Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Regulatory Mechanisms, Functions, and Therapeutic Implications
Simple Summary
Abstract
1. Introduction
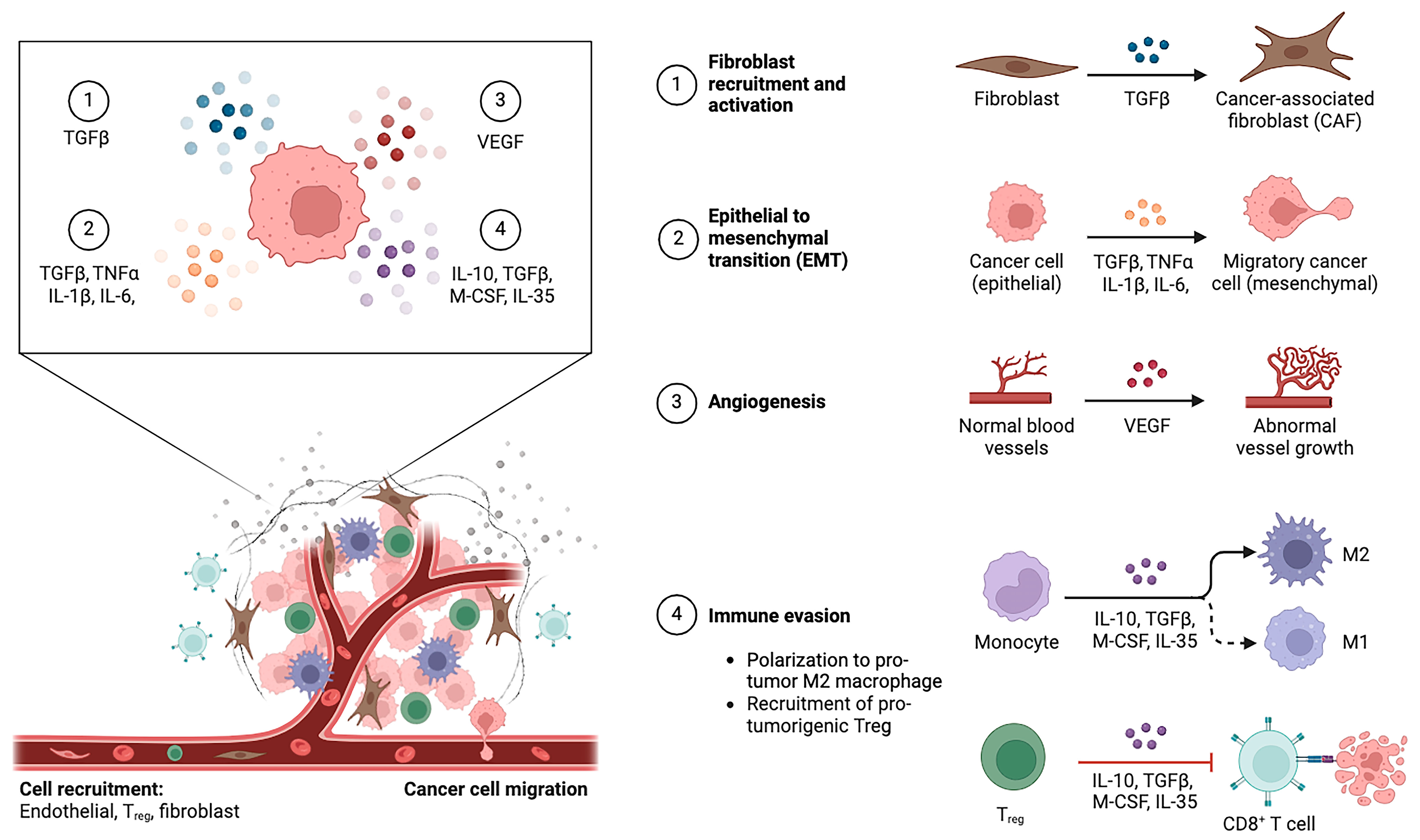
2. The Tumor Immune Microenvironment of Intrahepatic Cholangiocarcinoma
2.1. Cellular Elements of Tumor Immune Microenvironment
2.1.1. Cancer-Associated Fibroblasts
2.1.2. Tumor-Associated Macrophages
2.1.3. Tumor-Associated Neutrophils
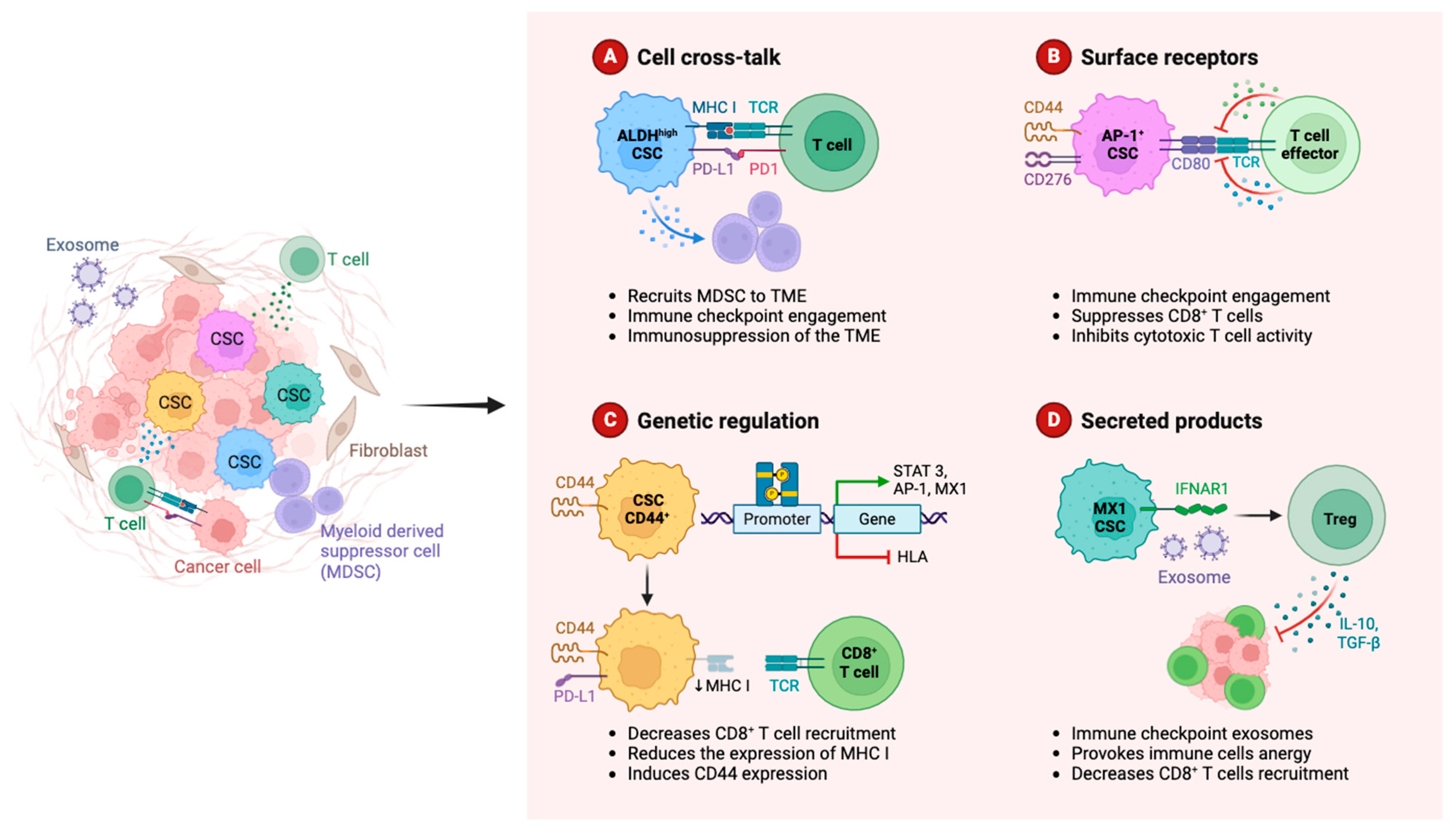
2.1.4. Myeloid-Derived Suppressor Cells
2.1.5. Natural Killers
2.1.6. Tumor-Infiltrating Lymphocytes (TILs)
2.1.7. Dendritic Cells
2.2. Non-Cellular Elements of Tumor Immune Microenvironment
2.2.1. Extracellular Matrix
2.2.2. Chemokines
2.2.3. Extracellular Vesicles
2.2.4. Platelet-Derived Growth Factor
2.2.5. Notch Signaling Pathway
3. Immune Evasion and Immunophenotyping in Intrahepatic Cholangiocarcinoma
4. Implications for Treatment and Future Research Avenues
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzo, A.; Brandi, G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: Reflections on a standard of care. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Sharman, R.; Shroff, R.T. Precision Medicine in Biliary Tract Cancer. J. Clin. Oncol. 2022, 40, 2716–2734. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e194–e203. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kwong, L.N.; Javle, M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr. Treat. Options Oncol. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Song, S.Y. Recent Advancement in Diagnosis of Biliary Tract Cancer through Pathological and Molecular Classifications. Cancers 2024, 16, 1761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guven, D.C.; Erul, E.; Kaygusuz, Y.; Akagunduz, B.; Kilickap, S.; De Luca, R.; Rizzo, A. Immune checkpoint inhibitor-related hearing loss: A systematic review and analysis of individual patient data. Support. Care Cancer 2023, 31, 624. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: A matter of debate. Br. J. Cancer 2022, 127, 1381–1382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, J.H.; Chan, L.C.; Song, M.S.; Hung, M.C. New Approaches on Cancer Immunotherapy. Cold Spring Harb. Perspect. Med. 2020, 10, a036863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, P.; Reck, M.; Overbeck, T.; Christopoulos, P.; Rittmeyer, A.; Lüders, H.; Kollmeier, J.; Kulhavy, J.; Kemper, M.; Reinmuth, N.; et al. Outcome of First-Line Treatment With Pembrolizumab According to KRAS/TP53 Mutational Status for Nonsquamous Programmed Death-Ligand 1-High (≥50%) NSCLC in the German National Network Genomic Medicine Lung Cancer. J. Thorac. Oncol. 2024, 19, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Mollica, V.; Massari, F. Expression of Programmed Cell Death Ligand 1 as a Predictive Biomarker in Metastatic Urothelial Carcinoma Patients Treated with First-line Immune Checkpoint Inhibitors Versus Chemotherapy: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2022, 8, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, D.; Liu, J.; Qiu, J.; Zhou, J.; Ying, J.; Shi, Y.; Wang, Z.; Lou, H.; Cui, J.; et al. Genomic alterations in biliary tract cancer predict prognosis and immunotherapy outcomes. J. Immunother. Cancer. 2021, 9, e003214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricci, A.D.; Rizzo, A.; Brandi, G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control 2020, 27, 1073274820948047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Queiroz, M.M.; Lima, N.F., Jr.; Biachi de Castria, T. Immunotherapy and Targeted Therapy for Advanced Biliary Tract Cancer: Adding New Flavors to the Pizza. Cancers 2023, 15, 1970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yue, S.; Zhang, Y.; Zhang, W. Recent Advances in Immunotherapy for Advanced Biliary Tract Cancer. Curr. Treat. Options Oncol. 2024, 25, 1089–1111. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Khalil, D.N.; Fabris, L.; Abou-Alfa, G.K. Rational development of combination therapies for biliary tract cancers. J. Hepatol. 2023, 78, 217–228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neureiter, D.; Mayr, C.; Kiesslich, T. The challenges of combinatory immunotherapy for biliary tract cancer. Expert. Opin. Investig. Drugs 2021, 30, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Carloni, R.; Rizzo, A.; Ricci, A.D.; Federico, A.D.; De Luca, R.; Guven, D.C.; Yalcin, S.; Brandi, G. Targeting tumor microenvironment for cholangiocarcinoma: Opportunities for precision medicine. Transl. Oncol. 