Dose Calculation Accuracy of Beam Models in RadCalc for a 1.5 T MR-Linac
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Beam Modeling in RadCalc
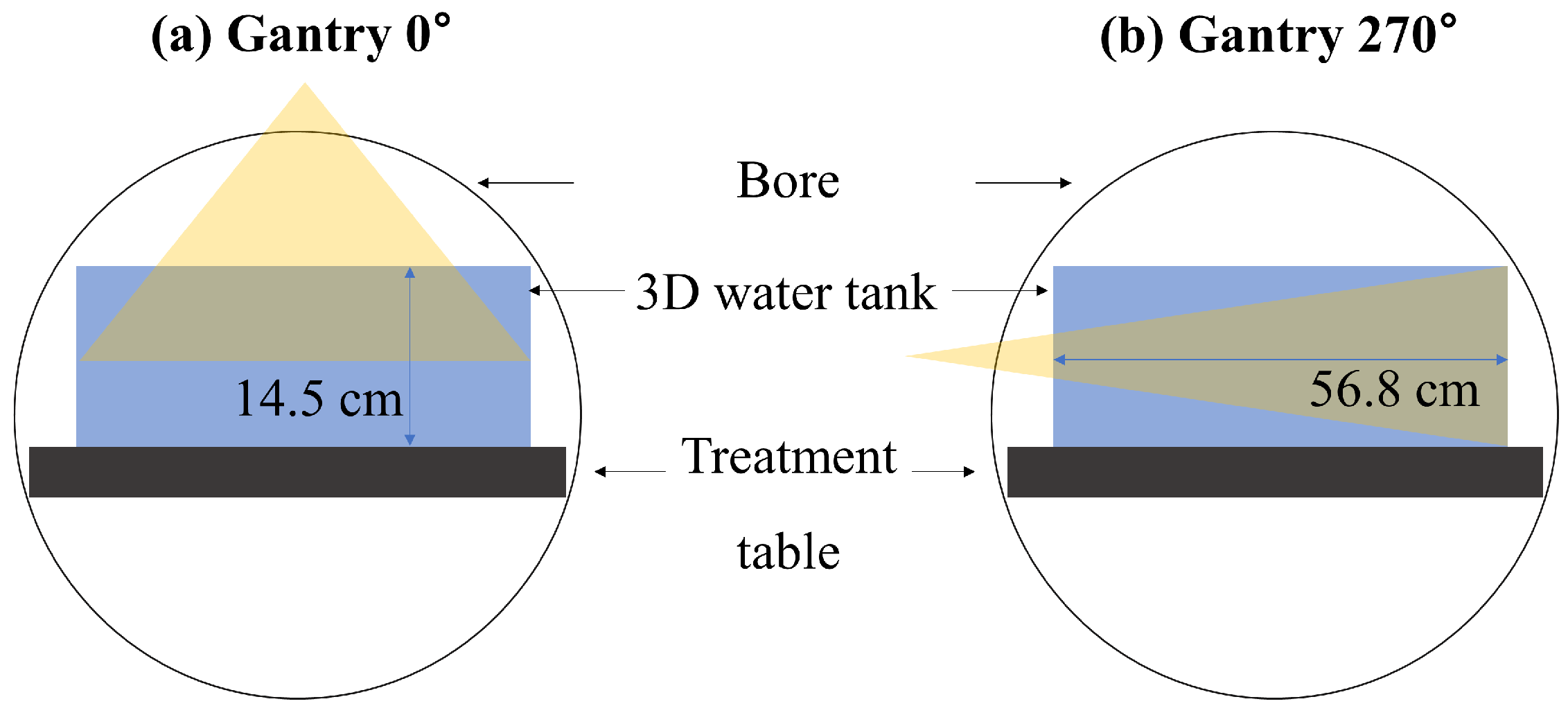
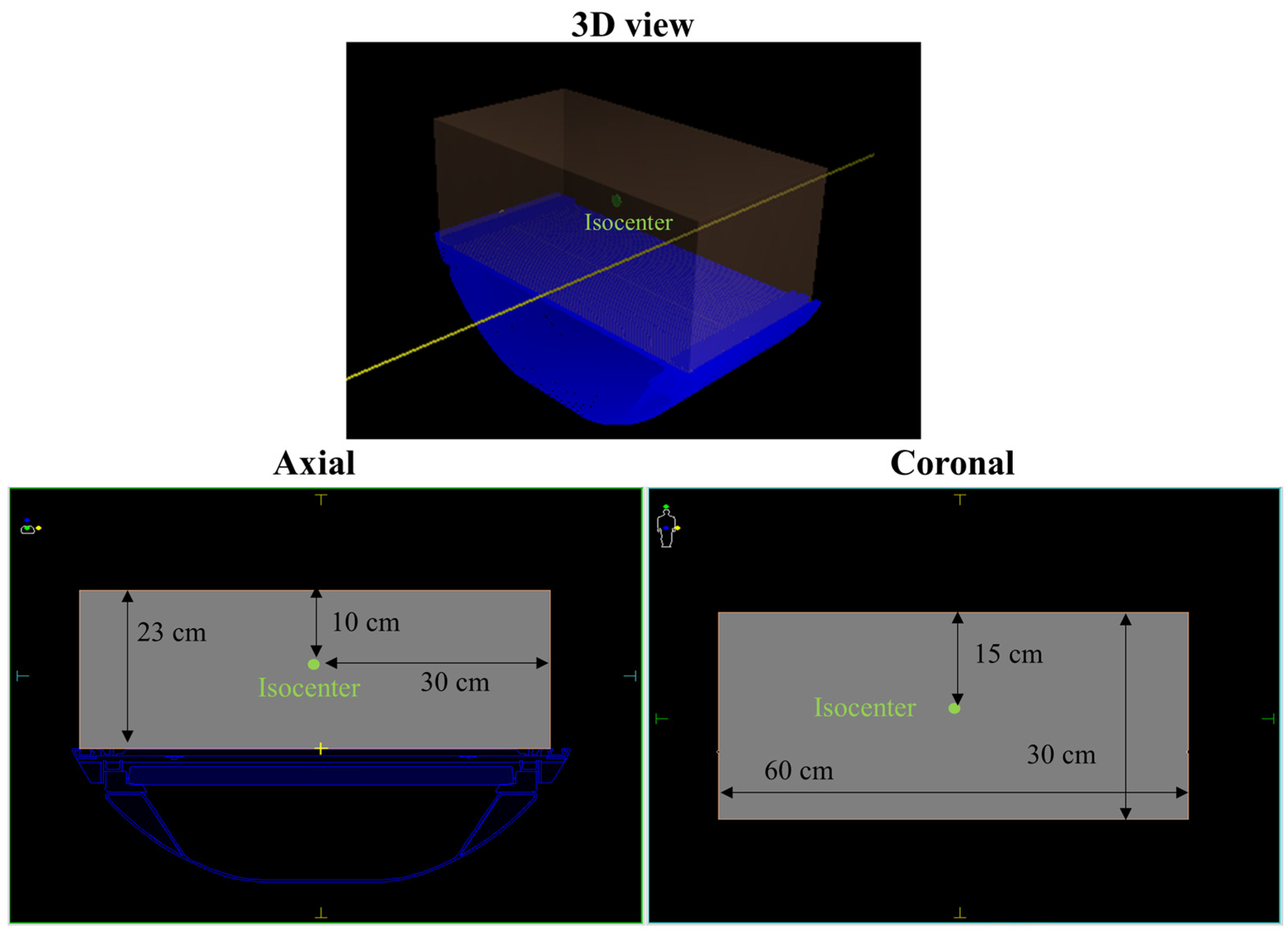
2.2. Evaluation of Beam Models for Virtual Phantom
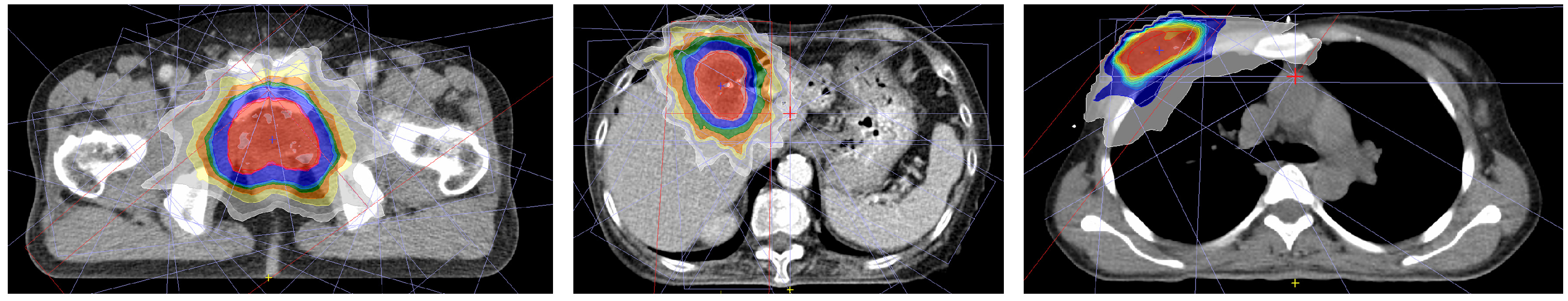
2.3. Evaluation of Beam Models for Patients
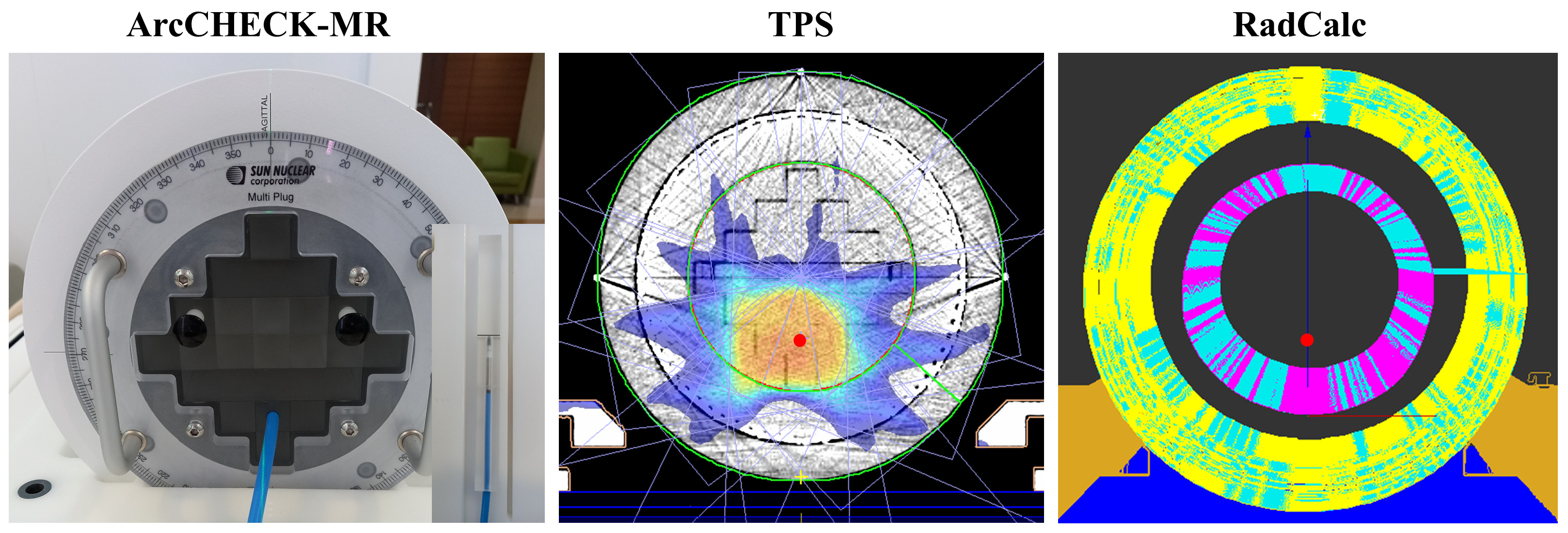
2.4. Correlation with PSQA Based on Measurement
3. Results
3.1. Evaluation of Beam Models in Virtual Phantom
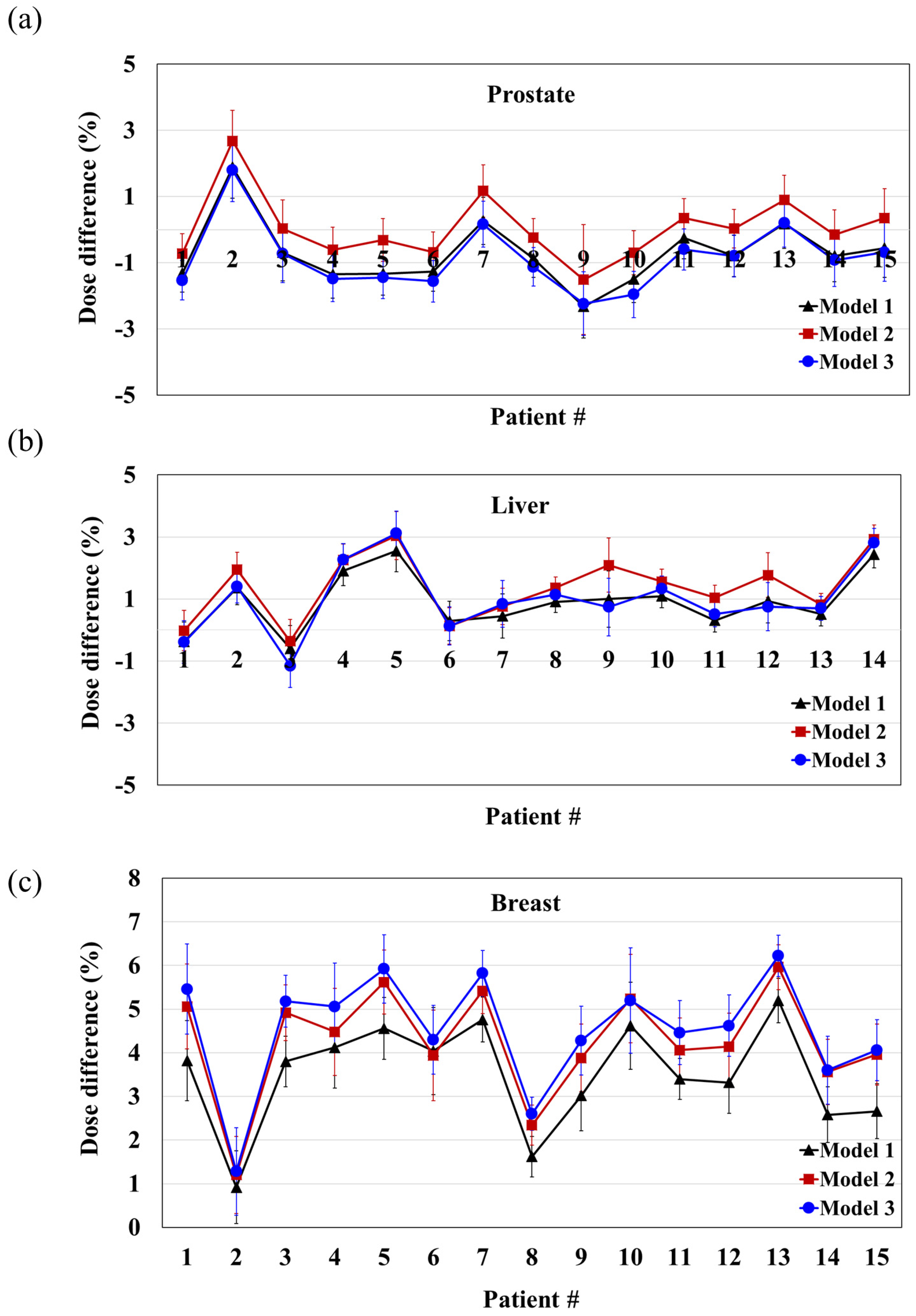
3.2. Evaluation of Beam Models in Patients
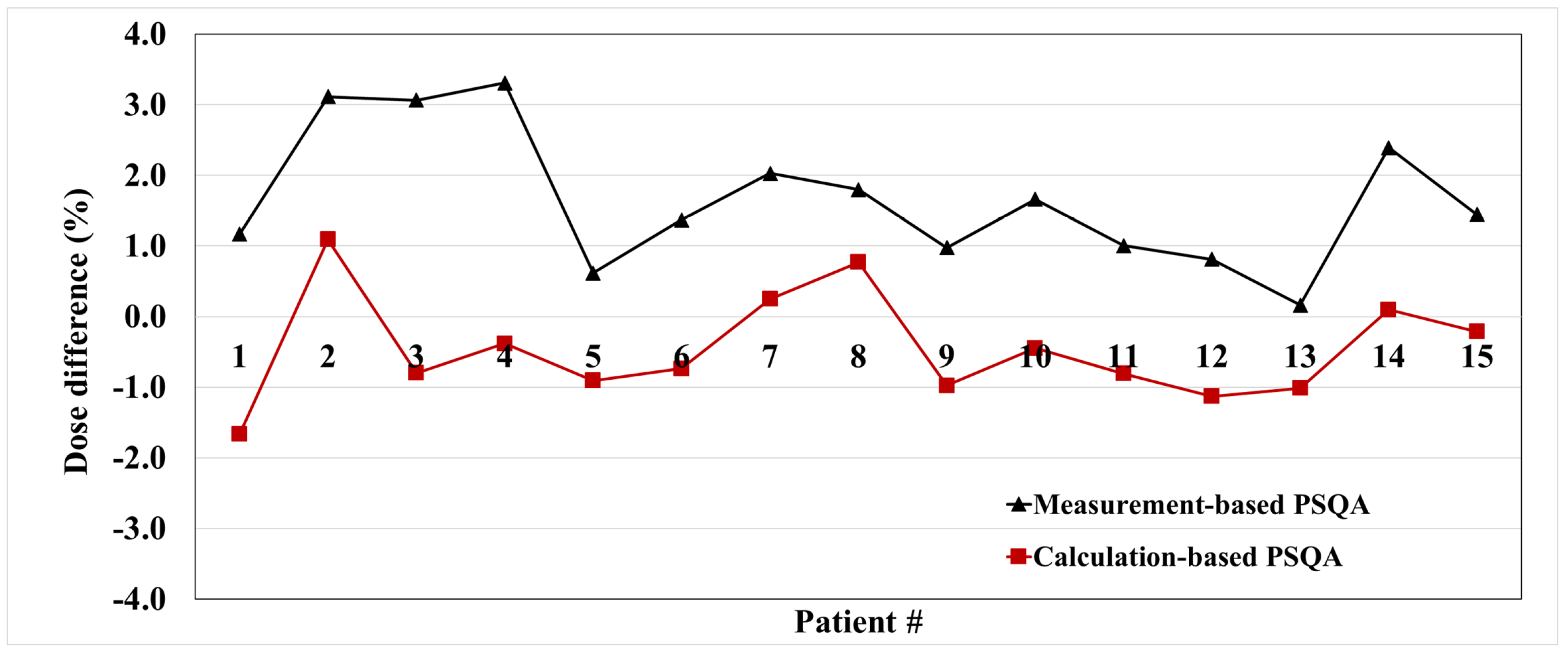
3.3. Correlation with PSQA Based on Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maziero, D.; Straza, M.W.; Ford, J.C.; Bovi, J.A.; Diwanji, T.; Stoyanova, R.; Paulson, E.S.; Mellon, E.A. MR-Guided Radiotherapy for Brain and Spine Tumors. Front. Oncol. 2021, 11, 626100. [Google Scholar] [CrossRef]
- Boldrini, L.; Corradini, S.; Gani, C.; Henke, L.; Hosni, A.; Romano, A.; Dawson, L. MR-guided radiotherapy for liver malignancies. Front. Oncol. 2021, 11, 616027. [Google Scholar] [CrossRef]
- Rammohan, N.; Randall, J.W.; Yadav, P. History of technological advancements towards MR-Linac: The future of image-guided radiotherapy. J. Clin. Med. 2022, 11, 4730. [Google Scholar] [CrossRef]
- Sung, J.; Choi, Y.; Kim, J.W.; Lee, I.J.; Lee, H. Compact bunker shielding assessment for 1.5 T MR-Linac. Sci. Rep. 2022, 12, 6712. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Buizza, G.; Landry, G.; Kamp, F.; Rabe, M.; Paganelli, C.; Baroni, G.; Reiner, M.; Keall, P.J.; van den Berg, C.A. Medical physics challenges in clinical MR-guided radiotherapy. Radiat. Oncol. 