoma | 1–100 μM | 5.16 μM | Promoted cell death and inhibited MAPK and HIF-1 activity. |
| Luo et al. [40] | The United States | OVCAR-3, A2780/CP70, A2780/wt | Human ovarian cancer | 20–160 μM | NI | Kaempferol induces apoptosis in ovarian cancer cells by regulating pro-apoptotic and anti-apoptotic protein expressions in the intrinsic apoptosis pathways and is a good candidate for the chemoprevention of ovarian cancers in humans. |
| Luo et al. [41] | The United States | A2780/CP70, OVCAR-3 | Human ovarian cancer | 10–25 µM | NI | Induced apoptosis. |
| Chen et al. [42] | Japan | U-2 OS | Human osteosarcoma | 25–100 μM | NI | Inhibited invasion, migration, and adhesion, reduced activities of MMP-2, MMP-9, and uPA, and altered phosphorylation of MAPK, p38, and JNK and decreased the DNA binding activity of AP-1. |
| Cho et al. [43] | Republic of Korea | HT-29 | Human colon adenocarcinoma | 60 μmol/L | NI | Decreased viability and proliferation and induced G1 cell cycle arrest in 6 h and G2/M arrest in 12 h. Inhibited CDK2, CDK4, cyclin D1, cyclin E, cyclin A, phosphorylation of Rb, Cdc25C, Cdc2, cyclin B1, and Cdc2. |
| Wang et al. [44] | China | HeLa, HepG2, A549 | Human cervical cancer, liver cancer, lung cancer | 5–20 μM | NI | Antagonized activities of estrogen-related receptors alpha and gamma (ERRα and ERRγ). |
| Jaramillo-Carmona et al. [45] | Spain | HCT-116 | Human colon carcinoma | 0–200 μM | 75 μM | The combination of quercetin and kaempferol exhibited greater cytotoxic efficacy than quercetin or kaempferol alone, and this effect was related to cell cycle arrest at G2/M. |
| Dang et al. [46] | China | 5637, T24, T24L | Human bladder cancer | 50–150 mM | NI | Induced apoptosis and cell cycle arrest. |
| Hang et al. [47] | China | A549 | Lung cancer | 10–60 μM | 72 μM | Inhibited proliferation and migration in a dose-dependent manner and modulated the expression of E-cadherin and vimentin. |
| Jo et al. [48] | Republic of Korea | A549 | Human lung cancer | 50 μM | NI | Blocked TGF-β1-induced migration, MMP-2 activity, and EMT |
| Kuo et al. [49] | Taiwan | A-549 | Human lung cancer | 7–112 μM | NI | Increased cell death. |
| Li et al. [50] | China | MDA-MB-231 | Human breast cancer | 10–40 μM | 204.7 mol/L. | Inhibited invasion by blocking the PKC/MAPK/AP-1 cascade and subsequent MMP-9 expression and activity. |
| Song et al. [51] | China | MKN28. SGC7901 | Human gastric cancer | 25–200 µM | 124.86 μM | Inhibited proliferation and promoted G2/M cell cycle arrest and apoptosis. |
| Lee et al. [52] | Republic of Korea | Miapaca-2, Panc-1, SNU-213 | Human pancreatic cancer | 0–200 μM | NI | Decreased viability and increased apoptosis, which were mediated by inhibition of EGFR-related Src, MAPK, and AKT pathways. |
| Liao et al. [53] | China | MCF-7, SGC-7901, Hela, A549 | Human breast cancer, gastric cancer, cervical cancer, lung cancer | 25–100 μg/mL | NI | Inhibited proliferation and induced apoptosis. |
| Han et al. [54] | China | HCC, HepG2, Huh-7, BEL7402 SMMC, Pri-1/-2/-3 | Human hepatocellular carcinoma | 5–100 μM | 25–50 μM | Induced AMPK, which led to Ulk1 phosphorylation, mTOR 1 complex inhibition, and cellular autophagy. |
| Kashafi et al. [55] | Iran | HeLa | Human cervical adenocarcinoma | 12–100 mM | 10.48 mM | Decreased viability and induced apoptosis through downregulation of PI3K/AKT and hTERT pathways. |
| Chuwa et al. [56] | Japan | HEC-265/HEC-108, HEC-180 | Human endometrial carcinoma | 36–72 µM | 83.65 μM | Induced sub-G1 cell accumulation and apoptosis. |
| Gao et al. [57] | The United States | A2780/CP70 | Human ovarian endometrioid adenocarcinoma | 40μmol/L | NI | Induced G2/M cell cycle arrest via Chk2/Cdc25C/Cdc2 pathway and Chk2/p21/Cdc2 pathway and apoptosis and p53 upregulation. |
| Kim et al. [58] | Republic of Korea | AGS, SNU-216, NCI-N87, SNU-638, MKN-74 | Human gastric cancer | 25–100 μM | NI | Promoted autophagy and cell death. |
| Lu et al. [59] | China | HCT116, HT29, YB5 | Human colorectal cancer | 1.25–5 M | NI | Regulated proliferation and migration. |
| Pham et al. [60] | Australia | A2780, H460, A431, MIA PaCa-2, Du145, HT29, MCF-7, BE2-C, SJ-G2, U87, SMA | Human ovarian cancer, lung cancer, skin cancer, pancreas cancer, prostate cancer, colon cancer, breast cancer, neuroblastoma, glioblastoma | 0–50 µM | 19–50 µM | Promoted cytotoxicity. |
| Thangavel et al. [61] | Republic of Korea | A549 | Human lung cancer | 25–150 μM | 10 μM | Inhibited proliferation. |
| Wu et al. [62] | China | EJ | Human bladder cancer | 10–320 µM | NI | Induced apoptosis and cell cycle arrest at S phase. |