2022, 25, 101514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kam, A.E.; Masood, A.; Shroff, R.T. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol. 2021, 6, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, B.; Wang, Y.; Zhang, X.; Jiang, H.; Han, F.; Li, C.; Lu, S. Identification and validation of inflammatory subtypes in intrahepatic cholangiocellular carcinoma. J. Transl. Med. 2024, 22, 730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 2022, 42, 1112–1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clapéron, A.; Mergey, M.; Aoudjehane, L.; Ho-Bouldoires, T.H.N.; Wendum, D.; Prignon, A.; Merabtene, F.; Firrincieli, D.; Desbois-Mouthon, C.; Scatton, O.; et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013, 58, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 333–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Chen, C.; Hu, W.; Tao, L.; Chen, J. Revealing the role of necroptosis microenvironment: FCGBP + tumor-associated macrophages drive primary liver cancer differentiation towards cHCC-CCA or iCCA. Apoptosis 2024, 29, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Serino, G.; Dituri, F.; Cigliano, A.; Ribback, S.; Wang, J.; Chen, X.; Calvisi, D.F.; Giannelli, G. Crenigacestat, a selective NOTCH1 inhibitor, reduces intrahepatic cholangiocarcinoma progression by blocking VEGFA/DLL4/MMP13 axis. Cell Death Differ. 2020, 27, 2330–2343. [Google Scholar] [CrossRef]
- Czekay, R.P.; Cheon, D.J.; Samarakoon, R.; Kutz, S.M.; Higgins, P.J. Cancer-Associated Fibroblasts: Mechanisms of Tumor Progression and Novel Therapeutic Targets. Cancers 2022, 14, 1231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancarella, S.; Serino, G.; Coletta, S.; Armentano, R.; Dituri, F.; Ardito, F.; Ruzzenente, A.; Fabregat, I.; Giannelli, G. The Tumor Microenvironment Drives Intrahepatic Cholangiocarcinoma Progression. Int. J. Mol. Sci. 2022, 23, 4187. [Google Scholar] [CrossRef]
- Khan, G.J.; Sun, L.; Khan, S.; Yuan, S.; Nongyue, H. Versatility of Cancer Associated Fibroblasts: Commendable Targets for Anti-tumor Therapy. Curr. Drug Targets 2018, 19, 1573–1588. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Cai, Q.; Chen, Y.; Shi, T.; Liu, W.; Mao, L.; Deng, B.; Ying, Z.; Gao, Y.; Luo, H.; et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology 2022, 75, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Perugorria, M.J.; Mertens, J.; Björkström, N.K.; Cramer, T.; Lleo, A.; Solinas, A.; Sänger, H.; Lukacs-Kornek, V.; Moncsek, A.; et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 63–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer. 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cencini, E.; Sicuranza, A.; Ciofini, S.; Fabbri, A.; Bocchia, M.; Gozzetti, A. Tumor-Associated Macrophages in Multiple Myeloma: Key Role in Disease Biology and Potential Therapeutic Implications. Curr. Oncol. 2023, 30, 6111–6133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Sun, Y.; Ma, Y.; Gao, C.; Zhang, Y.; Yang, X.; Zhao, X.; Wang, W.; Wang, L. Tumor-associated M2 macrophages in the immune microenvironment influence the progression of renal clear cell carcinoma by regulating M2 macrophage-associated genes. Front. Oncol. 2023, 13, 1157861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nam, S.J.; Kim, S.; Kwon, D.; Kim, H.; Kim, S.; Lee, E.; Kim, T.M.; Heo, D.S.; Park, S.H.; Lim, M.S.; et al. Prognostic implications of tumor-infiltrating macrophages, M2 macrophages, regulatory T-cells, and indoleamine 2,3-dioxygenase-positive cells in primary diffuse large B-cell lymphoma of the central nervous system. Oncoimmunology 2018, 7, e1442164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vadevoo, S.M.P.; Gunassekaran, G.R.; Yoo, J.D.; Kwon, T.H.; Hur, K.; Chae, S.; Lee, B. Epigenetic therapy reprograms M2-type tumor-associated macrophages into an M1-like phenotype by upregulating miR-7083-5p. Front. Immunol. 2022, 13, 976196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, C.; Xin, H.; Zhou, Z.; Hu, Z.; Sun, R.; Yao, N.; Sun, Q.; Borjigin, U.; Wu, X.; Fan, J.; et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 2022, 76, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, P.; Sun, R.; Li, J.; Hu, Z.; Xin, H.; Luo, C.; Zhou, J.; Fan, J.; Zhou, S. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J. Immunother. Cancer 2021, 9, e001946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, M.H.; Oh, E.; Kim, Y.; Nam, D.H.; Jeon, S.Y.; Yu, J.H.; Minn, D. Recent Advances in CAR-Based Solid Tumor Immunotherapy. Cells 2023, 12, 1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Wu, Y.; Hong, J.; Zhang, M. The prognostic significance of tumor-associated neutrophils and circulating neutrophils in glioblastoma (WHO CNS5 classification). BMC Cancer 2023, 23, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Bo, X.; Suo, T.; Liu, H.; Ni, X.; Shen, S.; Li, M.; Xu, J.; Liu, H.; Wang, Y. Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018, 109, 2266–2274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y.; Wei, Y.; Wang, Z.; Jing, Y.; He, H.; Yuan, J.; Li, R.; Zhao, Q.; Wei, L.; Yang, T.; et al. TGF-β regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell. Physiol. Biochem. 2015, 35, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.A.; Reijrink, M.; Abdulahad, W.H.; Hoekstra, E.S.; Slart, R.H.J.A.; Heerspink, H.J.L.; Westra, J.; Mulder, D.J. Angiogenic T cells are decreased in people with type 2 diabetes mellitus and recruited by the dipeptidyl peptidase-4 inhibitor Linagliptin: A subanalysis from a randomized, placebo-controlled trial (RELEASE study). Diabetes Obes. Metab. 2020, 22, 1220–1225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barcellos-Hoff, M.H.; Gulley, J.L. Molecular Pathways and Mechanisms of TGFβ in Cancer Therapy. Clin. Cancer Res. 2023, 29, 2025–2033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Du, J.; Shen, X. Targeting myeloid-derived suppressor cells in tumor immunotherapy: Current, future and beyond. Front. Immunol. 2023, 14, 1157537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrand-Rosenberg, S.; Lamb, T.J.; Pawelec, G. Here, There, and Everywhere: Myeloid-Derived Suppressor Cells in Immunology. J. Immunol. 2023, 210, 1183–1197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballbach, M.; Dannert, A.; Singh, A.; Siegmund, D.M.; Handgretinger, R.; Piali, L.; Rieber, N.; Hartl, D. Expression of checkpoint molecules on myeloid-derived suppressor cells. Immunol. Lett. 2017, 192, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Dongye, S.; Liu, X.; Xu, X.; Wang, L.; Jin, C.Q.; Yao, M.; Gong, Z.; Jiang, D.; Zhang, K.; et al. Immunotherapy of targeting MDSCs in tumor microenvironment. Front. Immunol. 2022, 13, 990463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, M.; Ramirez, M.E.; Sierra, R.A.; Raber, P.; Thevenot, P.; Al-Khami, A.A.; Sanchez-Pino, D.; Hernandez, C.; Wyczechowska, D.D.; Ochoa, A.C.; et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 2015, 75, 275–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayik, D.; Lauko, A.J.; Roversi, G.A.; Serbinowski, E.; Acevedo-Moreno, L.A.; Lanigan, C.; Orujov, M.; Lo, A.; Alban, T.J.; Kim, A.; et al. Hepatobiliary malignancies have distinct peripheral myeloid-derived suppressor cell signatures and tumor myeloid cell profiles. Sci. Rep. 2020, 10, 18848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rose, P.; van den Engel, N.K.; Kovács, J.R.; Hatz, R.A.; Boon, L.; Winter, H. Anti-Gr-1 Antibody Provides Short-Term Depletion of MDSC in Lymphodepleted Mice with Active-Specific Melanoma Therapy. Vaccines 2022, 10, 560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrova, V.; Groth, C.; Bitsch, R.; Arkhypov, I.; Simon, S.C.S.; Hetjens, S.; Müller, V.; Utikal, J.; Umansky, V. Immunosuppressive capacity of circulating MDSC predicts response to immune checkpoint inhibitors in melanoma patients. Front. Immunol. 2023, 14, 1065767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, H.K.; Pore, N.; Michelotti, E.F.; Walcheck, B. Anti-ADAM17 monoclonal antibody MEDI3622 increases IFNγ production by human NK cells in the presence of antibody-bound tumor cells. Cancer Immunol. Immunother. 