Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies
Abstract
Simple Summary
Abstract
1. Molecular Mechanisms Driving Castration-Resistant Prostate Cancer Cell
2. Global Statistics on the Incidence and Mortality of Prostate Cancer
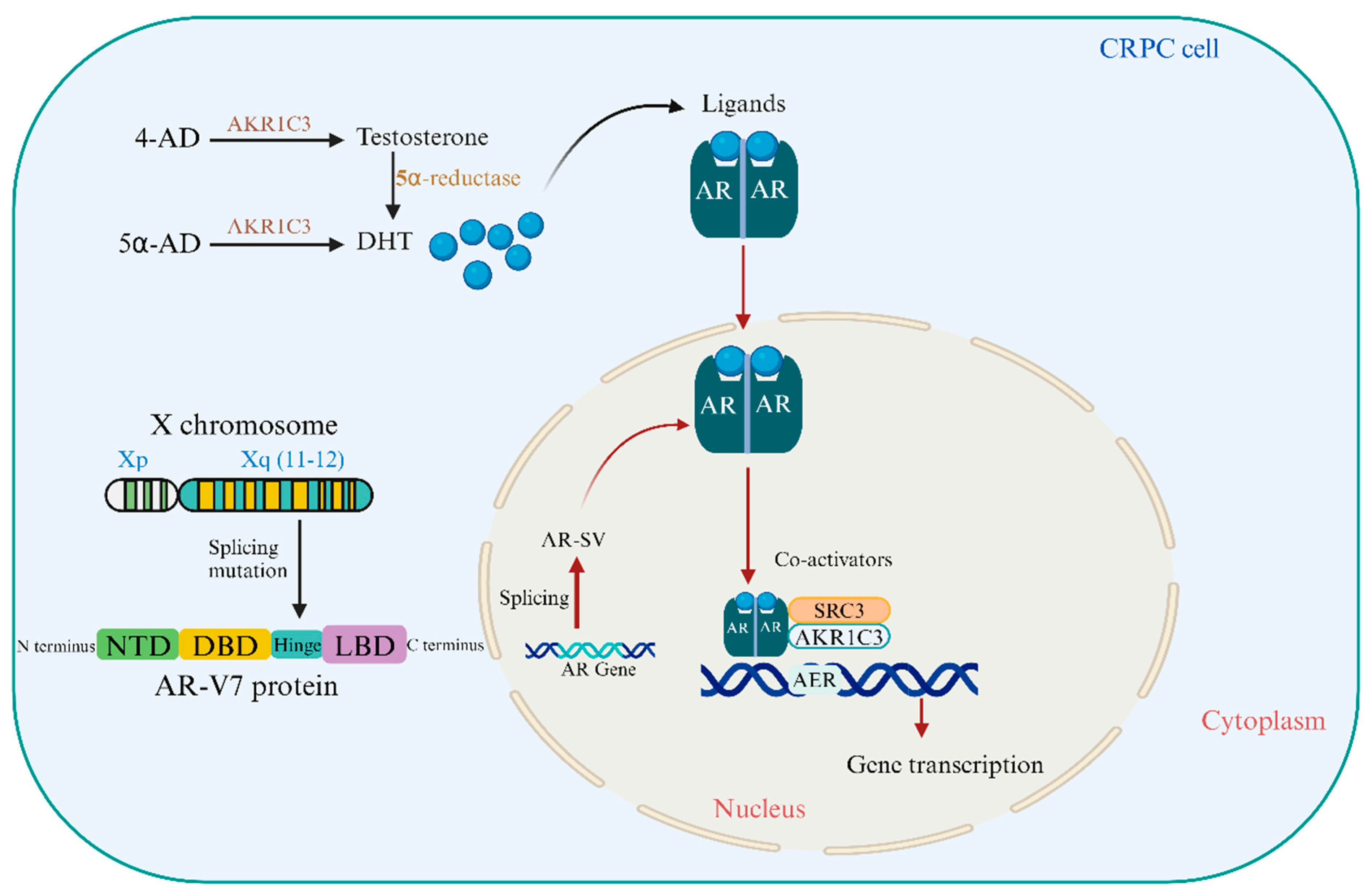
3. The Role of Androgen Receptor Variants and Coactivators in Prostate Cancer Progression
3.1. The Crucial Strategies That Can Target AR-V7 for the Treatment of CRPC
- AR-V7 Resistance: Two antisense oligonucleotides (AONs) were created to target AR pre-mRNA splicing enhancers, restoring sensitivity to androgen deprivation and inducing apoptosis in CRPC cell lines [31]. AKR1C3 and AR-V7 interact in prostate rebiopsy tissues, preventing degradation and repressing B4GALT1, a prostate cancer tumor suppressor gene essential for CRPC cell proliferation post-androgen deprivation [32].
- Targeting AR-V7 Translation/Transcription: AR-FL binds to ARBS2, an AR intron 2 enhancer, to inhibit chromatin. These negative autoregulatory mechanisms of ADT enhance AR-FL and AR-V mRNA simultaneously. The AR-FL protein boosts AR-FL and AR-V transcripts after ADT [33]. A tiny molecule SC912 interacts with full-length AR and AR-V7 via its N-terminal domain. Pan-AR targeting uses AR-NTD amino acids 507–531. SC912 prevented AR-V7 nuclear localization, DNA binding, and transcription. SC912 stopped AR-V7-positive CRPC cell growth, cell-cycle arrest, and death. SC912 inhibited AR signaling and CRPC xenograft development in AR-V7-expressing cells. These findings suggested SC912 could treat CRPC [34].
- Combination Therapies: New targeted, immunological, AR-targeting, and chemotherapy. Metastatic Castration-Resistant Prostate Cancer Treatment) and AFFINITY (Cabazitaxel/Prednisone Alone or With Custirsen for 2nd-line Prostate Cancer Chemotherapy). Reactivating AR activity is another docetaxel resistance mechanism. A gene mutation that makes AR translocation independent of microtubule control can produce docetaxel resistance. Drug interactions with cytotoxic treatment can cause fatal neutropenic enterocolitis [35].
- AR-V7-Targeted Immunotherapy: AR-V7-Targeted Immunotherapy (MVI-118) is a DNA vaccine for metastatic prostate cancer, promoting a CD8+T cell-mediated immune response against AR-overexpressing cancer cells. Genomic profiling can detect genetic changes and molecular subtypes, potentially predicting treatment response. Next-generation sequencing technologies can also reveal immune response landscapes and immunotherapy-predictive biomarkers in tumor microenvironments [5,36].
- Epigenetic Modulators: Emerging evidence links non-coding RNAs to CRPC development and treatment resistance, while AR-epigenetic pathways are specifically engaged in PC carcinogenesis [7]. Histone regulators including LSD1 and EZH2 can have a significant impact on AR expression and help CRPC re-programme AR activation. The expression of EZH2 and BRD4, which stimulates regulators and engages with acetylated histones to coactivate AR. Epigenetic alterations help PCa cells respond to AR signaling suppression. Recent research conducted that EZH2 can make CRPC tumors resistant to DNA-damaging therapies
- Proteolysis-Targeting Chimeras (PROTACs): In recent years, targeting AR protein for breakdown by AR-targeted proteolysis targeting chimeras (PROTACs) or small-molecule degraders has become an interesting and potentially useful way to treat metastatic CRPC (mCRPC) [37]. The way AR expression inhibitor functions are different from how PROTAC targets AR protein. AR expression inhibitor uses antisense oligonucleotides to target AR mRNA by binding the complementary region of AR mRNA, which significantly lowers mRNA production [38].