| Yang et al. [63] | China | A375 | Human melanoma | 10–80 µM | 20 µM | Induced apoptosis and G2/M cell cycle arrest and inhibited migration, with downregulation of mTOR, pmTOR, PI3K, p-PI3K, and AKT. |
| Abdullah et al. [64] | India | IMR32, Neuro2A | Human and mouse neuroblastoma | 25–100 μM | NI | Reduced proliferation and increased apoptosis along with induction of neuritogenesis. |
| Zhu et al. [65] | China | BT474, MDA-MB231 | Human breast cancer | 50 μmol/L | 43, >100 μmol/L | Induced apoptosis and DNA damage and increased the expression levels of H2AX, cleaved caspase-9, cleaved caspase-3, and p-ATM. |
| Chen et al. [66] | China | U87 MG, U251 | Human glioblastoma | 20–120 μM | 97.2, 79.2 μM | Suppressed proliferation, increased ROS, and decreased mitochondrial membrane potential. |
| Gutierrez-Uribe et al. [67] | Mexico | RKO | Human colon cancer | 0.01–1 μM | NI | miR31, miR92a, KRAS, and c-MYC were downregulated, whereas AMPK and APC were upregulated. |
| Kitakaze et al. [68] | Japan | HepG2 | Human hepatocellular carcinoma | 30 μM | NI | In combination with luteolin, inhibited the expression of drug-metabolizing enzymes. |
| Nair et al. [69] | India | HepG2, N1S1 | Human hepatocellular carcinoma | 6.25–50 µM | 9.61 µM | Increased the effect of sub-toxic concentrations of sorafenib. |
| Fouzder et al. [70] | India | A549, NCIH460 | Human colon adenocarcinoma | NI | NI | Induced ROS accumulation and apoptosis, which was related to modulation of Nrf2, GST, and NQO1. |
| Inden et al. [71] | Japan | N2A | Mouse neuroblastoma | 5 mM | NI | Induced autophagy by increasing lysosome biogenesis and directly blocked the formation of α-Syn amyloid fibrils. |
| Ju et al. [72] | Taiwan | Huh-7, SK-Hep-1 | Human hepatocellular carcinoma | 25–100 μM | NI | Reduced invasion, migration, MMP-9 expression and activity, and AKT phosphorylation. |
| Li et al. [73] | China | A549 | Human lung cancer | 0–200 μM | NI | Reduced cell viability, after cytoskeleton collapse and mitochondrial dysfunction. |
| Liu et al. [74] | China | SGC996, GBC-SD | Human gallbladder cancer | 100–200 μg/mL | NI | Promoted apoptosis due to cytochrome C release, activation of caspase-3 and caspase-9, and increase in BAX expression. |
| Wang et al. [75] | China | Mia PaCa-2, PANC-1 | Human pancreatic cancer | 0–1000 µM/L | 78.75, 79.07 μM | Induced ROS-dependent apoptosis via TGM2-mediated AKT/mTOR signaling. |
| Wu et al. [76] | China | HCT-116, DLD1 | Human colon cancer | 20–160 μM | 63.0, 98.3 μM | Inhibited glycolysis and proliferation by modulating miR-339–5p-hnRNPA1/PTBP1–PKM2 axis. |
| Yang et al. [77] | China | Huh-7, Huh-1, HepG2, HepG2.2.15, SK-Hep-1, PLC/PRF/5, HLE, HLF, Hep3B | Human liver cancer | 10–40 µM | 40 µM | Inhibited proliferation, migration, and invasion. |
| Zhang et al. [78] | China | BxPC-3, PANC-1 | Human pancreatic cancer | 6–192 µM | 155.21, 108.47 µM | Increased the effect of erlotinib via inhibiting PI3K/AKT and EGFR signaling. |
| Zhang et al. [79] | China | AGS, SKOV3IP1, MDA-MB-231 | Human gastric adenocarcinoma, ovarian cystadenocarcinoma, breast cancer | 0.5–4 μM | 6.83, 9.6 μM 11.12 μM | Reduced cell proliferation and inhibited TGF-β-induced cell migration, invasion, and EMT markers. |
| Ma et al. [80] | China | A549 | Non-small-cell lung cancer | 5–120 µM | 72.8 μM/L | Inhibited proliferation, migration, and invasion and promoted apoptosis. |
| Zhang et al. [81] | China | 22Rv1, PC-3, DU145 | Human prostate cancer | 10–40 µM | NI | Inhibited proliferation of androgen-dependent and androgen-independent prostate cancer cells. |
NI: not informed. Legend: TRAIL, tumor necrosis factor (TNF)-related apoptosis-inducing ligand; PARP, poly (ADP-ribose) polymerase; MnSOD, manganese-dependent superoxide dismutase; CDK1, cyclin-dependent kinase 1; MAPK, dual specificity mitogen-activated protein kinase 1; MEK1, Mitogen-Activated Protein Kinase Kinase 1; ELK1, ETS transcription factor ELK1; TGF-β, transforming growth factor-beta; EMT, epithelial–mesenchymal transition; EGFR, epidermal growth factor receptor; PI3K, phosphoinositide 3-kinase; phosphatidylinositol 3-kinase; PTBP1, polypyrimidine tract-binding protein 1–pyruvate kinase M2; mTOR, mechanistic target of rapamycin; AKT, protein kinase B; TGM2, transglutaminase 2 gene; MMP-9, matrix metalloproteinase-9; MMP-2, matrix metalloproteinase-2; ROS, reactive oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2; GST, glutathione S-transferases; NQO1, NAD(P)Hquinone-oxidoreductase-1; KRAS, Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; AMPK, AMP-activated protein kinase; APC, adenomatous polyposis coli; H2AX, H2A histone family member X; p-ATM, phospho-ATM; CHK2, checkpoint kinase 2; CDC25C, Cell Division Cycle 25C; CDC2, cyclin-dependent kinase 2; hTERT, Human Telomerase Reverse Transcriptase; PKC, protein kinase C; AP-1, activator protein-1; ERRα, estrogen-related receptor alpha; ERRγ, estrogen-related receptor gamma; JNK, c-Jun N-terminal kinase; uPA, urokinase-type plasminogen activator; HIF-1, hypoxia-inducible factor 1; VEGF, vascular endothelial growth factor; DR5, death receptor 5; DR4, death receptor 4; STAT3, transducer and activator of transcription-3.