2020, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, L.; Cusumano, D.; Cellini, F.; Azario, L.; Mattiucci, G.C.; Valentini, V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: State of the art, pearls and pitfalls. Radiat. Oncol. 2019, 14, 71. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Jurgenliemk-Schulz, I.M.; Bol, G.H.; Glitzner, M.; Kotte, A.N.T.J.; van Asselen, B.; de Boer, J.C.J.; Bluemink, J.J.; Hackett, S.L.; Moerland, M.A.; et al. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys. Med. Biol. 2017, 62, L41–L50. [Google Scholar] [CrossRef]
- Keizer, D.M.D.; Kerkmeijer, G.W.; Willigenburg, T.; van Lier, A.L.H.M.W.; den Hartogh, M.D.; van Zyp, J.R.N.V.; de Groot-van Breugel, E.N.; Raaymakers, B.W.; Lagendijk, J.J.W.; de Boer, J.C.J. Prostate intrafraction motion during the preparation and delivery of MR-guided radiotherapy sessions on a 1.5T MR-Linac. Radiother. Oncol. 2020, 151, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Intven, M.; van Otterloo, S.D.M.; Mook, S.; Doornaert, P.; de Groot-van Breugel, E.; Sikkes, G.; Willemsen-Bosman, M.; van Zijp, H.; Tijssen, R. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5 T MR-linac: Clinical implementation and initial experience. Radiother. Oncol. 2021, 154, 172–178. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.W.; Eppinga, W.S.C.; Tijssen, R.H.N.; Kerkmeijer, L.G.W.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Mittauer, K.E.; Hill, P.M.; Geurts, M.W.; De Costa, A.