3.2. Certain Processes Supporting CRPC Evolution Similar to AR-V7
3.3. Clinical Intervention Linked to AR-V7 in the Development of CRPC
3.3.1. AR Neutral CRPC
3.3.2. Neuroendocrine Prostate Cancer (NEPC)
3.3.3. AR Regulation in AR-Independent CRPCs
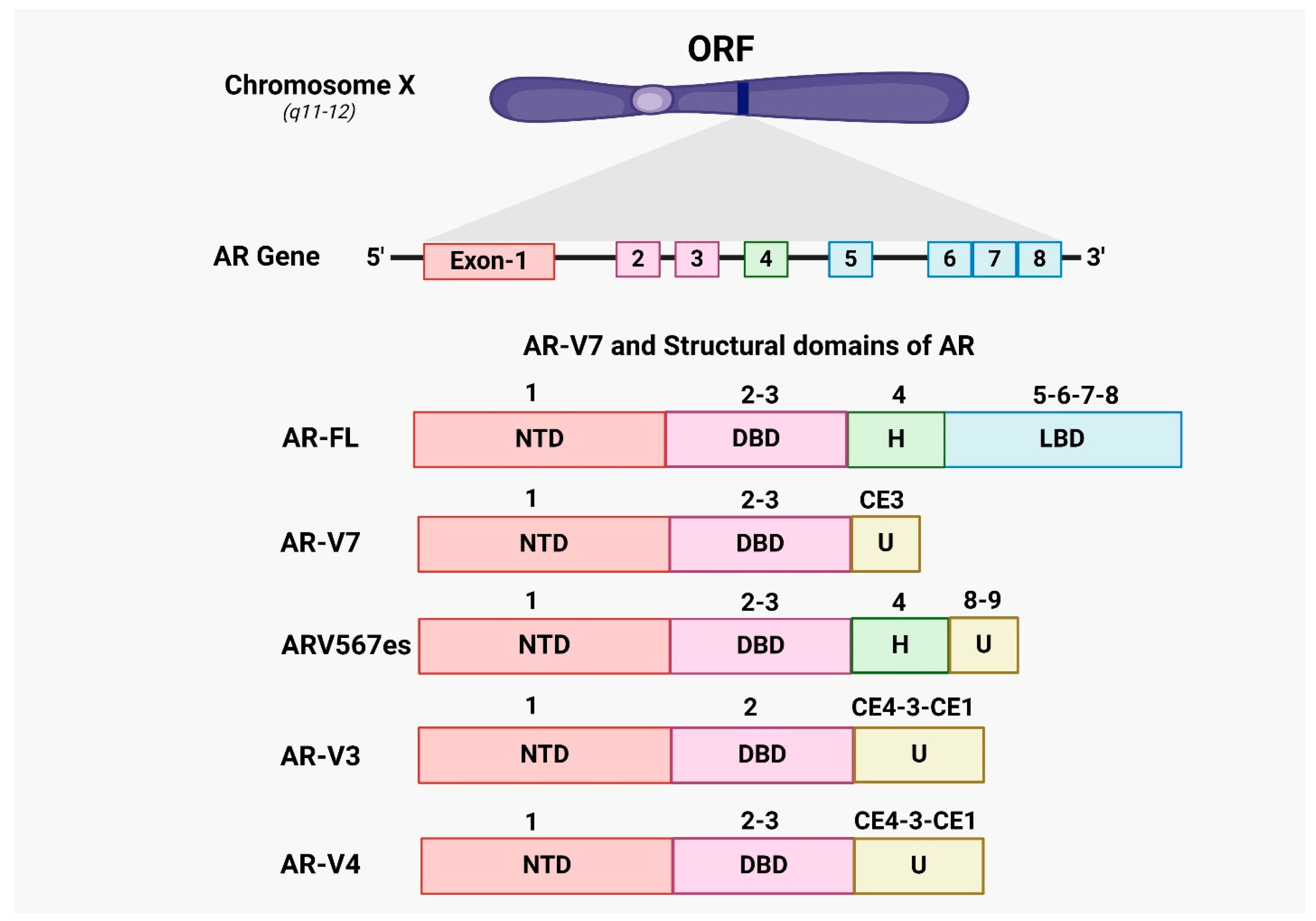
4. Androgen Receptor Splice Variants AR-V7 in CRPC Prostate Cancer
5. Adaptive Pathways and Treatment Options in CRPC Prostate Cancer
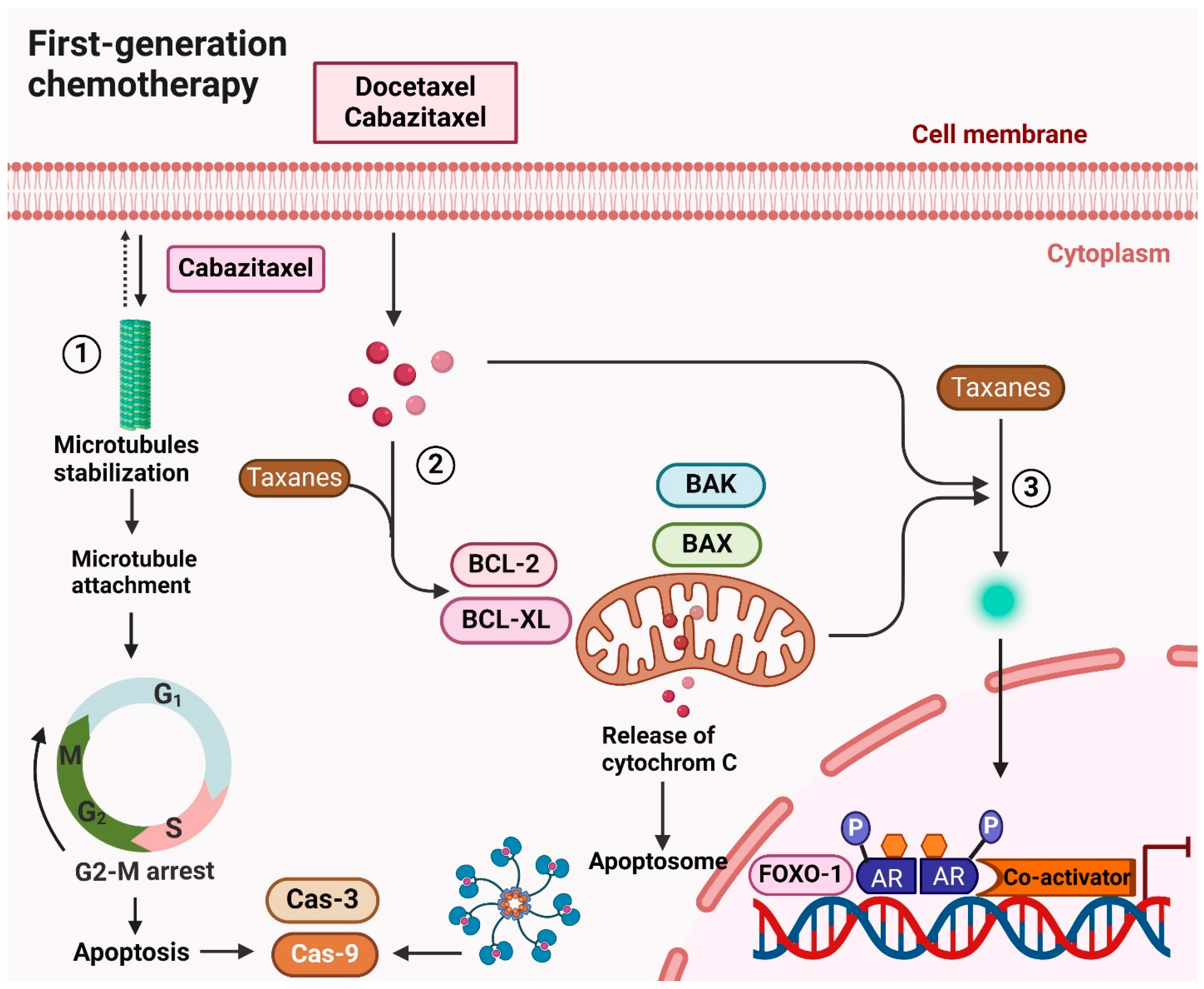
5.1. First-Generation Chemotherapy for Prostate Cancer
5.1.1. Docetaxel
5.1.2. Cabazitaxel
5.1.3. Mitoxantrone
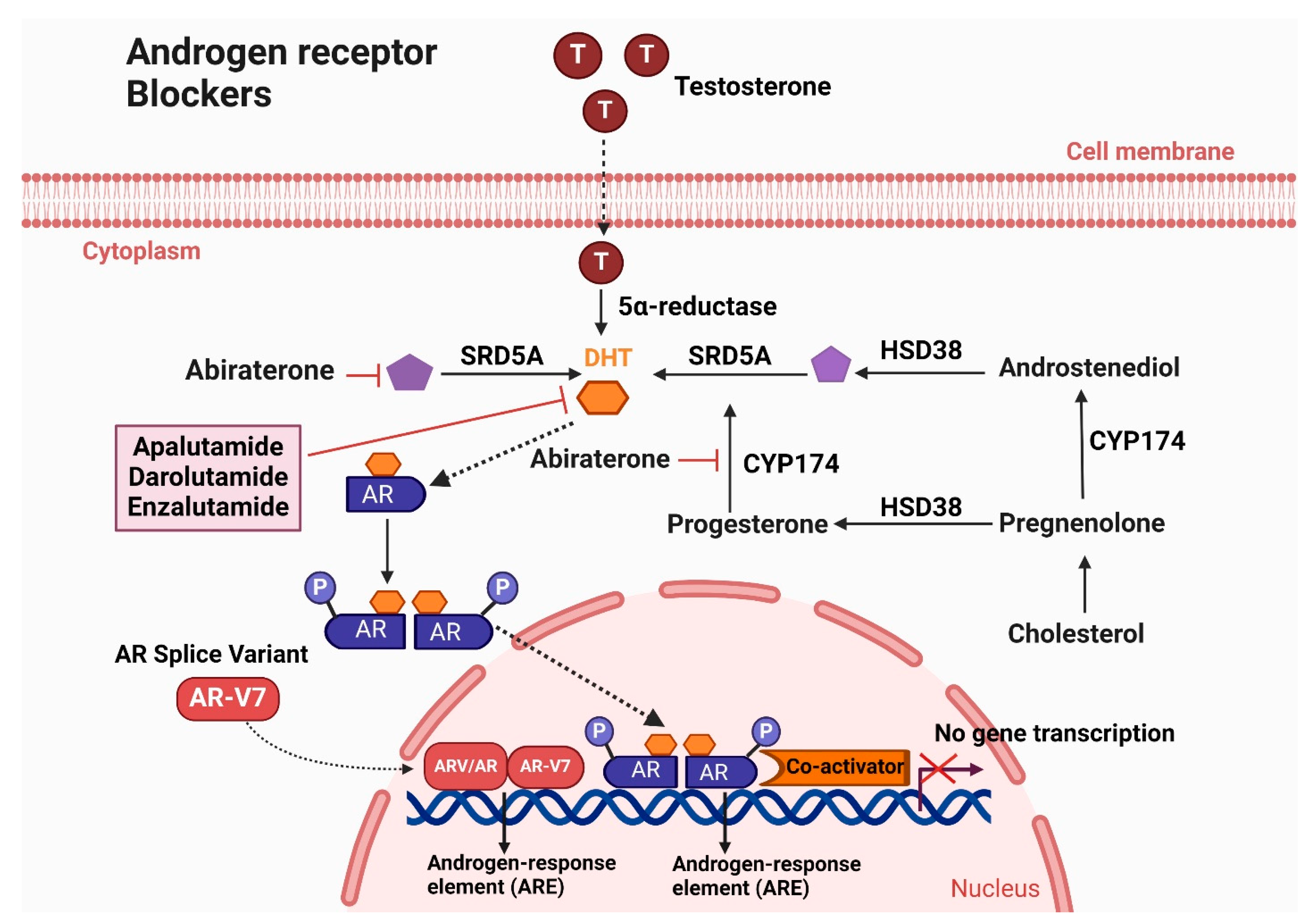
5.2. Androgen Receptor Blockers
5.2.1. Enzalutamide
5.2.2. Apalutamide
5.2.3. Darolutamide
5.3. Androgen Biosynthesis Inhibitors
Abiraterone Acetate
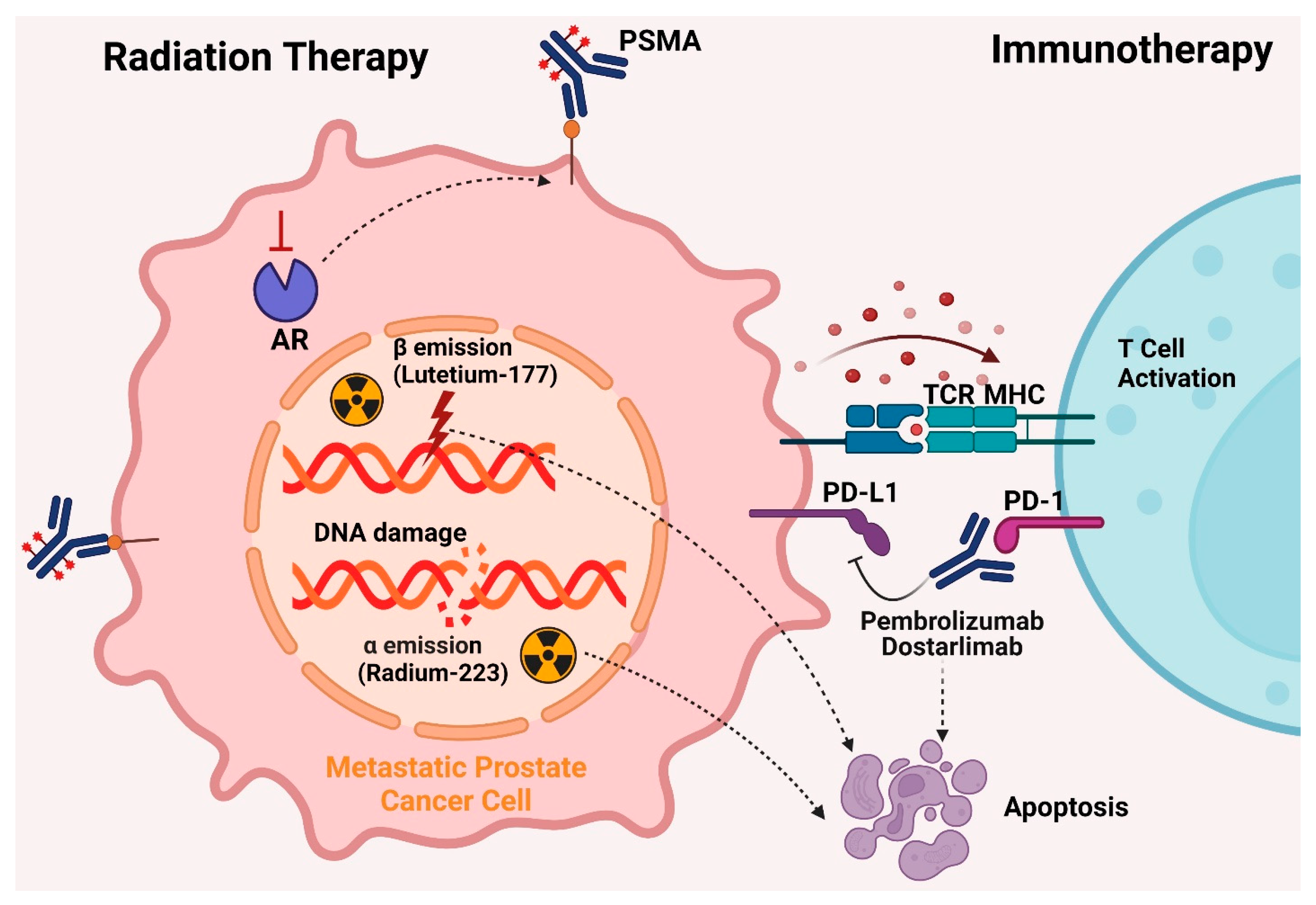
5.4. Radiation Therapy
5.4.1. Lutetium-177
5.4.2. Radium-223 Dichloride
5.5. Immunotherapy
5.5.1. Sipuleucel-T
5.5.2. Dostarlimab
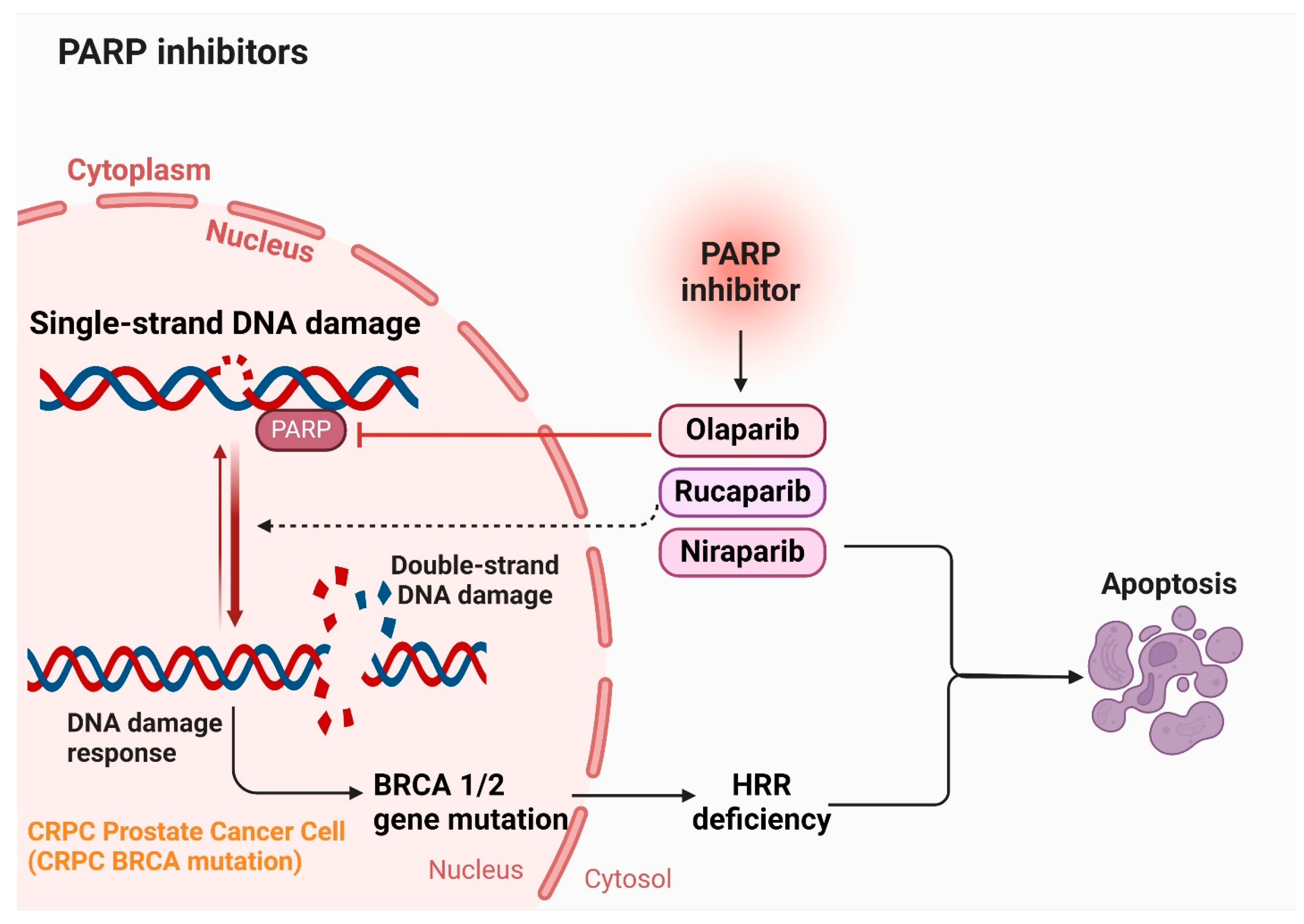
5.6. PARP Inhibitors
6. Role of Natural Compounds in Regulating AR-V7 in Prostate Cancer
7. The Effectiveness and Mechanism of Action of the Natural Compounds in Castration-Resistant Prostate Cancer
7.1. Berberine in Cancer Therapy
7.2. Curcumin in Cancer Therapy
7.3. Cryptotanshinone (CTS)
7.4. Epigallocatechin-3-Gallate (EGCG)
7.5. Fisetin
7.6. Genistein
7.7. Garcinia Mangostana/α-Mangosteen