Appendix D

Table A4.
Risk of bias assessment of the individual articles included in this systematic review (n = 64).
Table A4.
Risk of bias assessment of the individual articles included in this systematic review (n = 64).
| Author (Year) | Study Limitation | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Quality |
|---|---|---|---|---|---|---|
| Wang et al. [10] | Yes | Yes | No | Yes | Yes | +++ |
| Li et al. [12] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Park et al. [14] | Yes | Yes | Yes | No | Yes | +++ |
| Zheng et al. [17] | Yes | Yes | Yes | No | Yes | +++ |
| Nguyen et al. [22] | Yes | Yes | Yes | No | Yes | +++ |
| Ackland et al. [23] | Yes | Yes | Yes | No | Yes | +++ |
| Nakamura et al. [24] | Yes | Yes | Yes | No | Yes | +++ |
| Campbell et al. [25] | Yes | Yes | Yes | No | Yes | +++ |
| Oh et al. [26] | Yes | Yes | Yes | No | Yes | +++ |
| Leung et al. [27] | Yes | Yes | Yes | No | Yes | +++ |
| Li et al. [28] | Yes | Yes | No | Yes | Yes | +++ |
| Choi et al. [29] | Yes | Yes | Yes | No | Yes | +++ |
| Kim et al. [30] | Yes | Yes | Yes | No | Yes | +++ |
| Siegelin et al. [31] | Yes | Yes | Yes | No | Yes | +++ |
| Yoshida et al. [32] | Yes | Yes | Yes | No | Yes | +++ |
| Zhang et al. [33] | Yes | Yes | Yes | No | Yes | +++ |
| Luo et al. [34] | Yes | Yes | Yes | No | Yes | +++ |
| Huang et al. [35] | Yes | Yes | Yes | No | Yes | +++ |
| Kang et al. [36] | Yes | Yes | Yes | No | Yes | +++ |
| Luo et al. [37] | Yes | Yes | Yes | No | Yes | +++ |
| Macpherson et al. [38] | Yes | Yes | Yes | No | Yes | +++ |
| Mylonis et al. [39] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Luo et al. [40] | Yes | Yes | Yes | No | Yes | +++ |
| Luo et al. [41] | Yes | Yes | Yes | No | Yes | +++ |
| Chen et al. [42] | Yes | Yes | Yes | No | Yes | +++ |
| Cho et al. [43] | Yes | Yes | Yes | No | Yes | +++ |
| Wang et al. [44] | Yes | Yes | Yes | No | Yes | +++ |
| Jaramillo-Carmona et al. [45] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Dang et al. [46] | Yes | Yes | Yes | No | Yes | +++ |
| Hang et al. [47] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Jo et al. [48] | Yes | Yes | Yes | No | Yes | +++ |
| Kuo et al. [49] | Yes | Yes | Yes | No | Yes | +++ |
| Li et al. [50] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Song et al. [51] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Lee et al. [52] | Yes | Yes | Yes | No | Yes | +++ |
| Liao et al. [53] | Yes | Yes | No | No | Yes | ++ |
| Han et al. [54] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Kashafi et al. [55] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Chuwa et al. [56] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Gao et al. [57] | Yes | Yes | Yes | No | Yes | +++ |
| Kim et al. [58] | Yes | Yes | Yes | No | Yes | +++ |
| Lu et al. [59] | Yes | Yes | Yes | No | Yes | +++ |
| Pham et al. [60] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Thangavel et al. [61] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Wu et al. [62] | Yes | Yes | Yes | No | Yes | +++ |
| Yang et al. [63] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Abdullah et al. [64] | Yes | Yes | Yes | No | Yes | +++ |
| Zhu et al. [65] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Chen et al. [66] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Gutierrez-Uribe et al. [67] | Yes | Yes | Yes | No | Yes | +++ |
| Kitakaze et al. [68] | Yes | Yes | Yes | No | Yes | +++ |
| Nair et al. [69] | Yes | Yes | No | Yes | Yes | +++ |
| Fouzder et al. [70] | Yes | Yes | Yes | No | Yes | ++++ |
| Inden et al. [71] | Yes | Yes | Yes | No | Yes | +++ |
| Ju et al. [72] | Yes | Yes | Yes | No | Yes | +++ |
| Li et al. [73] | Yes | Yes | Yes | No | Yes | +++ |
| Liu et al. [74] | Yes | Yes | Yes | No | Yes | +++ |
| Wang et al. [75] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Wu et al. [76] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Yang et al. [77] | Yes | Yes | No | Yes | Yes | +++ |