2018, 67, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fahrner, R.; Trochsler, M.; Corazza, N.; Graubardt, N.; Keogh, A.; Candinas, D.; Brunner, T.; Stroka, D.; Beldi, G. Tumor necrosis factor-related apoptosis-inducing ligand on NK cells protects from hepatic ischemia-reperfusion injury. Transplantation 2014, 97, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Dianat-Moghadam, H.; Mahari, A.; Heidarifard, M.; Parnianfard, N.; Pourmousavi-Kh, L.; Rahbarghazi, R.; Amoozgar, Z. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021, 497, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Pietra, G.; Tumino, N.; Munari, E.; Mingari, M.C.; Moretta, L. Exploiting Human NK Cells in Tumor Therapy. Front. Immunol. 2020, 10, 3013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, N.; Kim, H.S. Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells. Front. Immunol. 2018, 9, 2041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, X.; Ning, W.; Liu, H.; Liu, X.; Luo, W.; Xia, N. Stepping forward: T-cell redirecting bispecific antibodies in cancer therapy. Acta Pharm. Sin. B 2024, 14, 2361–2377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsukagoshi, M.; Wada, S.; Yokobori, T.; Altan, B.; Ishii, N.; Watanabe, A.; Kubo, N.; Saito, F.; Araki, K.; Suzuki, H.; et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci. 2016, 107, 116–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vogler, M.; Shanmugalingam, S.; Särchen, V.; Reindl, L.M.; Grèze, V.; Buchinger, L.; Kühn, M.; Ullrich, E. Unleashing the power of NK cells in anticancer immunotherapy. J. Mol. Med. 2022, 100, 337–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panwong, S.; Wathikthinnakon, M.; Kaewkod, T.; Sawasdee, N.; Tragoolpua, Y.; Yenchitsomanus, P.T.; Panya, A. Cordycepin Sensitizes Cholangiocarcinoma Cells to Be Killed by Natural Killer-92 (NK-92) Cells. Molecules 2021, 26, 5973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiawpanit, C.; Wathikthinnakorn, M.; Sawasdee, N.; Phanthaphol, N.; Sujjitjoon, J.; Junking, M.; Yamabhai, M.; Panaampon, J.; Yenchitsomanus, P.T.; Panya, A. Precision immunotherapy for cholangiocarcinoma: Pioneering the use of human-derived anti-cMET single chain variable fragment in anti-cMET chimeric antigen receptor (CAR) NK cells. Int. Immunopharmacol. 2024, 136, 112273. [Google Scholar] [CrossRef] [PubMed]
- Li, B. Why do tumor-infiltrating lymphocytes have variable efficacy in the treatment of solid tumors? Front. Immunol. 2022, 13, 973881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levi, S.T.; Copeland, A.R.; Nah, S.; Crystal, J.S.; Ivey, G.D.; Lalani, A.; Jafferji, M.; White, B.S.; Parikh, N.B.; Leko, V.; et al. Neoantigen Identification and Response to Adoptive Cell Transfer in Anti-PD-1 Naïve and Experienced Patients with Metastatic Melanoma. Clin. Cancer Res. 2022, 28, 3042–3052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalili-Tanha, G.; Fiuji, H.; Gharib, M.; Moghbeli, M.; Khalili-Tanha, N.; Rahmani, F.; Shakour, N.; Maftooh, M.; Hassanian, S.M.; Asgharzadeh, F.; et al. Dual targeting of TGF-β and PD-L1 inhibits tumor growth in TGF-β/PD-L1-driven colorectal carcinoma. Life Sci. 2023, 328, 121865. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Watkins, R.; Vilgelm, A.E. Cell Therapy With TILs: Training and Taming T Cells to Fight Cancer. Front. Immunol. 2021, 12, 690499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer. 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Tian, Q.; Wang, B.Y.; Yang, J.; Zhao, S.D.; Yang, J. The prognostic significance of TILs as a biomarker in triple-negative breast cancer: What is the role of TILs in TME of TNBC? Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2885–2897. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Salazar, R.; Gruber, S.B. The prognostic value of TILs in stage III colon cancer must consider sidedness. Ann. Oncol. 2022, 33, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiawpanit, C.; Panwong, S.; Sawasdee, N.; Yenchitsomanus, P.T.; Panya, A. Genistein Sensitizes Human Cholangiocarcinoma Cell Lines to Be Susceptible to Natural Killer Cells. Biology 2022, 11, 1098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paillet, J.; Kroemer, G.; Pol, J.G. Immune contexture of cholangiocarcinoma. Curr. Opin. Gastroenterol. 