7.8. Ginsenosides
7.9. Honokiol
7.10. Luteolin
7.11. Quercetin
7.12. Resveratrol
7.13. Silibinin
7.14. Sulforaphane
7.15. Triptolide
8. Pro-Oxidative Activity of Natural Compound in Cancer Treatment
8.1. Cytotoxic Effects of Plant Polyphenol Compounds in Cancer Treatment
8.2. The Limitations and Challenges of Using Natural Compounds in Treating CRPC
8.3. Limitation of Castration-Resistant Prostate Cancer Treatment
9. Recommendations, Challenges, Future Perspectives, and Concluding Remarks
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-resistant prostate cancer: From uncovered resistance mechanisms to current treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Tang, P.; Tang, T.; Wang, Y.; Peng, S.; Wang, S.; Lan, W.; Wang, L.; Zhang, Y. Genetic profiling of hormone-sensitive and castration-resistant prostate cancers and identification of genetic mutations prone to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 180–187. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Munoz, E.; Ashong, D. Insight into Recent Advances in Degrading Androgen Receptor for Castration-Resistant Prostate Cancer. Cancers 2024, 16, 663. [Google Scholar] [CrossRef]
- Karaca, M. Regulation of Androgen Receptor Function by Tyrosine Phosphorylation. 2010. Available online: https://cdr.lib.unc.edu/concern/dissertations/dr26xz50m (accessed on 18 March 2013).