| Zhang et al. [78] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Zhang et al. [79] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Ma et al. [80] | Yes | Yes | Yes | Yes | Yes | ++++ |
| Zhang et al. [81] | Yes | Yes | Yes | No | Yes | +++ |
GRADE: Yes, no serious limitations; No, serious limitations. For overall quality of evidence: ++, low; +++, moderate; ++++, high.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W. Biological therapies in the treatment of cancer-update and new directions. Int. J. Mol. Sci. 2021, 22, 11694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Chen, J.Y.; Fu, L.Q.; Yan, M.J. Recent advances in natural therapeutic approaches for the treatment of cancer. J. Chemother. 2020, 332, 53–65. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination anticancer therapies using selected phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting drug chemo-resistance in cancer using natural products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Wang, Y.; Geng, J.; Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetics rats. Drug Dev. Res. 2019, 80, 294–309. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.E.; An, H.J.; Lee, C.J.; Cho, E.S.; Kang, H.C.; Lee, J.Y.; Lee, H.S.; Choi, J.S.; Kim, D.J.; et al. Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch. Pharm. Res. 2021, 44, 1091–1108. [Google Scholar] [CrossRef]
- Sengupta, B.; Biswas, P.; Roy, D.; Lovett, J.; Simington, L.; Fry, D.R.; Travis, K. Anticancer properties of kaempferol on cellular signaling pathways. Curr. Top. Med. Chem. 2022, 2, 2474–2482. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol can reverse the 5-FU resistance of colorectal cancer cells by inhibiting PKM2-mediated glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Pan, Y.; Yang, G.; Liu, Y.; Zou, J.; Zhao, H.; Yin, G.; Wu, Y.; Li, X.; Wei, Z.; et al. Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur. J. Pharmacol. 2022, 931, 175226. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef]
- Pavan, L.M.; Rêgo, D.F.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N. In vitro anti-tumor effects of statins on head and neck squamous cell carcinoma: A systematic review. PLoS ONE 2015, 10, e0130476. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell Physiol. 2003, 197, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ackland, M.L.; van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [Google Scholar] [PubMed]
- Nakamura, Y.; Chang, C.C.; Mori, T.; Sato, K.; Ohtsuki, K.; Upham, B.L.; Trosko, J.E. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 2005, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.K.; King, J.L.; Harmston, M.; Lila, M.A.; Erdman, J.W. Synergistic effects of flavonoids on cell proliferation in Hepa-1c1c7 and LNCaP cancer cell lines. J. Food Sci. 2006, 71, S358–S363. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, Y.P.; Chung, K.H. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharm. Res. 2006, 29, 354–362. [Google Scholar] [CrossRef]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liu, J.W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef]
- Kim, B.W.; Lee, E.R.; Min, H.M.; Jeong, H.S.; Ahn, J.Y.; Kim, J.H.; Choi, H.Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; von Deimling, A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. 2008, 7, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer. 2009, 61, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res. 2010, 24, S77–S82. [Google Scholar] [CrossRef]
- Luo, H.; Daddysman, M.K.; Rankin, G.O.; Jiang, B.H.; Chen, Y.C. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Int. 2010, 10, 16. [Google Scholar] [CrossRef]
- MacPherson, L.; Matthews, J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Lett. 2010, 299, 119–129. [Google Scholar] [CrossRef]
- Mylonis, I.; Lakka, A.; Tsakalof, A.; Simos, G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem. Biophys. Res. Commun. 2010, 398, 74–78. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Li, Z.; Depriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef]
- Chen, H.J.; Lin, C.M.; Lee, C.Y.; Shih, N.C.; Peng, S.F.; Tsuzuki, M.; Amagaya, S.; Huang, W.W.; Yang, J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. 2013, 30, 925–932. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, J.H. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Wang, J. Kaempferol inhibits cancer cell growth by antagonizing estrogen-related receptor α and γ activities. Cell Biol. Int. 2013, 37, 1190–1196. [Google Scholar] [CrossRef]