2020, 36, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Wan, L.; Wang, Z.; Wang, H.; Ge, C.; Liu, Y.; Hao, Y.; Zhang, D.; Shi, G.; et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.; Ko, M.; Won, J.Y.; Jo, Y.; Park, E.; Kim, H.; Choi, E.; Jung, U.J.; Jeon, J.; Kim, Y.; et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol. Ther. 2022, 30, 2800–2816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diggs, L.P.; Ruf, B.; Ma, C.; Heinrich, B.; Cui, L.; Zhang, Q.; McVey, J.C.; Wabitsch, S.; Heinrich, S.; Rosato, U.; et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 74, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z.Q.; Zhou, Z.J.; Luo, C.B.; Xin, H.Y.; Li, J.; Yu, S.Y.; Zhou, S.L. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int. 2020, 20, 582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, J.; Dai, Y.; Sang, C.; Song, G.; Xiang, B.; Zhang, M.; Dong, L.; Xia, X.; Ma, J.; Shen, X.; et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities. J. Immunother. Cancer. 2022, 10, e004892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, B.Y.; Wang, Z.T.; Chen, K.Z.; Song, Y.; Wu, J.F.; Zhang, D.; Sun, G.Q.; Zhou, J.; Fan, J.; Hu, B.; et al. Mobilization and activation of tumor-infiltrating dendritic cells inhibits lymph node metastasis in intrahepatic cholangiocarcinoma. Cell Death Discov. 2024, 10, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bryant, C.E.; Sutherland, S.; Kong, B.; Papadimitrious, M.S.; Fromm, P.D.; Hart, D.N.J. Dendritic cells as cancer therapeutics. Semin. Cell Dev. Biol. 2019, 86, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Radford, K.J. The role of dendritic cells in cancer. Int. Rev. Cell Mol. Biol. 2019, 348, 123–178. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Butterfield, L.H. Dendritic Cell-Based Cancer Vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Barnoud, C.; Cenerenti, M.; Sun, M.; Caffa, I.; Kizil, B.; Bill, R.; Liu, Y.; Pick, R.; Garnier, L.; et al. Dendritic cells direct circadian anti-tumour immune responses. Nature 2023, 614, 136–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzo, A.; Santoni, M.; Mollica, V.; Fiorentino, M.; Brandi, G.; Massari, F. Microbiota and prostate cancer. Semin. Cancer Biol. 2022, 86 Pt 3, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, L.; Kroemer, G.; Kepp, O. Conventional type 1 dendritic cells (cDC1) in cancer immunity. Biol. Direct. 2023, 18, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Junking, M.; Grainok, J.; Thepmalee, C.; Wongkham, S.; Yenchitsomanus, P.T. Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biol. 2017, 39, 1010428317733367. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Thepmalee, C.; Sawasdee, N.; Sujjitjoon, J.; Phanthaphol, N.; Junking, M.; Wongkham, S.; Yenchitsomanus, P.T. Cytotoxic activity of effector T cells against cholangiocarcinoma is enhanced by self-differentiated monocyte-derived dendritic cells. Cancer Immunol. Immunother. 2018, 67, 1579–1588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minini, M.; Fouassier, L. Cancer-Associated Fibroblasts and Extracellular Matrix: Therapeutical Strategies for Modulating the Cholangiocarcinoma Microenvironment. Curr. Oncol. 2023, 30, 4185–4196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantallops Vilà, P.; Ravichandra, A.; Agirre Lizaso, A.; Perugorria, M.J.; Affò, S. Heterogeneity, crosstalk, and targeting of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2024, 79, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Giguelay, A.; Turtoi, E.; Khelaf, L.; Tosato, G.; Dadi, I.; Chastel, T.; Poul, M.A.; Pratlong, M.; Nicolescu, S.; Severac, D.; et al. The landscape of cancer-associated fibroblasts in colorectal cancer liver metastases. Theranostics 2022, 12, 7624–7639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carpino, G.; Overi, D.; Melandro, F.; Grimaldi, A.; Cardinale, V.; Di Matteo, S.; Mennini, G.; Rossi, M.; Alvaro, D.; Barnaba, V.; et al. Matrisome analysis of intrahepatic cholangiocarcinoma unveils a peculiar cancer-associated extracellular matrix structure. Clin. Proteom. 2019, 16, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Tienderen, G.S.; Rosmark, O.; Lieshout, R.; Willemse, J.; de Weijer, F.; Elowsson Rendin, L.; Westergren-Thorsson, G.; Doukas, M.; Groot Koerkamp, B.; van Royen, M.E.; et al. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023, 158, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Ying, F.; Chan, M.S.M.; Lee, T.K.W. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma and Cholangiocarcinoma. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 985–999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, X.; Zhu, J.; Liu, W.; Peng, L.; Lu, C.; Sun, P.; Huang, L.; Nie, X.; Huang, S.; Guo, T.; et al. Proteome Landscapes of Human Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Mol. Cell. Proteom. 2023, 22, 100604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, C.; Ma, J.; Zhu, K.; Yu, L.; Zheng, B.; Rao, D.; Zhang, S.; Dong, L.; Gao, Q.; Zhang, X.; et al. Spatial immunophenotypes predict clinical outcome in intrahepatic cholangiocarcinoma. JHEP Rep. 2023, 5, 100762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Märkl, F.; Huynh, D.; Endres, S.; Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer 2022, 8, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drouillard, D.; Craig, B.T.; Dwinell, M.B. Physiology of chemokines in the cancer microenvironment. Am. J. Physiol. Cell Physiol. 2023, 324, C167–C182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masih, M.; Agarwal, S.; Kaur, R.; Gautam, P.K. Role of chemokines in breast cancer. Cytokine 2022, 155, 155909. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, J.; Wang, Y.; Xue, Y.; Yang, Q.; Yang, Q.; Zhao, H.; Huang, J.; Peng, X. Chemokines: Function and therapeutic potential in bone metastasis of lung cancer. Cytokine 2023, 172, 156403. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, U.; Mushtaq, U.; Mir, M.A.; Saleem, A.; Macha, M.A.; Lone, M.N.; Hamid, A.; Zargar, M.A.; Ahmad, S.M.; Wani, N.A. Chemokines in triple-negative breast cancer heterogeneity: New challenges for clinical implications. Semin. Cancer Biol. 2022, 86 Pt 2, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Yamashita, Y.I.; Yoshizumi, T.; Shiraishi, M.; Ohta, M.; Eguchi, S.; Aishima, S.; Fujioka, H.; Baba, H. CXCL12 expression in intrahepatic cholangiocarcinoma is associated with metastasis and poor prognosis. Cancer Sci. 2019, 110, 3197–3203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caligiuri, A.; Pastore, M.; Lori, G.; Raggi, C.; Di Maira, G.; Marra, F.; Gentilini, A. Role of Chemokines in the Biology of Cholangiocarcinoma. Cancers 2020, 12, 2215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, e2005709. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, F.; Feng, S.; Yang, H.; Mao, Y. Extracellular vesicles in hepatocellular cancer and cholangiocarcinoma. Ann. Transl. Med. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Vasileiadi, S.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Papageorgiou, E.G.; et al. The Arising Role of Extracellular Vesicles in Cholangiocarcinoma: A Rundown of the Current Knowledge Regarding Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 15563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, N.; Shu, L.; Liu, Z.; Shi, A.; Zhao, L.; Huang, S.; Sheng, G.; Yan, Z.; Song, Y.; Huang, F.; et al. The role of extracellular vesicles in cholangiocarcinoma tumor microenvironment. Front. Pharmacol. 2024, 14, 1336685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Shi, R.; Yin, Y.; Luo, H.; Cao, Y.; Lyu, Y.; Luo, H.; Zeng, X.; Wang, D. Clinical significance of small extracellular vesicles in cholangiocarcinoma. Front. Oncol. 2024, 14, 1334592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, L.; Hong, J.; Wu, J. Potential of extracellular vesicles and exosomes as diagnostic markers for cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 436–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gradilone, S.A. Extracellular vesicles as therapeutic carriers of microRNAs for cholangiocarcinoma. Hepatology 2017, 65, 404–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, X.; Tang, X.Y.; Qu, Z.Y.; Sun, Z.W.; Ji, C.F.; Li, Y.J.; Guo, S.D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Turrell, F.K.; Orha, R.; Guppy, N.J.; Gillespie, A.; Guelbert, M.; Starling, C.; Haider, S.; Isacke, C.M. Age-associated microenvironmental changes highlight the role of PDGF-C in ER+ breast cancer metastatic relapse. Nat. Cancer 2023, 4, 468–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaps, L.; Schuppan, D. Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, D.; Gimple, R.C.; Zhong, C.; Wu, Q.; Yang, K.; Prager, B.C.; Godugu, B.; Qiu, Z.; Zhao, L.; Zhang, G.; et al. PDGF signaling inhibits mitophagy in glioblastoma stem cells through N6-methyladenosine. Dev. Cell 2022, 57, 1466–1481.