- Daniels, V.A.; Luo, J.; Paller, C.J.; Kanayama, M. Therapeutic Approaches to Targeting Androgen Receptor Splice Variants. Cells 2024, 13, 104. [Google Scholar] [CrossRef]
- Obinata, D.; Takayama, K.; Inoue, S.; Takahashi, S. Exploring androgen receptor signaling pathway in prostate cancer: A path to new discoveries. Int. J. Urol. 2024, 31, 590–597. [Google Scholar] [CrossRef]
- Saker, Z.; Rizk, M.; Nabha, S. Androgen receptor-dependent mechanisms mediating therapy resistance in prostate cancer. In Therapy Resistance in Prostate Cancer; Elsevier: Amsterdam, The Netherlands, 2024; pp. 57–84. [Google Scholar]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L. 2022 update on prostate cancer epidemiology and risk factors—A systematic review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Yarnell, E.; Abascal, K. Holistic approaches to prostate cancer. Altern. Complement. Ther. 2008, 14, 164–180. [Google Scholar] [CrossRef]
- Barsouk, A.; Padala, S.A.; Vakiti, A.; Mohammed, A.; Saginala, K.; Thandra, K.C.; Rawla, P.; Barsouk, A. Epidemiology, staging and management of prostate cancer. Med. Sci. 2020, 8, 28. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Nyame, Y.A.; Cooperberg, M.R.; Cumberbatch, M.G.; Eggener, S.E.; Etzioni, R.; Gomez, S.L.; Haiman, C.; Huang, F.; Lee, C.T.; Litwin, M.S. Deconstructing, addressing, and eliminating racial and ethnic inequities in prostate cancer care. Eur. Urol. 2022, 82, 341–351. [Google Scholar] [CrossRef]
- Arenas-Gallo, C.; Owiredu, J.; Weinstein, I.; Lewicki, P.; Basourakos, S.P.; Vince, R., Jr.; Al Hussein Al Awamlh, B.; Schumacher, F.R.; Spratt, D.E.; Barbieri, C.E. Race and prostate cancer: Genomic landscape. Nat. Rev. Urol. 2022, 19, 547–561. [Google Scholar] [CrossRef]
- Shah, N.; Ioffe, V. Re: Trends in Prostate Cancer Incidence Rates and Prevalence of Prostate Specific Antigen Screening by Socioeconomic Status and Regions in the United States, 2004 to 2013: KA Houston, J. King, J. Li and A. Jemal: J Urol 2018; 199: 676–682. J. Urol. 2018, 200, 202. [Google Scholar]
- Di Pietro, G.; Chornokur, G.; Kumar, N.B.; Davis, C.; Park, J.Y. Racial differences in the diagnosis and treatment of prostate cancer. Int. Neurourol. J. 2016, 20, S112. [Google Scholar] [CrossRef]
- Conti, D.V.; Darst, B.F.; Moss, L.C.; Saunders, E.J.; Sheng, X.; Chou, A.; Schumacher, F.R.; Olama, A.A.A.; Benlloch, S.; Dadaev, T. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 2021, 53, 65–75. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Basourakos, S.P.; Lewicki, P.J.; Vince, R.; Spratt, D.E.; Barbieri, C.E.; Shoag, J.E. Race and genetic alterations in prostate cancer. JCO Precis. Oncol. 2021, 5, 1650–1653. [Google Scholar] [CrossRef]
- Patki, S.; Aquilina, J.; Thorne, R.; Aristidou, I.; Rodrigues, F.B.; Warren, H.; Bex, A.; Kasivisvanathan, V.; Moore, C.; Gurusamy, K. A systematic review of patient race, ethnicity, socioeconomic status, and educational attainment in prostate cancer treatment randomised trials—Is the evidence base applicable to the general patient population? Eur. Urol. Open Sci. 2023, 54, 56–64. [Google Scholar] [CrossRef]
- Ahaghotu, C.; Tyler, R.; Sartor, O. African American participation in oncology clinical trials—Focus on prostate cancer: Implications, barriers, and potential solutions. Clin. Genitourin. Cancer 2016, 14, 105–116. [Google Scholar] [CrossRef]
- Halabi, S.; Small, E.J.; Vogelzang, N.J.; Barrier, R.C., Jr.; George, S.L.; Gilligan, T.D. Impact of race on survival in men with metastatic hormone-refractory prostate cancer. Urology 2004, 64, 212–217. [Google Scholar] [CrossRef]
- Puente Vazquez, J.; Grande Pulido, E.; Anton Aparicio, L. Cytokine and endocrine signaling in prostate cancer. Med. Oncol. 2012, 29, 1956–1963. [Google Scholar] [CrossRef]
- Grant, C.M.; Kyprianou, N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl. Androl. Urol. 2013, 2, 202. [Google Scholar] [PubMed]
- Bellone, M.; Caputo, S. Crosstalk between prostate cancer stem cells and immune cells: Implications for tumor progression and resistance to immunotherapy. In Cancer Stem Cell Resistance to Targeted Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 173–221. [Google Scholar]
- Chen, Y.; Lan, T. Molecular origin, expression regulation, and biological function of androgen receptor splicing variant 7 in prostate cancer. Urol. Int. 2021, 105, 337–353. [Google Scholar] [CrossRef]
- Paschalis, A.; Sharp, A.; Welti, J.C.; Neeb, A.; Raj, G.V.; Luo, J.; Plymate, S.R.; de Bono, J.S. Alternative splicing in prostate cancer. Nat. Rev. Clin. Oncol. 2018, 15, 663–675. [Google Scholar] [CrossRef]
- Rawat, C.; Heemers, H.V. Alternative splicing in prostate cancer progression and therapeutic resistance. Oncogene 2024, 43, 1655–1668. [Google Scholar] [CrossRef]
- Haile, S.; Sadar, M.D. Androgen receptor and its splice variants in prostate cancer. Cell. Mol. Life Sci. 2011, 68, 3971–3981. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365. [Google Scholar]
- Zheng, Z.; Li, J.; Liu, Y.; Shi, Z.; Xuan, Z.; Yang, K.; Xu, C.; Bai, Y.; Fu, M.; Xiao, Q. The crucial role of AR-V7 in enzalutamide-resistance of castration-resistant prostate cancer. Cancers 2022, 14, 4877. [Google Scholar] [CrossRef]
- Luna Velez, M.V.; Paulino da Silva Filho, O.; Verhaegh, G.W.; van Hooij, O.; El Boujnouni, N.; Brock, R.; Schalken, J.A. Delivery of antisense oligonucleotides for splice-correction of androgen receptor pre-mRNA in castration-resistant prostate cancer models using cell-penetrating peptides. Prostate 2022, 82, 657–665. [Google Scholar] [CrossRef]
- Wang, B.; Wu, S.; Fang, Y.; Sun, G.; He, D.; Hsieh, J.T.; Wang, X.; Zeng, H.; Wu, K. The AKR1C3/AR-V7 complex maintains CRPC tumour growth by repressing B4GALT1 expression. J. Cell. Mol. Med. 2020, 24, 12032–12043. [Google Scholar] [CrossRef]
- Ma, T.; Bai, S.; Qi, Y.; Zhan, Y.; Ungerleider, N.; Zhang, D.Y.; Neklesa, T.; Corey, E.; Dehm, S.M.; Zhang, K. Increased transcription and high translation efficiency lead to accumulation of androgen receptor splice variant after androgen deprivation therapy. Cancer Lett. 2021, 504, 37–48. [Google Scholar] [CrossRef]
- Yi, Q.; Han, X.; Yu, H.G.; Chen, H.-Y.; Qiu, D.; Su, J.; Lin, R.; Batist, G.; Wu, J.H. SC912 inhibits AR-V7 activity in castration-resistant prostate cancer by targeting the androgen receptor N-terminal domain. Oncogene 2024, 43, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Li, S.; Liu, Q.; Wu, F. The emerging role of cross-resistance between taxanes and AR-targeting therapy in metastatic prostate cancer. J. Clin. Urol. 2024, 17, 182–189. [Google Scholar] [CrossRef]
- Cheng, B.; Huang, H. Expanding horizons in overcoming therapeutic resistance in castration-resistant prostate cancer: Targeting the androgen receptor-regulated tumor immune microenvironment. Cancer Biol. Med. 2023, 20, 568. [Google Scholar] [CrossRef] [PubMed]
- Labaf, M.; Li, M.; Ting, L.; Karno, B.; Zhang, S.; Gao, S.; Patalano, S.; Macoska, J.A.; Zarringhalam, K.; Han, D. Increased AR expression in castration-resistant prostate cancer rapidly induces AR signaling reprogramming with the collaboration of EZH2. Front. Oncol. 2022, 12, 1021845. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Ha, S.; Zhu, J.; Tao, W.; Fu, Z.; Wei, H.; Hou, Q.; Luo, G.; Xiang, H. Structure–Activity Relationship (SAR) Studies of Novel Monovalent AR/AR-V7 Dual Degraders with Potent Efficacy against Advanced Prostate Cancer. J. Med. Chem. 2024, 67, 5567–5590. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, H.; Kanesaka, M.; Imamura, Y.; Sakamoto, S.; Ichikawa, T.; Kaneda, A. Epigenetic modifications in prostate cancer. Int. J. Urol. 2021, 28, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Shen, X.; Wang, Z.; Chen, Y.; Weng, Y.; Yu, F.; Tang, Y.; Lu, P.; Liu, M.; Wang, L. Tumor cell-intrinsic epigenetic dysregulation shapes cancer-associated fibroblasts heterogeneity to metabolically support pancreatic cancer. Cancer Cell 2024, 42, 869–884.e9. [Google Scholar] [CrossRef]
- Enikeeva, K.; Rafikova, G.; Sharifyanova, Y.; Mulyukova, D.; Vanzin, A.; Pavlov, V. Epigenetics as a Key Factor in Prostate Cancer. Adv. Biol. 2024, 8, 2300520. [Google Scholar] [CrossRef]
- Doumat, G.; Abou Chawareb, E.; Sebai, T.N.; Hout, M.; Merhe, A.; Omarzai, Y. Epigenetic changes driving therapy resistance in prostate cancer. In Therapy Resistance in Prostate Cancer; Elsevier: Amsterdam, The Netherlands, 2024; pp. 85–106. [Google Scholar]
- Tao, L.; Zhou, Y.; Luo, Y.; Qiu, J.; Xiao, Y.; Zou, J.; Zhang, Y.; Liu, X.; Yang, X.; Gou, K. Epigenetic regulation in cancer therapy: From mechanisms to clinical advances. MedComm–Oncology 2024, 3, e59. [Google Scholar] [CrossRef]
- Uo, T.; Plymate, S.R.; Sprenger, C.C. The potential of AR-V7 as a therapeutic target. Expert Opin. Ther. Targets 2018, 22, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Niu, Y.; Lee, S.O.; Chang, C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat. Rev. 2014, 40, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Vellky, J.E.; Ricke, W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Luo, J. Decoding the androgen receptor splice variants. Transl. Androl. Urol. 2013, 2, 178. [Google Scholar] [PubMed]