-M.; Kimple, R.J.; Bassetti, M.F.; Bayouth, J.E. STAT-ART: The promise and practice of a rapid palliative single session of mr-guided online adaptive radiotherapy (ART). Front. Oncol. 2019, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wong, Y.S.; Lam, W.W.; Geng, H.; Huang, C.Y.; Tang, K.K.; Law, W.K.; Ho, C.C.; Nam, P.H.; Cheung, K.Y.; et al. Initial clinical experience of patient-specific QA of treatment delivery in online adaptive radiotherapy using a 1.5 T MR-Linac. Biomed. Phys. Eng. Express 2021, 7, 035022. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.L.; Heaton, R.; Fraser, M.W.; Murty Goddu, S.; Kirby, T.H.; Lam, K.L.; Molineu, A.; Zhu, T.C. Verification of monitor unit calculations for non-IMRT clinical radiotherapy: Report of AAPM Task Group 114. Med. Phys. 2011, 38, 504–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Stathakis, S.; Clark, J.R.; Feng, W.; Georg, D.; Holmes, S.M.; Kry, S.F.; Ma, C.M.C.; Miften, M.; Mihailidis, D. Report of AAPM Task Group 219 on independent calculation-based dose/MU verification for IMRT. Med. Phys. 2021, 48, e808–e829. [Google Scholar] [CrossRef] [PubMed]
- Hackett, S.; van Asselen, B.; Feist, G.; Pencea, S.; Akhiat, H.; Wolthaus, J.; Kotte, A.; Bol, G.; Lagendijk, J.; Raaymakers, B. SU-F-J-148: A Collapsed Cone Algorithm Can Be Used for Quality Assurance for Monaco Treatment Plans for the MR-Linac. Med. Phys. 2016, 43, 3441. [Google Scholar] [CrossRef]