- Jaramillo-Carmonal, S.; Rocio Abia, S.L.; Rodriguez-Arcos, R.; Jimenez, A.; Guillen, R.; Muriana, F.J.G. Combination of quercetin and kaempferol enhances in vitro cytotoxicity on human colon cancer (HCT-116) cells. Rec. Nat. Prod. 2014, 8, 262–271. [Google Scholar]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Hang, M.; Zhao, F.; Chen, S.B.; Sun, Q.; Zhang, C.X. Kaempferol modulates the metastasis of human non-small cell lung cancer cells by inhibiting epithelial-mesenchymal transition. Bangladesh J. Pharmacol. 2015, 10, 267–270. [Google Scholar] [CrossRef][Green Version]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.K.; Kim, B.C. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Tsai, Y.C.; Wu, H.C.; Ho, Y.J.; Chen, Y.S.; Yao, C.H.; Yao, C.H. Radiosensitization of non-small cell lung cancer by kaempferol. Oncol. Rep. 2015, 34, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H. Kaempferol Inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Han, B.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Wang, X.J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget 2017, 16, 86227–86239. [Google Scholar] [CrossRef]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef]
- Chuwa, A.H.; Sone, K.; Oda, K.; Tanikawa, M.; Kukita, A.; Kojima, M.; Oki, S.; Fukuda, T.; Takeuchi, M.; Miyasaka, A.; et al. Kaempferol, a natural dietary flavonoid, suppresses 17β-estradiol-induced survivin expression and causes apoptotic cell death in endometrial cancer. Oncol. Lett. 2018, 16, 6195–6201. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, Y.; Ou, R.; Feng, Q.; Ji, L.; Zheng, H.; Guo, Y.; Qi, X.; Kong, A.N.; Liu, Z. DACT2 epigenetic stimulator exerts dual efficacy for colorectal cancer prevention and treatment. Pharmacol. Res. 2018, 129, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief. 2018, 21, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Viswanath, B.; Kim, S. Synthesis and characterization of kaempferol-based ruthenium (II) complex: A facile approach for superior anticancer application. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 87–94. [Google Scholar] [CrossRef]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, P.; Sun, J.; Guo, L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J. BUON 2018, 23, 218–223. [Google Scholar]
- Abdullah, A.; Talwar, P.; d’Hellencourt, C.L.; Ravanan, P. IRE1α is critical for Kaempferol-induced neuroblastoma differentiation. FEBS J. 2019, 286, 1375–1392. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, J.; Yang, L.; Teng, M.; Lai, Z.Q.; Chen, X.; He, J. Anti-glioblastoma activity of kaempferol via programmed cell death induction: Involvement of autophagy and pyroptosis. Front. Bioeng. Biotechnol. 2020, 8, 614419. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Uribe, J.A.; Salinas-Santander, M.; Serna-Guerrero, D.; Serna-Saldivar, S.R.O.; Rivas-Estilla, A.M.; Rios-Ibarra, C.P. Inhibition of miR31 and miR92a as oncological biomarkers in RKO colon cancer cells treated with kaempferol-3-O-glycoside isolated from black bean. J. Med. Food. 2020, 23, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Makiyama, A.; Nakai, R.; Kimuraa, Y.; Ashida, H. Kaempferol modulates TCDD- and t-BHQ-induced drug-metabolizing enzymes and luteolin enhances this effect. Food Funct. 2020, 11, 3668–3680. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Anto, R.J.; Sabitha, M.; Lekshmi, R.N. Kaempferol-mediated sensitization enhances chemotherapeutic efficacy of sorafenib against hepatocellular carcinoma: An in silico and in vitro approach. Adv. Pharm. Bull. 2020, 10, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Takagi, A.; Kitai, H.; Ito, T.; Kurita, H.; Honda, R.; Kamatari, Y.O.; Nozaki, S.; Wen, X.; Hijioka, M.; et al. Kaempferol has potent protective and antifibrillogenic effects for α-synuclein neurotoxicity in vitro. Int. J. Mol. Sci. 2021, 22, 11484. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.C.; Ho, Y.C.; Chen, P.N.; Lee, H.L.; Lai, S.Y.; Yang, S.F.; Yeh, C.B. Kaempferol inhibits the cell migration of human hepatocellular carcinoma cells by suppressing MMP-9 and Akt signaling. Environ. Toxicol. 2021, 36, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, X.; Wang, Y.; Zheng, X.; Chu, Q. Kaempferol-3-O-rutinoside, a flavone derived from Tetrastigma hemsleyanum, suppresses lung adenocarcinoma via the calcium signaling pathway. Food Funct. 2021, 12, 8351–8365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Yao, G.L.; Zhai, J.M.; Hu, D.W.; Fan, Y.G. Kaempferol suppresses proliferation and induces apoptosis and DNA damage in human gallbladder cancer cells through the CDK4/CDK6/cyclin D1 pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, M.; Li, C.; Li, H.; Dai, Y.; Cui, K.; Li, Z. Kaempferol reverses aerobic glycolysis via mir-339-5p-mediated pkm alternative splicing in colon cancer cells. J. Agric. Food Chem. 2021, 69, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xing, J.; Aikemu, B.; Sun, J.; Zheng, M. Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncol. Rep. 2021, 45, 32. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Chen, M.; Chen, F.; Liu, B.; Shen, C. Kaempferol potentiates the sensitivity of pancreatic cancer cells to erlotinib via inhibition of the PI3K/AKT signaling pathway and epidermal growth factor receptor. Inflammopharmacology 2021, 29, 1587–1601. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, Y.; Yang, L.; Chen, Z.; Li, T.; Gu, M.; Li, C.; Liu, M.; Li, R. Kaempferol 3-O-gentiobioside, an ALK5 inhibitor, affects the proliferation, migration, and invasion of tumor cells via blockade of the TGF-β/ALK5/Smad signaling pathway. Phytother. Res. 2021, 35, 6310–6323. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic acid modified nanostructured lipid carrier for targeting delivery of kaempferol to NSCLC: Preparation, optimization, characterization, and performance evaluation in vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Fang, W.; Liang, K.; Li, X.; Zhang, F.; Pang, Y.; Fang, G.; Wang, X. Kaempferol suppresses androgen-dependent and androgen-independent prostate cancer by regulating Ki67 expression. Mol. Biol. Rep. 2022, 49, 4607–4617. [Google Scholar] [CrossRef]
- Dick, M.; Jamal, H.; Liu, Y.R.; Celli, J.P.; Lilge, L. On the need for standardized reporting of photophysical parameters of in vitro photodynamic therapy studies. Photodiagnosis Photodyn. Ther. 2023, 41, 103263. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid nanoparticles: A promising approach for cancer therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.W.; Wang, M.; Arroo, R.R.J.; Zou, L.; et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2773–2789. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Sacerdote, C.; Ricceri, F.; Weiderpass, E.; Roswall, N.; Buckland, G.; St-Jules, D.E.; Overvad, K.; Kyrø, C.; Fagherazzi, G.; et al. Flavonoid and lignan intake in relation to bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Cancer 2014, 111, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Dalla Pozza, E.; Scupoli, M.T.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Useros, J.; Li, W.; Cabeza-Morales, M.; Garcia-Foncillas, J. Oxidative stress: A new target for pancreatic cancer prognosis and treatment. J. Clin. Med. 2017, 6, 29. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Qiu, H.; Zhu, L.; Ren, Z.; Zhao, W.; Zhang, T.; Liu, L.; Liu, L. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food Funct. 2015, 6, 1518–1525. [Google Scholar] [CrossRef]
- Sun, Z.L.; Dong, J.L.; Wu, J. Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion. Biomed. Pharmacother. 2017, 85, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, P.N.; Chen, M.K.; Yang, W.E.; Tang, C.H.; Yang, S.F.; Hsieh, Y.S. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Jin, H.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biol. 2016, 37, 10247–10256. [Google Scholar] [CrossRef]
- Pearson, G.W. Control of invasion by epithelial-to-mesenchymal transition programs during metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef]
- Ayob, Z.; Mohd Bohari, S.P.; Abd Samad, A.; Jamil, S. Cytotoxic Activities against Breast Cancer Cells of Local Justicia gendarussa Crude Extracts. Evid. Based Complement. Altern. Med. 2014, 2014, 732980. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Romero, J.R.; Chattopadhyay, N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol. Cell. Endocrinol. 2008, 287, 57–64. [Google Scholar] [CrossRef]
- Bezdieniezhnykh, N.; Lykhova, A.; Semesiuk, N.; Okhrimenko, R.; Kudryavets, Y. Establishment and characterization of new breast and ovarian cancer cell lines as a model for studying cellular plasticity in vitro. Exp. Oncol. 2016, 38, 94–100. [Google Scholar] [CrossRef]
- Boadi, W.Y.; Amartey, P.K.; Lo, A. Effect of quercetin, genistein and kaempferol on glutathione and glutathione-redox cycle enzymes in 3T3-L1 preadipocytes. Drug Chem. Toxicol. 2016, 39, 239–247. [Google Scholar] [CrossRef]
- Boadi, W.Y.; Stevenson, C.; Johnson, D.; Mohamed, M.A. Flavonoids Reduce Lipid Peroxides and Increase Glutathione Levels in Pooled Human Liver Microsomes (HLMs). Adv. Biol. Chem. 2021, 11, 283–295. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Pop, L.; Raduly, L.; Barbat, A.; Moldovan, A.; et al. Inhibitory Effect of CAPE and Kaempferol in Colon Cancer Cell Lines-Possible Implications in New Therapeutic Strategies. Int. J. Mol. Sci 2019, 20, 1199. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.; Rodríguez, C.; Olmedo, I.; Carrasco-Rojas, J.; Rojas, D.; Molina-Berríos, A.; Díaz-Dosque, M.; Jara, J.A. Kaempferol Induces Cell Death and Sensitizes Human Head and Neck Squamous Cell Carcinoma Cell Lines to Cisplatin. Adv. Exp. Med. Biol. 2021, 1326, 95–109. [Google Scholar] [CrossRef]