- Ho, Y.; Dehm, S.M. Androgen receptor rearrangement and splicing variants in resistance to endocrine therapies in prostate cancer. Endocrinology 2017, 158, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Cato, L.; de Tribolet-Hardy, J.; Lee, I.; Rottenberg, J.T.; Coleman, I.; Melchers, D.; Houtman, R.; Xiao, T.; Li, W.; Uo, T. ARv7 represses tumor-suppressor genes in castration-resistant prostate cancer. Cancer Cell 2019, 35, 401–413.e406. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2019, 129, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sprenger, C.C.; Vessella, R.L.; Haugk, K.; Soriano, K.; Mostaghel, E.A.; Page, S.T.; Coleman, I.M.; Nguyen, H.M.; Sun, H. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010, 120, 2715–2730. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion–resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Agarwal, N.; Di Lorenzo, G.; Sonpavde, G.; Bellmunt, J. New agents for prostate cancer. Ann. Oncol. 2014, 25, 1700–1709. [Google Scholar] [CrossRef]
- Frieling, J.S.; Basanta, D.; Lynch, C.C. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control 2015, 22, 109–120. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Bruin, M.A.; Mohmaed Ali, M.I.; van Nuland, M.; Jacobs, B.A.; Lucas, L.; Dezentje, V.O.; de Feijter, J.M.; Rosing, H.; Bergman, A.M.; Beijnen, J.H. Cortisol as Biomarker for CYP17-Inhibition is Associated with Therapy Outcome of Abiraterone Acetate. Pharm. Res. 2023, 40, 3001–3010. [Google Scholar] [CrossRef]
- Hoy, S.M. Abiraterone acetate: A review of its use in patients with metastatic castration-resistant prostate cancer. Drugs 2013, 73, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.-D.; Zhang, S.-H.; Zeng, N.; Lu, Y.-C.; Qin, B.-L.; Wang, S.-G. Novel androgen receptor inhibitors for metastatic hormone-sensitive prostate cancer: Current application and future perspectives. Biomed. Pharmacother. 2023, 168, 115806. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Armstrong, A.J.; Sartor, O.; de Bono, J.; Kaplan, E.; Lin, C.-Y.; Solomon, N.C.; Small, E.J. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J. Clin. Oncol. 2013, 31, 3944. [Google Scholar] [CrossRef]
- Shao, L.; Kahraman, N.; Yan, G.; Wang, J.; Ozpolat, B.; Ittmann, M. Targeting the TMPRSS2/ERG fusion mRNA using liposomal nanovectors enhances docetaxel treatment in prostate cancer. Prostate 2020, 80, 65–73. [Google Scholar] [CrossRef]
- Lallous, N.; Snow, O.; Sanchez, C.; Parra Nuñez, A.K.; Sun, B.; Hussain, A.; Lee, J.; Morin, H.; Leblanc, E.; Gleave, M.E. Evaluation of darolutamide (Odm201) efficiency on androgen receptor mutants reported to date in prostate cancer patients. Cancers 2021, 13, 2939. [Google Scholar] [CrossRef] [PubMed]
- Lyou, Y.; Dorff, T.B. Hormonal manipulation in androgen signaling: A narrative review on using novel androgen therapy agents to optimize clinical outcomes and minimize side effects for prostate cancer patients. Transl. Androl. Urol. 2021, 10, 3199. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A. FDA approval summary: Lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2023, 29, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Ravi, P.; Sonpavde, G.; Jacene, H. Lutetium Lu 177 vipivotide tetraxetan for metastatic castration-resistant prostate cancer. Expert Rev. Anticancer Ther. 2022, 22, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Scher, H.I.; Sandhu, S.; Efstathiou, E.; Lara, P.N.; Evan, Y.Y.; George, D.J.; Chi, K.N.; Saad, F.; Ståhl, O. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 362–373. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, A.L.; Tangutoori, S.; Baldwin, P.; Qiao, J.; Gharagouzloo, C.; Seitzer, N.; Clohessy, J.G.; Makrigiorgos, G.M.; Cormack, R.; Pandolfi, P.P. Nanoformulation of olaparib amplifies PARP inhibition and sensitizes PTEN/TP53-deficient prostate cancer to radiation. Mol. Cancer Ther. 2017, 16, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S. Overview of checkpoint inhibitors mechanism of action: Role of immune-related adverse events and their treatment on progression of underlying cancer. Front. Med. 2022, 9, 875974. [Google Scholar] [CrossRef] [PubMed]
- Rehman, L.u.; Nisar, M.H.; Fatima, W.; Sarfraz, A.; Azeem, N.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Immunotherapy for prostate cancer: A current systematic review and patient centric perspectives. J. Clin. Med. 2023, 12, 1446. [Google Scholar] [CrossRef]
- Sindhu, K.K.; Nehlsen, A.D.; Stock, R.G. Radium-223 for metastatic castrate-resistant prostate cancer. Pract. Radiat. Oncol. 2022, 12, 312–316. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U. Rucaparib or physician’s choice in metastatic prostate cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Subudhi, S.K.; Pieczonka, C.M.; Karsh, L.I.; Quinn, D.I.; Hafron, J.M.; Wilfehrt, H.M.; Harmon, M.; Sheikh, N.A.; Shore, N.D. Combination treatment with sipuleucel-t and abiraterone acetate or enzalutamide for metastatic castration-resistant prostate cancer: STAMP and STRIDE Trials. Clin. Cancer Res. 2023, 29, 2426–2434. [Google Scholar] [CrossRef]
- de Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1250–1264. [Google Scholar] [CrossRef]
- Vardanyan, R.; Hruby, V. Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Dhillon, S. Decitabine/cedazuridine: First approval. Drugs 2020, 80, 1373–1378. [Google Scholar] [CrossRef]
- Baldwin, E.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Varnai, R.; Koskinen, L.M.; Mäntylä, L.E.; Szabo, I.; FitzGerald, L.M.; Sipeky, C. Pharmacogenomic biomarkers in docetaxel treatment of prostate cancer: From discovery to implementation. Genes 2019, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Capasso, A. Vinorelbine in cancer therapy. Curr. Drug Targets 2012, 13, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.M.; Frey, F.J. Clinical pharmacokinetics of prednisone and prednisolone. Clin. Pharmacokinet. 1990, 19, 126–146. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; D’Alessio, A.; Maiello, M.R.; Gallo, M.; Chicchinelli, N.; Pergameno, M.; Piccirilli, M.S.; Normanno, N. Evaluation of the pharmacokinetics of ixabepilone for the treatment of breast cancer. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1177–1185. [Google Scholar] [CrossRef]
- Azarenko, O.; Smiyun, G.; Mah, J.; Wilson, L.; Jordan, M.A. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol. Cancer Ther. 2014, 13, 2092–2103. [Google Scholar] [CrossRef]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Tannock, I.F. Chemotherapy-based treatment for castration-resistant prostate cancer. J. Clin. Oncol. 2011, 29, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Rouyer, M.; Oudard, S.; Joly, F.; Fizazi, K.; Tubach, F.; Jove, J.; Lacueille, C.; Lamarque, S.; Guiard, E.; Balestra, A. Overall and progression-free survival with cabazitaxel in metastatic castration-resistant prostate cancer in routine clinical practice: The FUJI cohort. Br. J. Cancer 2019, 121, 1001–1008. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Horbinski, C.M.; Garzotto, M.; Qian, D.Z.; Beer, T.M.; Kyprianou, N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010, 70, 7992–8002. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, C.J.; Omlin, A.G.; Altavilla, A.; Lorente, D.; Ferraldeschi, R.; Bianchini, D.; Dearnaley, D.; Parker, C.; De Bono, J.S.; Attard, G. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur. Urol. 2014, 66, 459–465. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Wei, W.; Liu, Q.; Wang, Y.; Zhang, Y.; Lian, F.; Liu, F.; Li, C.; Ying, K. Mitoxantrone triggers immunogenic prostate cancer cell death via p53-dependent PERK expression. Cell. Oncol. 2020, 43, 1099–1116. [Google Scholar] [CrossRef]
- Green, A.K.; Corty, R.W.; Wood, W.A.; Meeneghan, M.; Reeder-Hayes, K.E.; Basch, E.; Milowsky, M.I.; Dusetzina, S.B. Comparative effectiveness of mitoxantrone plus prednisone versus prednisone alone in metastatic castrate-resistant prostate cancer after docetaxel failure. Oncologist 2015, 20, 516–522. [Google Scholar] [CrossRef]
- Devos, G.; Devlies, W.; De Meerleer, G.; Baldewijns, M.; Gevaert, T.; Moris, L.; Milonas, D.; Van Poppel, H.; Berghen, C.; Everaerts, W. Neoadjuvant hormonal therapy before radical prostatectomy in high-risk prostate cancer. Nat. Rev. Urol. 2021, 18, 739–762. [Google Scholar] [CrossRef]
- Nuvola, G.; Santoni, M.; Rizzo, M.; Rosellini, M.; Mollica, V.; Rizzo, A.; Marchetti, A.; Battelli, N.; Massari, F. Adapting to hormone-therapy resistance for adopting the right therapeutic strategy in advanced prostate cancer. Expert Rev. Anticancer Ther. 2023, 23, 593–600. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, M.; Liao, J.; Tang, Q.; Lei, Y.; Gong, Z.; Shan, L.; Duan, M.; Chai, X.; Pang, J. Discovery of a novel androgen receptor antagonist manifesting evidence to disrupt the dimerization of the ligand-binding domain via attenuating the hydrogen-bonding network between the two monomers. J. Med. Chem. 2021, 64, 17221–17238. [Google Scholar] [CrossRef]