- Pollitt, A.; Budgell, G.J.; Pooler, A.; Wood, J.; Chuter, R.; McWilliam, A. Feasibility of Mobius 3D as an independent MU checker for the adaptive work flow on the MR-Linac. Radiother. Oncol. 2018, 127, S1191–S1192. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Ding, S.; Liu, H.; Liu, B.; Xia, Y.; Song, T.; Huang, X. Feasibility of using a commercial collapsed cone dose engine for 1.5 T MR-LINAC online independent dose verification. Phys. Med. 2020, 80, 288–296. [Google Scholar] [CrossRef]
- Wang, Y.; Mazur, T.R.; Green, O.; Hu, Y.; Li, H.; Rodriguez, V.; Wooten, H.O.; Yang, D.; Zhao, T.; Mutic, S. A GPU-accelerated Monte Carlo dose calculation platform and its application toward validating an MRI-guided radiation therapy beam model. Med. Phys. 2016, 43, 4040–4052. [Google Scholar] [CrossRef]
- Friedel, M.; Nachbar, M.; Mönnich, D.; Dohm, O.; Thorwarth, D. Development and validation of a 1.5 T MR-Linac full accelerator head and cryostat model for Monte Carlo dose simulations. Med. Phys. 2019, 46, 5304–5313. [Google Scholar] [CrossRef]
- Chen, G.P.; Ahunbay, E.; Li, X.A. Development and performance of a software tool for quality assurance of online replanning with a conventional Linac or MR-Linac. Med. Phys. 2016, 43, 1713–1719. [Google Scholar] [CrossRef]
- Graves, S.A.; Snyder, J.E.; Boczkowski, A.; St-Aubin, J.; Wang, D.; Yaddanapudi, S.; Hyer, D.E. Commissioning and performance evaluation of RadCalc for the Elekta unity MRI-linac. J. Appl. Clin. Med. Phys. 2019, 20, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, M.; Lai, Y.; Trivedi, Z.; Wu, J.; Foo, T.; Gonzalez, Y.; Reynolds, R.; Park, C.; Yan, Y.; et al. ART2Dose: A comprehensive dose verification platformfor online adaptive radiotherapy. Med. Phys. 2024, 51, 18–30. [Google Scholar] [CrossRef]