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Dou, Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Huang, H.L.; HuangFu, W.C.; Yen, S.C.; Ngo, S.T.; Wu, Y.W.; Lin, T.E.; Sung, T.Y.; Lien, S.T.; Tseng, H.J.; et al. Biological Evaluation of Selected Flavonoids as Inhibitors of MNKs Targeting Acute Myeloid Leukemia. J. Nat. Prod. 2020, 83, 2967–2975. [Google Scholar] [CrossRef]
- Chien, H.W.; Wang, K.; Chang, Y.Y.; Hsieh, Y.H.; Yu, N.Y.; Yang, S.F.; Lin, H.W. Kaempferol suppresses cell migration through the activation of the ERK signaling pathways in ARPE-19 cells. Environ. Toxicol. 2019, 34, 312–318. [Google Scholar] [CrossRef]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell. Pathol. (Amst). 2019, 2019, 1907698. [Google Scholar] [CrossRef]
- de Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Dimas, K.; Demetzos, C.; Mitaku, S.; Marselos, M.; Tzavaras, T.; Kokkinopoulos, D. Cytotoxic activity of kaempferol glycosides against human leukaemic cell lines in vitro. Pharmacol. Res. 2000, 41, 85–88. [Google Scholar] [CrossRef]
- Halimah, E.; Diantini, A.; Destiani, D.P.; Pradipta, I.S.; Sastramihardja, H.S.; Lestari, K.; Subarnas, A.; Abdulah, R.; Koyama, H. Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. Biomed. Rep. 2015, 3, 115–117. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. RETRACTED: Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef]
- Huang, D.; Yang, S.; Dai, Z.; Li, W.; Wang, R. Cytotoxicity of Kaempferol-3-O-rhamnoside against nasopharyngeal cancer via inhibition of EGFR-TK. Int. J. Clin. Exp. Pathol. 2017, 10, 5462–5470. [Google Scholar]
- Jeong, J.C.; Kim, M.S.; Kim, T.H.; Kim, Y.K. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem. Res. 2009, 34, 991–1001. [Google Scholar] [CrossRef]
- Jin, Y.; Zhai, Z.; Jia, H.; Lai, J.; Si, X.; Wu, Z. Kaempferol attenuates diquat-induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food Funct. 2021, 12, 6889–6899. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.J.; Kim, B.G.; An, B.C.; Chong, Y.; Ahn, J.H. Engineering of flavonoid O-methyltransferase for a novel regioselectivity. Mol. Cells 2010, 30, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.H.; Sedighi, S.; Moradzadeh, M. A comparative study of anti-leukemic effects of kaempferol and epigallocatechin-3-gallate (EGCG) on human leukemia HL-60 cells. Avicenna J. Phytomed. 2021, 11, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Y.; Lee, E.R.; Kim, J.H.; Jung, J.W.; Lim, J.; Kim, S.K.; Cho, S.G.; Kim, K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol. 2009, 611, 17–21. [Google Scholar] [CrossRef]
- Kang, H.J.; Youn, Y.K.; Hong, M.K.; Kim, L.S. Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. J. Korean Med Sci. 2011, 26, 893–899. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Choi, S.U.; Ryu, S.Y. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch. Pharmacal Res. 2004, 27, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Suh, K.S.; Choi, M.C.; Chon, S.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, J.W.; Kim, Y.S. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytother. Res. 2010, 24, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kwon, M.; Choi, J.S.; Jeong, H.O.; Chung, H.Y.; Kim, H.R. Kaempferol Isolated from Nelumbo nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes. J. Med. Food 2015, 18, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. Chem. Biol. Interact. 2009, 177, 121–127. [Google Scholar] [CrossRef]
- Lin, F.; Luo, X.; Tsun, A.; Li, Z.; Li, D.; Li, B. Kaempferol enhances the suppressive function of Treg cells by inhibiting FOXP3 phosphorylation. Int. Immunopharmacol. 2015, 28, 859–865. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, L.; Qiu, Q.; Guo, S. Isolation and identification of antiproliferative compounds from the roots of Tetrastigma hemsleyanum against MDA-MB-435S cell lines. Pak. J. Pharm. Sci. 2016, 29, 1171–1175. [Google Scholar]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Ye, L.; Chen, X.; Jia, W.; Zhao, Y.; Samorodov, A.V.; Zhang, Y.; Hu, X.; Zhuang, F.; et al. Kaempferol attenuates streptozotocin-induced diabetic nephropathy by downregulating TRAF6 expression: The role of TRAF6 in diabetic nephropathy. J. Ethnopharmacol. 2021, 268, 113553. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, A.; Wang, X.; Sun, H. A kaempferol-3-O-β-d-glucoside, intervention effect of astragalin on estradiol metabolism. Steroids 2019, 149, 108413. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Roshangar, L. Kaempferol: A potential agent in the prevention of colorectal cancer. Physiol. Rep. 2022, 10, e15488. [Google Scholar] [CrossRef]