- Gim, H.J.; Park, J.; Jung, M.E.; Houk, K. Conformational dynamics of androgen receptors bound to agonists and antagonists. Sci. Rep. 2021, 11, 15887. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Beer, T.M.; Taplin, M.-E.; Efstathiou, E.; Hirmand, M.; Forer, D.; Scher, H.I. Long-term safety and antitumor activity in the phase 1–2 study of enzalutamide in pre-and post-docetaxel castration-resistant prostate cancer. Eur. Urol. 2015, 68, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Pilon, D.; Behl, A.S.; Ellis, L.A.; Robitaille, M.-N.; Lefebvre, P.; Dawson, N.A. Assessment of real-world central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am. Health Drug Benefits 2017, 10, 143. [Google Scholar] [PubMed]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cai, L. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012, 72, 1494–1503. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Moilanen, A.-M.; Riikonen, R.; Oksala, R.; Ravanti, L.; Aho, E.; Wohlfahrt, G.; Nykänen, P.S.; Törmäkangas, O.P.; Palvimo, J.J.; Kallio, P.J. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci. Rep. 2015, 5, 12007. [Google Scholar] [CrossRef]
- Fizazi, K.; Albiges, L.; Loriot, Y.; Massard, C. ODM-201: A new-generation androgen receptor inhibitor in castration-resistant prostate cancer. Expert Rev. Anticancer Ther. 2015, 15, 1007–1017. [Google Scholar] [CrossRef]
- Zurth, C.; Sandmann, S.; Trummel, D.; Seidel, D.; Gieschen, H. Blood-brain barrier penetration of [14C] darolutamide compared with [14C] enzalutamide in rats using whole body autoradiography. J. Clin. Oncol. 2018, 36, 345. [Google Scholar] [CrossRef]
- Darne, C.P.; Velaparthi, U.; Saulnier, M.; Frennesson, D.; Liu, P.; Huang, A.; Tokarski, J.; Fura, A.; Spires, T.; Newitt, J. The discovery of BMS-737 as a potent, CYP17 lyase-selective inhibitor for the treatment of castration-resistant prostate cancer. Bioorganic Med. Chem. Lett. 2022, 75, 128951. [Google Scholar]
- Wróbel, T.M.; Jørgensen, F.S.; Pandey, A.V.; Grudzińska, A.; Sharma, K.; Yakubu, J.; Björkling, F. Non-steroidal CYP17A1 inhibitors: Discovery and assessment. J. Med. Chem. 2023, 66, 6542–6566. [Google Scholar] [CrossRef]
- Pezaro, C.J.; Mukherji, D.; De Bono, J.S. Abiraterone acetate: Redefining hormone treatment for advanced prostate cancer. Drug Discov. Today 2012, 17, 221–226. [Google Scholar] [CrossRef]
- Vanneste, B.G.; Van Limbergen, E.J.; van Lin, E.N.; van Roermund, J.G.; Lambin, P. Prostate cancer radiation therapy: What do clinicians have to know? BioMed Res. Int. 2016, 2016, 6829875. [Google Scholar] [CrossRef]
- Ferini, G.; Pergolizzi, S. A ten-year-long update on radiation proctitis among prostate cancer patients treated with curative external beam radiotherapy. In Vivo 2021, 35, 1379–1391. [Google Scholar] [CrossRef]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Achchillage, C.K.H.W.; Ren, C. Prostate Cancer, Castration-Resistant Prostate Cancer (CRPC), Radium-223 Dichloride Injection for Bone Metastasized Prostate Cancer. J. Cancer Ther. 2023, 14, 429–442. [Google Scholar] [CrossRef]
- Höllriegl, V.; Petoussi-Henss, N.; Hürkamp, K.; Ocampo Ramos, J.C.; Li, W.B. Radiopharmacokinetic modelling and radiation dose assessment of 223Ra used for treatment of metastatic castration-resistant prostate cancer. EJNMMI Phys. 2021, 8, 44. [Google Scholar] [CrossRef]
- Nilsson, S.; Cislo, P.; Sartor, O.; Vogelzang, N.; Coleman, R.; O’Sullivan, J.; Reuning-Scherer, J.; Shan, M.; Zhan, L.; Parker, C. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann. Oncol. 2016, 27, 868–874. [Google Scholar] [CrossRef]
- Westdorp, H.; Sköld, A.E.; Snijer, B.A.; Franik, S.; Mulder, S.F.; Major, P.P.; Foley, R.; Gerritsen, W.R.; de Vries, I.J.M. Immunotherapy for prostate cancer: Lessons from responses to tumor-associated antigens. Front. Immunol. 2014, 5, 85682. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2009, 115, 3670–3679. [Google Scholar] [CrossRef]
- Gulley, J.L.; Madan, R.A.; Pachynski, R.; Mulders, P.; Sheikh, N.A.; Trager, J.; Drake, C.G. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. JNCI J. Natl. Cancer Inst. 2017, 109, djw261. [Google Scholar] [CrossRef]
- GuhaThakurta, D.; Sheikh, N.A.; Fan, L.-Q.; Kandadi, H.; Meagher, T.C.; Hall, S.J.; Kantoff, P.W.; Higano, C.S.; Small, E.J.; Gardner, T.A. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin. Cancer Res. 2015, 21, 3619–3630. [Google Scholar] [CrossRef]
- Fong, L.; Carroll, P.; Weinberg, V.; Chan, S.; Lewis, J.; Corman, J.; Amling, C.L.; Stephenson, R.A.; Simko, J.; Sheikh, N.A. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl. Cancer Inst. 2014, 106, dju268. [Google Scholar] [CrossRef]
- Mark, D.; Samson, D.J.; Bonnell, C.J.; Ziegler, K.M.; Aronson, N. Outcomes of Sipuleucel-T Therapy. 2015. Available online: https://europepmc.org/article/nbk/nbk280060?report=printable&client=bot&client=bot&client=bot (accessed on 18 March 2013).
- González del Alba, A.; Méndez-Vidal, M.; Vazquez, S.; Castro, E.; Climent, M.; Gallardo, E.; Gonzalez-Billalabeitia, E.; Lorente, D.; Maroto, J.; Arranz, J. SEOM clinical guidelines for the treatment of advanced prostate cancer (2020). Clin. Transl. Oncol. 2021, 23, 969–979. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A. FDA approval summary: Pembrolizumab for the treatment of tumor mutational burden–high solid tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Azuma, M.; Krummel, M.F.; Bluestone, J.A. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR–induced stop signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. FDA approval summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune checkpoint inhibitors in prostate cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti–PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Curtin, N.J. Targeting the DNA damage response for cancer therapy. Biochem. Soc. Trans. 2023, 51, 207–221. [Google Scholar] [CrossRef]
- Wu, M.S.; Goldberg, H. Role of Rucaparib in the Treatment of Prostate Cancer: Clinical Perspectives and Considerations. Cancer Manag. Res. 2022, 3159–3174. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Hörnberg, E.; Ylitalo, E.B.; Crnalic, S.; Antti, H.; Stattin, P.; Widmark, A.; Bergh, A.; Wikström, P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE 2011, 6, e19059. [Google Scholar] [CrossRef]
- Li, J.; Cao, B.; Liu, X.; Fu, X.; Xiong, Z.; Chen, L.; Sartor, O.; Dong, Y.; Zhang, H. Berberine suppresses androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2011, 10, 1346–1356. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Srivastava, S.K.; Pai, S.; Singh, S.; Singh, A.P. Therapies targeted to androgen receptor signaling axis in prostate cancer: Progress, challenges, and hope. Cancers 2019, 12, 51. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, T.; Zhang, J. Recent advances in dietary androgen receptor inhibitors. Med. Res. Rev. 2024, 44, 1446–1500. [Google Scholar] [CrossRef]
- Xu, D.; Lin, T.-H.; Li, S.; Da, J.; Wen, X.-Q.; Ding, J.; Chang, C.; Yeh, S. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 2012, 316, 11–22. [Google Scholar] [CrossRef]
- McCarty, M.F.; Hejazi, J.; Rastmanesh, R. Beyond androgen deprivation: Ancillary integrative strategies for targeting the androgen receptor addiction of prostate cancer. Integr. Cancer Ther. 2014, 13, 386–395. [Google Scholar] [CrossRef]
- Hao, Q.; Wu, Y.; Vadgama, J.V.; Wang, P. Phytochemicals in inhibition of prostate cancer: Evidence from molecular mechanisms studies. Biomolecules 2022, 12, 1306. [Google Scholar] [CrossRef]
- Jameel, M.; Fatma, H.; Nadtochii, L.A.; Siddique, H.R. Molecular Insight into Prostate Cancer: Preventive Role of Selective Bioactive Molecules. Life 2023, 13, 1976. [Google Scholar] [CrossRef]
- VanOpstall, C.; Perike, S.; Brechka, H.; Gillard, M.; Lamperis, S.; Zhu, B.; Brown, R.; Bhanvadia, R.; Vander Griend, D.J. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. Elife 2020, 9, e53600. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Che, C.-T. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef]
- Hahm, E.R.; Karlsson, A.I.; Bonner, M.Y.; Arbiser, J.L.; Singh, S.V. Honokiol inhibits androgen receptor activity in prostate cancer cells. Prostate 2014, 74, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Naiki, T.; Kato, H.; Iida, K.; Etani, T.; Nagayasu, Y.; Suzuki, S.; Yamashita, Y.; Inaguma, S.; Onishi, M. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis 2020, 41, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Lou, W.; Gao, A.C.; Nadiminty, N. Quercetin targets hnRNPA1 to overcome enzalutamide resistance in prostate cancer cells. Mol. Cancer Ther. 2017, 16, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tian, H.; Lin, S.; Mo, J.; Li, Z.; Chen, X.; Liu, J. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol. Lett. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.-S.; Young, C.Y. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis 2001, 22, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Khurana, N.; Kim, H.; Chandra, P.K.; Talwar, S.; Sharma, P.; Abdel-Mageed, A.B.; Sikka, S.C.; Mondal, D. Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer. Oncol. Rep. 2017, 38, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical 1. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, W.; Liu, J.; Liu, D.; Cui, Y.; Huang, R.; Yan, J.; Lei, M. Triptolide inhibits the AR signaling pathway to suppress the proliferation of enzalutamide resistant prostate cancer cells. Theranostics 2017, 7, 1914. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Gao, A.C. Adaptive pathways and emerging strategies overcoming treatment resistance in castration resistant prostate cancer. Asian J. Urol. 2016, 3, 185–194. [Google Scholar] [CrossRef]