- Price, A.T.; Knutson, N.C.; Kim, T.; Green, O.L. Commissioning a secondary dose calculation software for a 0.35 T MR-linac. J. Appl. Clin. Med. Phys. 2022, 23, e13452. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gu, X.; Graves, Y.J.; Folkerts, M.; Jiang, S.B. GPU-based fast Monte Carlo simulation for radiotherapy dose calculation. Phys. Med. Biol. 2011, 56, 7017. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Wang, B.; Liu, H.; Huang, X.; Song, T. Extension and validation of a GPU-Monte Carlo dose engine gDPM for 1.5 T MR-LINAC online independent dose verification. Med. Phys. 2021, 48, 6174–6183. [Google Scholar] [CrossRef]
- Clements, M.; Schupp, N.; Tattersall, M.; Brown, A.; Larson, R. Monaco treatment planning system tools and optimization processes. Med. Dosim. 2018, 43, 106–117. [Google Scholar] [CrossRef]
- Currie, M.P. Verification of an Independent Monitor Unit Calculation Program for IMRT Quality Assurance. Master’s Thesis, University of Wollongong, Wollongong, Australia, 2007. [Google Scholar]
- Kung, J.; Chen, G.; Kuchnir, F. A monitor unit verification calculation in intensity modulated radiotherapy as a dosimetry quality assurance. Med. Phys. 2000, 27, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Sarfehnia, A.; Paudel, M.R.; Kim, A.; Hissoiny, S.; Sahgal, A.; Keller, B. Evaluation of a commercial MRI Linac based Monte Carlo dose calculation algorithm with geant 4. Med. Phys. 2016, 43, 894–907. [Google Scholar] [CrossRef]
- Yamazaki, T.; Itano, M.; Ishibashi, S.; Higuchi, Y.; Yamashita, M.; Kosaka, M.; Kobayashi, N.; Tachibana, H. Impact of Different Independent Dose Verification Software Programs for Secondary Check. Igaku Butsuri Nihon Igaku Butsuri Gakkai Kikanshi = Jpn. J. Med. Phys. Off. J. Jpn. Soc. Med. Phys. 2017, 36, 197–206. [Google Scholar]