- Park, J.S.; Rho, H.S.; Kim, D.H.; Chang, I.S. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J. Agric. Food Chem. 2006, 54, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, B.S.; Kondath, S.; Anantanarayanan, R.; Rajaram, R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem. 2015, 50, 1966–1976. [Google Scholar] [CrossRef]
- Rahul, F.N.; Jyoti, S.; Siddique, Y.H. Effect of kaempferol on the transgenic Drosophila model of Parkinson’s disease. Sci. Rep. 2020, 10, 13793. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Schaffner, W.; Hertel, C. Phytoestrogen kaempferol (3,4’,5,7-tetrahydroxyflavone) protects PC12 and T47D cells from beta-amyloid-induced toxicity. J. Neurosci. Res. 1999, 57, 399–404. [Google Scholar] [CrossRef]
- Saraei, R.; Rahman, H.S.; Soleimani, M.; Asghari-Jafarabadi, M.; Naimi, A.; Hassanzadeh, A.; Solali, S. Kaempferol sensitizes tumor necrosis factor-related apoptosis-inducing ligand-resistance chronic myelogenous leukemia cells to apoptosis. Mol. Biol. Rep. 2022, 49, 19–29. [Google Scholar] [CrossRef]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef]
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomed. Pharmacother. 2019, 109, 1610–1619. [Google Scholar] [CrossRef]
- Stapel, J.; Oppermann, C.; Richter, D.U.; Ruth, W.; Briese, V. Polyphenol compounds with anti-carcinogenic qualities: Effects of quercetin (flavonol), chrysin (flavon), kaempferol (flavanol), naringenin (flavanon) and hesperidin (flavanoid) on in vitro breast cancer. J. Med. Plants Res. 2013, 7, 2187–2196. [Google Scholar] [CrossRef]
- Swanson, H.I.; Choi, E.Y.; Helton, W.B.; Gairola, C.G.; Valentino, J. Impact of apigenin and kaempferol on human head and neck squamous cell carcinoma. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 214–220. [Google Scholar] [CrossRef]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef]
- Tu, L.Y.; Pi, J.; Jin, H.; Cai, J.Y.; Deng, S.P. Synthesis, characterization and anticancer activity of kaempferol-zinc(II) complex. Bioorg. Med. Chem. Lett. 2016, 26, 2730–2734. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef]
- Wang, T.; Liu, B.; Zhang, C.; Fang, T.; Ding, Y.; Song, G.; Zhang, S.; Shi, L.; Deng, X.; Wang, J. Kaempferol-Driven Inhibition of Listeriolysin O Pore Formation and Inflammation Suppresses Listeria monocytogenes Infection. Microbiol. Spectr. 2022, 10, e0181022. [Google Scholar] [CrossRef]
- Wu, W.; Yang, B.; Qiao, Y.; Zhou, Q.; He, H.; He, M. Kaempferol protects mitochondria and alleviates damages against endotheliotoxicity induced by doxorubicin. Biomed. Pharmacother. 2020, 126, 110040. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Q.; Deng, X.; Shi, K.; Zhang, W.; Jiang, Y.; Ma, X.; Zeng, J.; Wang, X. Old wine in new bottles: Kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacol. Res. 2022, 175, 106005. [Google Scholar] [CrossRef]
- Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215–21226. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Yang, M.; Zhang, W.; Li, M.; Xu, Y.; Li, J.; Kang, J.; Zhang, J.; Guo, S. Exploration in the Mechanism of Kaempferol for the Treatment of Gastric Cancer Based on Network Pharmacology. Biomed. Res. Int. 2020, 2020, 5891016. [Google Scholar] [CrossRef] [PubMed]
- Yusof, H.M.; Sarah, N.M.L.; Lam, T.W.; Kassim, M.N.I. Hypolipidemic effects of quercetin and kaempferol in human hepatocellular carcinoma (HepG2) cells. Int. Food Res. J. 2017, 25, 241–245. [Google Scholar]
- Zeng, Z.C.; Jiang, J.; Wang, X.J.; Wei, K.N.; Liang, H.S.; Zeng, L.X.; Xu, Y.; Xie, S.J.; Meng, Z.; Yang, X.J.; et al. Kaempferol ameliorates in-vitro and in-vivo postovulatory oocyte ageing in mice. Reprod. Biomed. Online 2022, 45, 1065–1083. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C. Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. Braz. J. Med Biol. Res. 2019, 52, e7843. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, Y.; Liu, Y.; Ma, L.; Meng, L. Molecular Mechanism Investigation on Monomer Kaempferol of the Traditional Medicine Dingqing Tablet in Promoting Apoptosis of Acute Myeloid Leukemia HL-60 Cells. Evid. Based Complement. Altern. Med. 2022, 2022, 8383315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).