- Jiang, C.; Masood, M.; Rasul, A.; Wei, W.; Wang, Y.; Ali, M.; Mustaqeem, M.; Li, J.; Li, X. Altholactone inhibits NF-κB and STAT3 activation and induces reactive oxygen species-mediated apoptosis in prostate cancer DU145 cells. Molecules 2017, 22, 240. [Google Scholar] [CrossRef]
- Qiu, Y.-Y.; Tang, L.-Q.; Wei, W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol. Cell. Endocrinol. 2017, 443, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, L.; Wang, Y.; Zhang, H.; Xu, D.; Zhao, X.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar] [PubMed]
- Liu, C.-H.; Tang, W.-C.; Sia, P.; Huang, C.-C.; Yang, P.-M.; Wu, M.-H.; Lai, I.-L.; Lee, K.-H. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.-M.; Kim, D. Berberine inhibited radioresistant effects and enhanced anti-tumor effects in the irradiated-human prostate cancer cells. Toxicol. Res. 2010, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Y.; Zheng, X.; Shao, D.; Zhao, Y.; Wang, Z.; Dawulieti, J.; Liu, W.; Sun, M.; Sun, W. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019, 100, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Naqvi, S.T.Q.; Muhammad, S.A.; Qadir, M.; Naqvi, S.T. Curcumin: A polyphenol with molecular targets for cancer control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Shtivelman, E.; Beer, T.M.; Evans, C.P. Molecular pathways and targets in prostate cancer. Oncotarget 2014, 5, 7217. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From chemoprevention to chemotherapy: Common targets and common goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of prostate cancer by natural agents: Evidence from molecular and epidemiological studies. Anticancer Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef]
- Imran, M.; Ullah, A.; Saeed, F.; Nadeem, M.; Arshad, M.U.; Suleria, H.A.R. Cucurmin, anticancer, & antitumor perspectives: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1271–1293. [Google Scholar] [PubMed]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and its derivatives as potential therapeutic agents in prostate, colon and breast cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- McCarty, M.F. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integr. Cancer Ther. 2004, 3, 349–380. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Guo, Q.; Liu, P. Cryptotanshinone Induces Apoptosis and Autophagic Cell Death in Prostate Cancer Cells. Med One 2017, 2, e170019. [Google Scholar] [CrossRef]
- Shin, D.-S.; Kim, H.-N.; Shin, K.D.; Yoon, Y.J.; Kim, S.-J.; Han, D.C.; Kwon, B.-M. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Hsieh, C.Y.; Huang, K.E.; Chang, C.; Kang, H.Y. Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer 2012, 131, 1423–1434. [Google Scholar] [CrossRef]
- Stuart, E.C.; Scandlyn, M.J.; Rosengren, R.J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006, 79, 2329–2336. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, S.; Yu, Y.; Xu, G. (−)-Epigallocatechin-3-gallate suppresses prostate cancer cell growth via activating miR-520a-3p. Rev. Bras. De Farmacogn. 2020, 30, 528–536. [Google Scholar] [CrossRef]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E. Epigallocatechin-3-gallate therapeutic potential in cancer: Mechanism of action and clinical implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Gupta, S.; Ahmad, N.; Nieminen, A.-L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef]
- Marchetti, C.; Gavazzo, P.; Burlando, B. Epigallocatechin-3-gallate mobilizes intracellular Ca2+ in prostate cancer cells through combined Ca2+ entry and Ca2+-induced Ca2+ release. Life Sci. 2020, 258, 118232. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015, 367, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Asim, M.; Afaq, F.; Abu Zaid, M.; Mukhtar, H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008, 68, 8555–8563. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar] [PubMed]
- Chien, C.-S.; Shen, K.-H.; Huang, J.-S.; Ko, S.-C.; Shih, Y.-W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell. Biochem. 2010, 333, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Adhami, V.M.; Lall, R.K.; Sechi, M.; Joshi, D.C.; Haidar, O.M.; Syed, D.N.; Siddiqui, I.A.; Chiu, S.-Y.; Mukhtar, H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget 2014, 5, 2462. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Fleshner, N.; Nelson, C.; Saour, B.; Musquera, M.; Venkateswaran, V.; Klotz, L. Antiproliferative mechanisms of the flavonoids 2, 2′-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr. Cancer 2010, 62, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol./Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Banyś, K.; Jelińska, M.; Wrzosek, M.; Skrajnowska, D.; Wrzesień, R.; Bielecki, W.; Bobrowska-Korczak, B. Inflammation Factors and Genistein Supplementation in Cancer—Preliminary Research. Curr. Issues Mol. Biol. 2024, 46, 2166–2180. [Google Scholar] [CrossRef]
- Shete, V.; Mahajan, N.M.; Shivhare, R.; Akkewar, A.; Gupta, A.; Gurav, S. Genistein: A promising phytoconstituent with reference to its bioactivities. Phytother. Res. 2024. [Google Scholar] [CrossRef]
- Pavese, J.M.; Krishna, S.N.; Bergan, R.C. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am. J. Clin. Nutr. 2014, 100, 431S–436S. [Google Scholar] [CrossRef] [PubMed]
- Naing, S.; Sandech, N.; Maiuthed, A.; Chongruchiroj, S.; Pratuangdejkul, J.; Lomarat, P. Garcinia mangostana L. Pericarp Extract and its active compound α-Mangostin as potential inhibitors of Immune Checkpoint programmed death Ligand-1. Molecules 2023, 28, 6991. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Petiwala, S.M.; Yan, M.; Won, J.H.; Petukhov, P.A.; Johnson, J.J. Gartanin, an isoprenylated xanthone from the mangosteen fruit (Garcinia mangostana), is an androgen receptor degradation enhancer. Mol. Nutr. Food Res. 2016, 60, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Nauman, M.C.; Won, J.H.; Petiwala, S.M.; Vemu, B.; Lee, H.; Sverdlov, M.; Johnson, J.J. α-Mangostin Promotes In Vitro and In Vivo Degradation of Androgen Receptor and AR-V7 Splice Variant in Prostate Cancer Cells. Cancers 2023, 15, 2118. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Qi, Y.; Yang, Y.; Liu, X.; Xu, D.; Guo, W.; Zhan, Y.; Xiong, Z.; Zhang, A.; Wang, A.R. 20 (S)-protopanaxadiol inhibition of progression and growth of castration-resistant prostate cancer. PLoS ONE 2014, 9, e111201. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Arbiser, J.L.; Sun, S.Y.; Zayzafoon, M.; Johnstone, P.A.; Fujisawa, M.; Gotoh, A.; Weksler, B.; Zhau, H.E.; Chung, L.W. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, F.; Ali, S.; Kumar, V.; Elasbali, A.M.; Alhassan, H.H.; Alharethi, S.H.; Islam, A.; Hassan, M.I. Pharmacological features, health benefits and clinical implications of honokiol. J. Biomol. Struct. Dyn. 2023, 41, 7511–7533. [Google Scholar] [CrossRef]
- Herrmann, D.; Schreiber, A.; Ciotkowska, A.; Strittmatter, F.; Waidelich, R.; Stief, C.G.; Gratzke, C.; Hennenberg, M. Honokiol, a constituent of Magnolia species, inhibits adrenergic contraction of human prostate strips and induces stromal cell death. Prostate Int. 2014, 2, 140–146. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Cheon, C.; Ko, S.-G. Synergistic effects of natural products in combination with anticancer agents in prostate cancer: A scoping review. Front. Pharmacol. 2022, 13, 963317. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121. [Google Scholar] [CrossRef]
- Inala, M.S.R.; Pamidimukkala, K. In vitro combination effects of plant-derived quercetin with synthetic bicalutamide on prostate cancer and normal cell lines: In silico comparison. Silico Pharmacol. 2024, 12, 22. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, A.; Butt, N.A.; Zhang, L.; Williams, R.; Rimando, A.M.; Biswas, P.K.; Levenson, A.S. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: In silico approach to understand biological actions. Mol. BioSystems 2016, 12, 1702–1709. [Google Scholar] [CrossRef]
- Vancauwenberghe, E.; Noyer, L.; Derouiche, S.; Lemonnier, L.; Gosset, P.; Sadofsky, L.R.; Mariot, P.; Warnier, M.; Bokhobza, A.; Slomianny, C. Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF). Mol. Carcinog. 2017, 56, 1851–1867. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; Soto, A.; Gil-Araujo, B.; Gallego, B.; Chiloeches, A.; Lasa, M. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem. Toxicol. 2019, 124, 273–279. [Google Scholar] [CrossRef]
- Amiri, H.; Javid, H.; Einafshar, E.; Ghavidel, F.; Rajabian, A.; Hashemy, S.I.; Hosseini, H. Development and Evaluation of PLGA Nanoparticles Surfaced Modified with Chitosan-Folic Acid for Improved Delivery of Resveratrol to Prostate Cancer Cells. BioNanoScience 2024, 14, 988–998. [Google Scholar] [CrossRef]
- Rahman Siddique, H.; Nanda, S.; Parray, A.; Saleem, M. Androgen receptor in human health: A potential therapeutic target. Curr. Drug Targets 2012, 13, 1907–1916. [Google Scholar] [CrossRef]