- Haslam, J.; Bonta, D.; Lujan, A.; Rash, C.; Jackson, W.; Roeske, J. Comparison of dose calculated by an intensity modulated radiotherapy treatment planning system and an independent monitor unit verification program. J. Appl. Clin. Med. Phys. 2003, 4, 224–230. [Google Scholar] [CrossRef]
- Cao, W.; Chen, L.; Chen, L.; Deng, X. The feasibility study of independent check for intensity-modulated radiotherapy. Chin. J. Radiat. Oncol. 2011, 20, 521–524. [Google Scholar]
| Parameter | Setting |
|---|---|
| Source axis distance | 143.5 cm |
| Couch vertical zero position | 0.0 cm |
| Volume average dose options | 0.1 cm, automatically select best value |
| Clarkson radial sampling distance | 0.1 cm |
| Clarkson angular sampling increment | 5° |
| Clarkson radius used for primary dose | 0.5 cm |
| Clarkson pixel size for intensity map | 0.25 cm |
| Clarkson max angular step between control points | 2.5° |
| Clarkson max leaf position change between control points | 0.2 cm |
| Jaw transmission | 0.006 |
| MLC leaf transmission | 0.006 |
| Energy value (MV) | 7 MV FFF |
| Reference SSD | 133.5 |
| Dmax depth | 1.4 cm |
| Reference depth | 10 cm |
| Calibration @ reference | 0.87 cGy/MU |
| Leaf width/position | 0.717 cm width, range from −28.341 to +28.341 |
| Allow fluence corrections for selected machine | Yes |
| Beam Modeling Parameters | Model | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Sc, Sp, OAR | MD (same data except model 6) | Pre-configuration data | |||||