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cadamuro, M.; Brivio, S.; Mertens, J.; Vismara, M.; Moncsek, A.; Milani, C.; Fingas, C.; Cristina Malerba, M.; Nardo, G.; Dall’Olmo, L.; et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J. Hepatol. 2019, 70, 700–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, J.; Xiao, G.; Yang, C.; Liu, Q.; Lv, C.; Yu, X.; Zhou, Z.; Lin, S.; Bai, Z.; Lin, H.; et al. Cancer-Associated Fibroblasts Promote Lymphatic Metastasis in Cholangiocarcinoma via the PDGF-BB/PDGFR-β Mediated Paracrine Signaling Network. Aging Dis. 2024, 15, 369–389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, A.; Liu, Z.; Fan, Z.; Li, K.; Liu, X.; Tang, Y.; Hu, J.; Li, X.; Shu, L.; Zhao, L.; et al. Function of mast cell and bile-cholangiocarcinoma interplay in cholangiocarcinoma microenvironment. Gut 2024, 73, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.F. Imatinib Mesylate. Recent Results Cancer Res. 2018, 212, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Luo, Y.; Liu, Y.C.; Chen, Y.H.; Li, Y.T.; Hu, X.Y.; You, X.Y.; Yu, S.S.; Li, Z.Y.; Chen, L.; et al. Imatinib inhibits pericyte-fibroblast transition and inflammation and promotes axon regeneration by blocking the PDGF-BB/PDGFRβ pathway in spinal cord injury. Inflamm. Regen. 2022, 42, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, B.; Malato, Y.; Calvisi, D.F.; Naqvi, S.; Razumilava, N.; Ribback, S.; Gores, G.J.; Dombrowski, F.; Evert, M.; Chen, X.; et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Invest. 2012, 122, 2911–2915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Serino, G.; Gigante, I.; Cigliano, A.; Ribback, S.; Sanese, P.; Grossi, V.; Simone, C.; Armentano, R.; Evert, M.; et al. CD90 is regulated by notch1 and hallmarks a more aggressive intrahepatic cholangiocarcinoma phenotype. J. Exp. Clin. Cancer Res. 2022, 41, 65, Erratum in J. Exp. Clin. Cancer Res. 2023, 42, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancarella, S.; Gigante, I.; Serino, G.; Pizzuto, E.; Dituri, F.; Valentini, M.F.; Wang, J.; Chen, X.; Armentano, R.; Calvisi, D.F.; et al. Crenigacestat blocking notch pathway reduces liver fibrosis in the surrounding ecosystem of intrahepatic CCA viaTGF-β inhibition. J. Exp. Clin. Cancer Res. 2022, 41, 331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, R.; Wang, D.; Han, S.; Gu, Y.; Li, Z.; Deng, L.; Yin, A.; Gao, Y.; Li, X.; Yu, Y.; et al. MiR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAFs. Int. J. Biol. Sci. 2022, 18, 43–64, Erratum in Int. J. Biol. Sci. 2022, 18, 4466–4467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Peng, L.; Dong, L.; Liu, D.; Ma, J.; Lin, J.; Chen, X.; Lin, P.; Song, G.; Zhang, M.; et al. Geospatial Immune Heterogeneity Reflects the Diverse Tumor-Immune Interactions in Intrahepatic Cholangiocarcinoma. Cancer Discov. 2022, 12, 2350–2371. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.L.; Valle, J.W.; Ilyas, S.I. Immunobiology of cholangiocarcinoma. J. Hepatol. 2023, 79, 867–875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, X.; Ji, C.; Ren, D.; Ke, A.; Yang, Z. Circular RNA circEIF3C promotes intrahepatic cholangiocarcinoma progression and immune evasion via the miR-34a-5p/B7-H4 axis. Genes Dis. 2022, 10, 370–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mauro, E.; Forner, A. Immunotherapy in biliary tract cancer: The race has begun! Liver Int. 2023, 43, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Gani, F.; Nagarajan, N.; Kim, Y.; Zhu, Q.; Luan, L.; Bhaijjee, F.; Anders, R.A.; Pawlik, T.M. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2016, 23, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yang, S.; Wang, S.; Wu, J.; Zheng, B.; Wang, K.; Shen, S.; Jeong, S.; Li, Z.; Zhu, Y.; et al. M6A Demethylase ALKBH5 Regulates PD-L1 Expression and Tumor Immunoenvironment in Intrahepatic Cholangiocarcinoma. Cancer Res. 2021, 81, 4778–4793. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Hwang, H.; Kim, Y.H.; Yoo, B.C.; Han, N.; Kong, S.Y.; Baek, M.J.; Kim, K.H.; Lee, M.R.; Park, J.G.; et al. Refining Classification of Cholangiocarcinoma Subtypes via Proteogenomic Integration Reveals New Therapeutic Prospects. Gastroenterology 2023, 164, 1293–1309, Erratum in Gastroenterology 2023, 165, 1313. [Google Scholar] [CrossRef] [PubMed]