- Khurana, N.; Talwar, S.; Chandra, P.K.; Sharma, P.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells. Int. J. Oncol. 2016, 49, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Khurana, N.; Sikka, S.C. Targeting crosstalk between Nrf-2, NF-κB and androgen receptor signaling in prostate cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Interplay between SOX9, Wnt/β-catenin and androgen receptor signaling in castration-resistant prostate cancer. Int. J. Mol. Sci. 2019, 20, 2066. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Geethangili, M.; Fang, S.-H.; Tzeng, Y.-M. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: A comparative study. Food Chem. Toxicol. 2007, 45, 1770–1776. [Google Scholar] [CrossRef]
- Mia, M.A.R.; Dey, D.; Sakib, M.R.; Biswas, M.Y.; Prottay, A.A.S.; Paul, N.; Rimti, F.H.; Abdullah, Y.; Biswas, P.; Iftehimul, M. The efficacy of natural bioactive compounds against prostate cancer: Molecular targets and synergistic activities. Phytother. Res. 2023, 37, 5724–5754. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Long, Y.; Gao, R.; Liu, Q.; Tian, X.; Liu, B.; Zhou, Q. Nanodelivery System of Traditional Chinese Medicine Bioactive Compounds: Application in the Treatment of Prostate Cancer. Phytomedicine 2024, 155554. [Google Scholar] [CrossRef]
- Khan, T.A.; Bhar, K.; Samanta, R.; Bhatt, S.; Singh, M.; Rani, R.; Kumar, V.; Sharma, A.K. A bis-quinoline ruthenium (ii) arene complex with submicromolar cytotoxicity in castration-resistant prostate cancer cells. Chem. Commun. 2024, 60, 1579–1582. [Google Scholar] [CrossRef]
- Karantanos, T.; Evans, C.P.; Tombal, B.; Thompson, T.C.; Montironi, R.; Isaacs, W.B. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 2015, 67, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Costa, L.; Maurício, M.J.; Figueira, L.; Ramos, R.; Martins-da-Silva, C. Nonmetastatic castration-resistant prostate cancer: Current challenges and trends. Clin. Drug Investig. 2022, 42, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.C.; Ramos, I.B.; Silva, J.M.; Barra, W.F.; Riggins, G.J.; Palande, V.; Pinho, C.T.; Frenkel-Morgenstern, M.; Santos, S.E.; Assumpcao, P.P.; et al. Current perspectives on circulating tumor DNA, precision medicine, and personalized clinical management of cancer. Mol. Cancer Res. 2020, 18, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.E.; Dorff, T.B.; Quinn, D.I.; Diaz, P.M.; Castellanos, O.O.; Agus, D.B. Safety and efficacy of docetaxel, bevacizumab, and everolimus for castration-resistant prostate cancer (CRPC). Clin. Genitourin. Cancer 2018, 16, e11–e21. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef]
| Drug (Brand) Name | Initial Approval Date by US-FDA | Mechanism of Action | Mode of Administration | Reference |
|---|---|---|---|---|
| Abiraterone Acetate (Zytiga) | 28 April 2011 | ↓ 17α-hydroxylase CYP17 enzyme, testosterone, adrenal androgen, DHEA, androstenedione, DHT, PI3-kinase/Akt, Bcl-2, and c-Met, AR nuclear translocation, AR-DNA binding domain, PSA, and AR-V7 | Oral | [58,59,60] |
| Apalutamide (Erleada) | 14 February 2018 | ↓ AR at the ligand binding domain, AR nuclear translocation, DNA binding, AR-mediated transcription, co-factor recruitment, AR-V7 | Oral | [61] |
| Cabazitaxel (Jevtana) | 17 June 2010 | ↓ microtubular depolymerization, AR-V7, PSA, AR, Foxo-1, MCAK, and HSET | IV | [3,4,62,63] |
| Docetaxel (Taxotere) | 19 May 2004 | ↑ Bcl-2, nuclear AR ↓ microtubular depolymerization, Bcl-2, Bcl-xL, TMPRSS2-ERG, AR-V7, PSA | IV | [5,62,64] |
| Darolutamide (Nubeqa) | 30 July 2019 | ↓ AR-mediated transcription, AR nuclear translocation, F877L, 7878A, T878G levels | Oral | [6,65] |
| Dostarlimab | 22 April 2021 | ↓ PD-1 receptor, PD-L1 and PD-L2 ligand | [7,8] | |
| Enzalutamide (Xtandi) | 31 August 2012 | ↓ AR nuclear translocation, AR-DNA binding, full-length AR translocation, co-factor recruitment, AR-V7, PSA | Oral | [9,51] |
| Leuprolide acetate (Lupron Depot) | 1985 | ↓ LH, FSH, GnRH receptors, androgen synthesis | IV | [66] |
| Lutetium Lu-177 Vipivotide tetraxetan (Pluvicto) | 23 March 2022 | ↓ PSMA, PSA | IV | [67,68] |
| Mitoxantrone (Novantrone) | 1996 | ↓ topoisomerase II inhibitor, DNA replication | IV | [1] |
| Niraparib (Zejula) | 27 March 2017 | ↑ PARP-DNA complexes ↓PARP | Oral | [69] |
| Olaparib (Lynparza) | 19 December 2014 | ↓ PARP and PARP-DNA binding, ADT, HR, PTEN, TP53 | Oral | [70,71] |
| Pembrolizumab | 4 September 2014 | ↓ PD-1 receptor, PD-L1, PD-L2 ligand | [72,73] | |
| Radium-223 | 2013 | ↓ PSA | IV | [1,74] |
| Rucaparib (Rubraca) | 19 December 2016 | ↓ BRCA2, ATM, CHEK2, and BRCA1 | Oral | [75] |
| Sipuleucel-T | April 2010 | ↑ antigen-presenting cells (APCs) | IV | [76] |
| Talazoparib (Talzenna) | 16 October 2018 | ↓ PARP | Oral | [77] |
| Types of Drugs | Name as Example | Mechanism of Action | Reference |
|---|---|---|---|
| Alkylating agents | Mechlorethamine melphalan | ↑ nucleotide mismatching, ↑ DNA fragmentation | [78] |
| Antimetabolites | Decitabine | ↑ Inhibit DNA methyltransferase, ↑ DNA hypomethylation, ↑ S Phase of cells | [79] |
| Topoisomerase (ii) inhibitors | Etoposide | ↑ Inhibit both α and β isoforms of topoisomerase II | [80] |
| Mitotic inhibitors | Docetaxel | ↓Mitosis, ↑ apoptosis ↓ Bcl-2 | [81] |
| Mitotic Inhibitors (Vinca Alkaloids) | Vinorelbine | ↓ Mitosis, ↓ Bcl-2, ↑ BAX [5] | [82] |
| Corticosteroids | Prednisone | ↓ gene expression, ↓ Inflammatory transcription | [83] |
| Synthetic Analog | Ixabepilone | ↑ Microtubule stability | [84] |
| Scientific Name/Chemical Compound | Plant Source | Plant Part | Efficacy | Mechanism | References |
|---|---|---|---|---|---|
| Berberine | Induction of apoptosis, suppression of cell proliferation, CRPC xenografts, and AR splice variant | ↓ AR, AR translocation, AR-fL, ARΔLBDs, and HSP90 | [138,139] | ||
| Curcumin/Curcuma longa | Inhibition of cell proliferation, programmed cell death, G2/M cell cycle arrest, and inflammation | ↓ AR, PSA, ARE, CYP11A1, and HSD3B2 | [140] | ||
| Cryptotanshinone (CTS) | Danshen | Roots | Suppression of cell proliferation, full-length AR transactivation, and cancer growth in xenograft PCa model | ↓ AR, TMPRSS2, TMEPA1, and PSA | [141] |
| Epigallocatechin-3-gallate (EGCG) | Green tea | Leaves | Reduction of histone acetylation and cell proliferation in xenografts | ↓ AR, nuclear translocation receptor, and NF-κB | [142] |
| Fisetin | Strawberries, apples, persimmons, onions, kiwi, and cucumbers | leaves, stems, and fruits | Slow down therapy resistance, invasion, and migration of cancer cells | MMP-2, MMP-9, PI3K/AKT, NF-κB pathway, AR, and PSA | [143,144] |
| Garcinia mangostana/α-Mangosteen | Purple mangosteen | Stimulation of programmed cell death, inhibition of nuclear translocation, and tumor growth in CRPC | ↓ AR, AR-V7, BiP, and GRP78 | [145] | |
| Ginsenosides Rg3 | Panax ginseng, Panax quinquefolius, Panax japonicus, Panax notoginseng, Panax cocos, and Pfaffia paniculata | Roots | Induction of cell cycle arrest in the G1 phase | ↓ AR, AR-Vs, 5α-reductase, PSA, PCNA ↑p21, p27, caspase 3 | [146] |
| Honokiol | Magnolia grandiflora and Magnolia dealbata. | Induction of programmed cell death, suppression of cell viability, stimulation of nuclear translocation of AR | ↓ AR, AR translocation, transcriptional activity of AR | [147] | |
| luteolin | Inhibition of cell proliferation, CRPC tumor growth, and induction of apoptosis | ↓ AR-V7 via miR-8080 ↑ Sensitize enzalutamide | [148] | ||
| Quercetin | Suppression of PCa progression, increase the proportion of apoptotic cells | ↓ hnRNPA1, AR-V7, AR, HSP70, PSA | [149] | ||
| Resveratrol | Grapes, Blueberries, Peanuts | Enhancement of apoptosis, slow down the growth of CRPC cells, and prevention of tumor formation in vivo | ↓ PI3K/AKT, AR-V7, AR, PSA | [150] | |
| Silibinin | Silybum marianum or milk thistle | Inhibition of cell proliferation, induction of cell cycle arrest and apoptosis in CRPC | ↓ AR, PSA | [151] | |
| Sulforaphane | Broccoli, Brussels sprouts, and cabbage | Vegetables | Suppression of cell proliferation, migration, and clonogenic potential | ↓ AR-V7, HSP90 ↑ Nrf2 | [152,153] |
| Triptolide | Tripterygium wilfordii | Induction of apoptotic cell death, inhibition of CRPC tumor growth | ↓ AR, AR-V7 at Ser via XPB/CDK7, PSA/KLK3, TFIIH and RNA Pol II recruitment | [154] | |
| Ursolic acid | Reduction of cell proliferation in vitro and xenograft tumor growth in animal models | ↓ IκB kinase, IκBα, NF-κB, TNF receptor-associated factor, IkappaBalpha kinase, p65, cyclin D1, cyclooxygenase 2, and matrix metalloproteinase 9 | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Akter, K.; Ahmed, K.R.; Fahim, M.M.H.; Aktary, N.; Park, M.N.; Shin, S.-W.; Kim, B. Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies. Cancers 2024, 16, 2777. https://doi.org/10.3390/cancers16162777
Rahman M, Akter K, Ahmed KR, Fahim MMH, Aktary N, Park MN, Shin S-W, Kim B. Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies. Cancers. 2024; 16(16):2777. https://doi.org/10.3390/cancers16162777
Chicago/Turabian StyleRahman, Muntajin, Khadija Akter, Kazi Rejvee Ahmed, Md. Maharub Hossain Fahim, Nahida Aktary, Moon Nyeo Park, Sang-Won Shin, and Bonglee Kim. 2024. "Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies" Cancers 16, no. 16: 2777. https://doi.org/10.3390/cancers16162777
APA StyleRahman, M., Akter, K., Ahmed, K. R., Fahim, M. M. H., Aktary, N., Park, M. N., Shin, S.-W., & Kim, B. (2024). Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies. Cancers, 16(16), 2777. https://doi.org/10.3390/cancers16162777