| PDD | Data type | CD | CD | MD | MD | MD | |
| Gantry angle | 0° | 0° | 270° | 0° | 0° and 270° | ||
| Post-processed | Smoothing | Fitting | - | - | - | ||
| Parameters | Prostate (n = 15) | Liver (n = 14) | Breast (n = 15) | |
|---|---|---|---|---|
| Gender | Male | 15 (100%) | 9 (64%) | 0 (0%) |
| Female | 0 (0%) | 5 (36%) | 15 (100%) | |
| Age | 30~39 | 0 (0%) | 0 (0%) | 3 (20%) |
| 40~49 | 0 (0%) | 0 (0%) | 3 (20%) | |
| 50~59 | 2 (13%) | 1 (7%) | 5 (33%) | |
| 60~69 | 5 (33%) | 6 (43%) | 4 (27%) | |
| 70~79 | 8 (53%) | 5 (36%) | 0 (0%) | |
| 80~89 | 0 (0%) | 2 (14%) | 0 (0%) | |
| Stage | T0~Tis | 0 (0%) | 0 (0%) | 5 (33%) |
| T1~T2 | 6 (40%) | 7 (50%) | 10 (67%) | |
| T3~T4 | 9 (60%) | 7 (50%) | 0 (0%) | |
| Target location | Right (Breast) | - | - | 10 (67%) |
| Left (Breast) | - | - | 5 (33%) | |
| Treatment technique | Palliative RT | 0 (0%) | 6 (43%) | 0 (0%) |
| Postoperative RT (boost) | 0 (0%) | 0 (0%) | 15 (100%) | |
| Salvage RT | 15 (100%) | 8 (57%) | 0 (0%) | |
| Prescribed dose | Only 57.6 Gy or 57.6 Gy for target and 45.6 Gy for LN | 30 Gy~50 Gy | 10 Gy | |
| Number of fractions | 24 | 10 | 5 | |
| Total number of plans used in this study | 360 | 140 | 75 | |
| Gantry Angle | 0° | 270° | Mean | SD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field Size (cm2) | 2 × 2 | 3 × 3 | 5 × 5 | 10 × 10 | 15 × 15 | 20 × 20 | 22 × 22 | 40 × 22 | 50 × 22 | 2 × 2 | 3 × 3 | 5 × 5 | 10 × 10 | 16 × 16 | ||
| Model 1 | −0.9 | −2.4 | −1.0 | −0.8 | −1.1 | −1.0 | −1.2 | −0.8 | −0.5 | 1.1 | −2.9 | −0.4 | −0.8 | −1.9 | −1.0 | 0.9 |
| Model 2 | −0.9 | −2.4 | −1.0 | −0.8 | −1.1 | −1.0 | −1.3 | −0.8 | −0.5 | 2.0 | −1.7 | 0.8 | 1.2 | −0.1 | −0.5 | 1.2 |