- Wardell, C.P.; Fujita, M.; Yamada, T.; Simbolo, M.; Fassan, M.; Karlic, R.; Polak, P.; Kim, J.; Hatanaka, Y.; Maejima, K.; et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 2018, 68, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Fu, F.; Wang, J.; Dong, C. Definition of immune molecular subtypes with distinct immune microenvironment, recurrence, and PANoptosis features to aid clinical therapeutic decision-making. Front. Genet. 2022, 13, 1007108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 2022, 40, 70–87.e15. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Chen, X.; Wang, H.; Lin, Y.; Chen, L.; Yuan, K.; Liao, M.; Jiang, H.; Peng, J.; Wu, Z.; et al. Whole-Genome DNA Methylation Profiling of Intrahepatic Cholangiocarcinoma Reveals Prognostic Subtypes with Distinct Biological Drivers. Cancer Res. 2024, 84, 1747–1763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Ma, Z.; Li, C.; Wang, C.; Jiang, W.; Chang, J.; Han, S.; Lu, Z.; Shao, Z.; Wang, Y.; et al. The genomic landscape of cholangiocarcinoma reveals the disruption of post-transcriptional modifiers. Nat. Commun. 2022, 13, 3061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahipal, A.; Tella, S.H.; Kommalapati, A.; Anaya, D.; Kim, R. FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat. Rev. 2019, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Ran, P.; Chen, L.; Wang, Y.; Yu, Z.; Cai, K.; Feng, J.; Qin, Z.; Yin, Y.; Tan, S.; et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology 2023, 77, 411–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208, Erratum in J. Hepatol. 2023, 79, 1342. [CrossRef] [PubMed]
- Kitagawa, A.; Osawa, T.; Noda, M.; Kobayashi, Y.; Aki, S.; Nakano, Y.; Saito, T.; Shimizu, D.; Komatsu, H.; Sugaya, M.; et al. Convergent genomic diversity and novel BCAA metabolism in intrahepatic cholangiocarcinoma. Br. J. Cancer. 2023, 128, 2206–2217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning cold tumors hot: From molecular mechanisms to clinical applications. Trends Immunol. 2022, 43, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greten, T.F.; Schwabe, R.; Bardeesy, N.; Ma, L.; Goyal, L.; Kelley, R.K.; Wang, X.W. Immunology and immunotherapy of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; Rizzo, A.; Brandi, G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): A new Pandora’s box? ESMO Open 2020, 5, e001042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Rajabi, R.; Sun, W. Immunotherapy in cholangiocarcinoma. Curr. Opin. Gastroenterol. 2021, 37, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Jiang, T.; Lu, T.; Wang, H.; Cui, X.; Pan, Y.; Xu, M.; Pei, M.; Ding, Z.; Feng, X.; et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front. Immunol. 2023, 14, 1166497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 527–536. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.D.; Rizzo, A.; Schirizzi, A.; D’Alessandro, R.; Frega, G.; Brandi, G.; Shahini, E.; Cozzolongo, R.; Lotesoriere, C.; Giannelli, G. Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Regulatory Mechanisms, Functions, and Therapeutic Implications. Cancers 2024, 16, 3542. https://doi.org/10.3390/cancers16203542
Ricci AD, Rizzo A, Schirizzi A, D’Alessandro R, Frega G, Brandi G, Shahini E, Cozzolongo R, Lotesoriere C, Giannelli G. Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Regulatory Mechanisms, Functions, and Therapeutic Implications. Cancers. 2024; 16(20):3542. https://doi.org/10.3390/cancers16203542
Chicago/Turabian StyleRicci, Angela Dalia, Alessandro Rizzo, Annalisa Schirizzi, Rosalba D’Alessandro, Giorgio Frega, Giovanni Brandi, Endrit Shahini, Raffaele Cozzolongo, Claudio Lotesoriere, and Gianluigi Giannelli. 2024. "Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Regulatory Mechanisms, Functions, and Therapeutic Implications" Cancers 16, no. 20: 3542. https://doi.org/10.3390/cancers16203542
APA StyleRicci, A. D., Rizzo, A., Schirizzi, A., D’Alessandro, R., Frega, G., Brandi, G., Shahini, E., Cozzolongo, R., Lotesoriere, C., & Giannelli, G. (2024). Tumor Immune Microenvironment in Intrahepatic Cholangiocarcinoma: Regulatory Mechanisms, Functions, and Therapeutic Implications. Cancers, 16(20), 3542. https://doi.org/10.3390/cancers16203542