| Model 3 | −1.0 | −2.6 | −1.2 | −0.9 | −1.2 | −1.1 | −1.3 | −0.9 | −0.5 | 4.3 | −0.9 | −0.2 | −1.9 | −4.0 | −1.0 | 1.8 |
| Model 4 | −0.9 | −2.4 | −1.0 | −0.8 | −1.1 | −1.0 | −1.3 | −0.8 | −0.5 | −40.8 | −38.4 | −52.3 | −72.8 | −73.7 | −20.6 | 28.7 |
| Model 5 | −0.9 | −2.5 | −1.1 | −0.8 | −1.1 | −1.0 | −1.3 | −0.8 | −0.5 | −39.8 | −11.0 | −38.1 | −44.9 | −38.8 | −13.0 | 18.2 |
| Model 6 | −10.3 | −10.4 | −9.2 | −8.7 | −9.2 | −9.0 | −9.2 | −9.0 | −8.7 | −10.2 | −10.1 | −7.9 | −7.8 | −9.1 | −9.2 | 0.8 |
| Site | Model | Average Dose Difference (%) | SD |
|---|---|---|---|
| Prostate | 1 | −0.71 | 1.0 |
| 2 | 0.04 | 1.0 | |
| 3 | −0.86 | 1.0 | |
| Liver | 1 | 0.91 | 0.9 |
| 2 | 1.43 | 1.0 | |
| 3 | 1.01 | 1.2 | |
| Breast | 1 | 3.50 | 1.2 |
| 2 | 4.25 | 1.3 | |
| 3 | 4.54 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.; Choi, Y.; Kim, J.W.; Lee, H. Dose Calculation Accuracy of Beam Models in RadCalc for a 1.5 T MR-Linac. Cancers 2024, 16, 526. https://doi.org/10.3390/cancers16030526
Sung J, Choi Y, Kim JW, Lee H. Dose Calculation Accuracy of Beam Models in RadCalc for a 1.5 T MR-Linac. Cancers. 2024; 16(3):526. https://doi.org/10.3390/cancers16030526
Chicago/Turabian StyleSung, Jiwon, Yeonho Choi, Jun Won Kim, and Ho Lee. 2024. "Dose Calculation Accuracy of Beam Models in RadCalc for a 1.5 T MR-Linac" Cancers 16, no. 3: 526. https://doi.org/10.3390/cancers16030526
APA StyleSung, J., Choi, Y., Kim, J. W., & Lee, H. (2024). Dose Calculation Accuracy of Beam Models in RadCalc for a 1.5 T MR-Linac. Cancers, 16(3), 526. https://doi.org/10.3390/cancers16030526







