An Early Increase in IL-10 and TNF-α Levels Following Atezolizumab Plus Bevacizumab Treatment Predicts Survival in Advanced Hepatocellular Carcinoma Patients: A Prospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
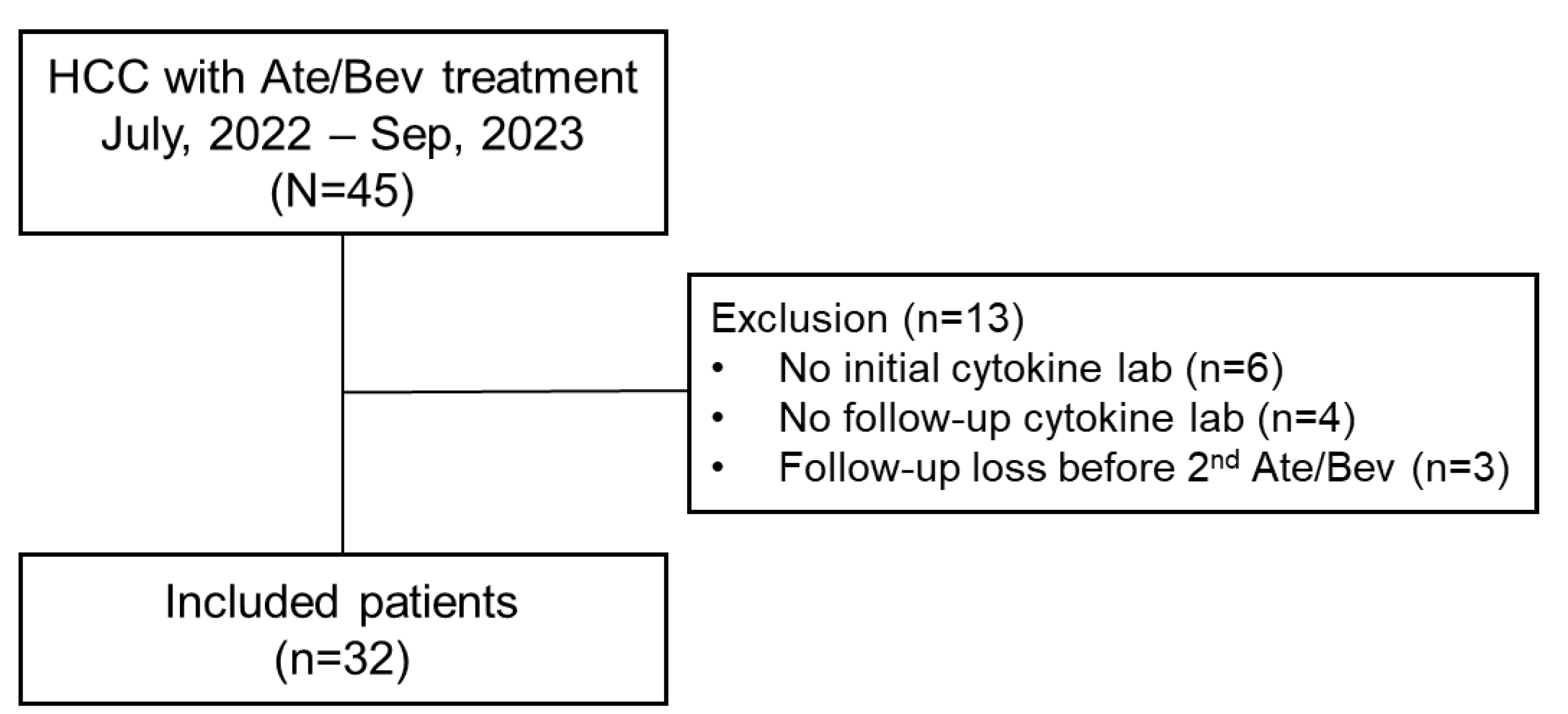
2.1. Patients
2.2. Clinical and Laboratory Data
2.3. Cytokine Analysis
2.4. Outcomes Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. OS According to Changes in Cytokine Levels
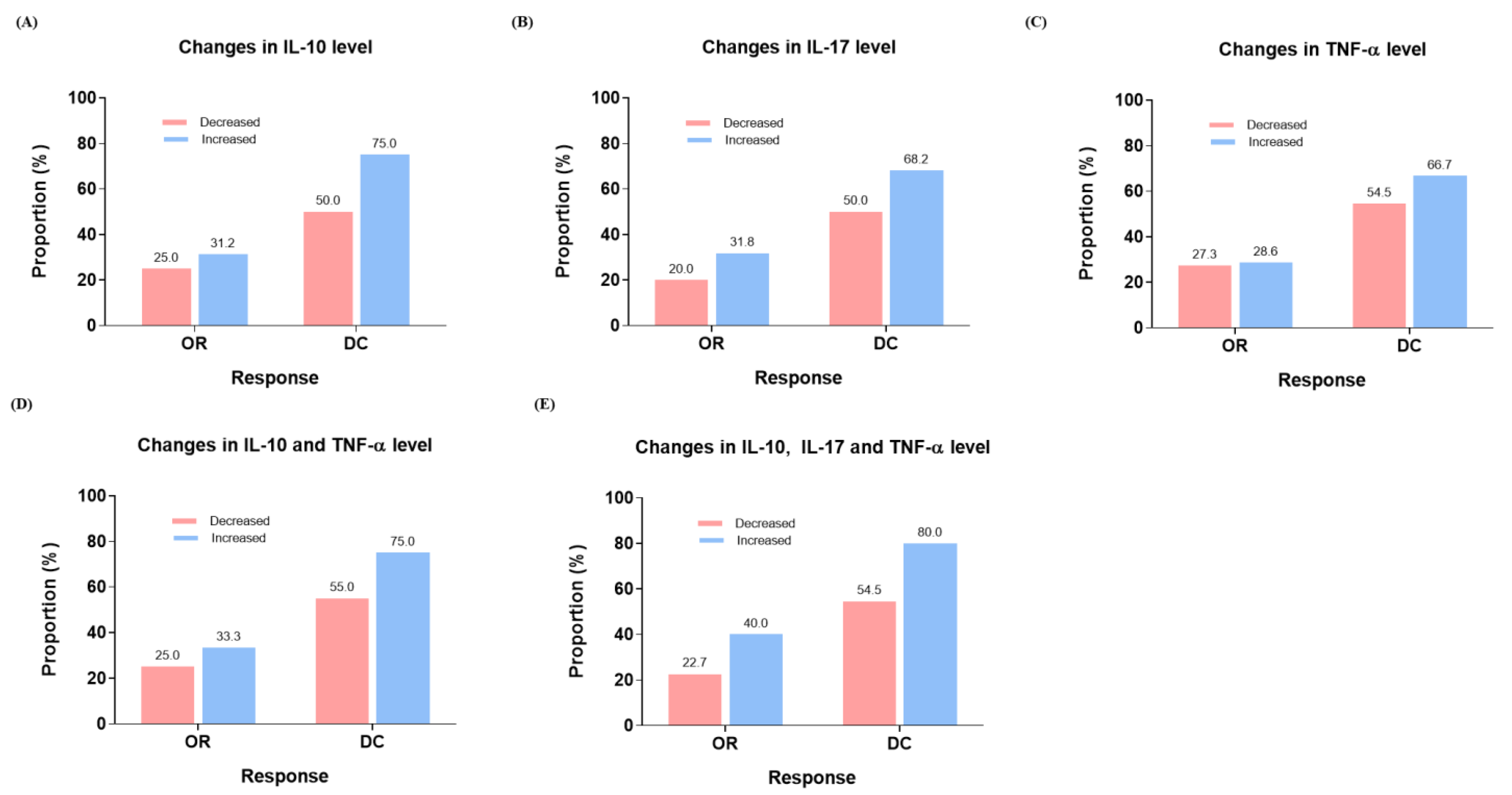
3.3. PFS at 6 Months and Treatment Response According to Changes in Cytokine Levels
3.4. Correlation Between Cytokine Levels
3.5. OS and PFS at 6 Months According to Combination of Cytokine Level Changes
3.6. Predicting Factors for OS Including Changes in Cytokine Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Ahn, J.C.; Tran, N.H.; Yang, J.D. Systemic therapy in advanced hepatocellular carcinoma. Clin. Mol. Hepatol. 2023, 29, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2021, 70, 204–214. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.H.; He, A.R.; Ryoo, B.Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Rico Montanari, N.; Anugwom, C.M.; Boonstra, A.; Debes, J.D. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers 2021, 13, 4876. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lee, S.K.; Jhun, J.; Lee, S.Y.; Choi, S.; Choi, S.S.; Park, M.S.; Lee, S.Y.; Cho, K.H.; Lee, A.R.; Ahn, J.; et al. A decrease in functional microbiomes represented as Faecalibacterium affects immune homeostasis in long-term stable liver transplant patients. Gut Microbes 2022, 14, 2102885. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, R.; Wang, Y.; Jiao, M.; Li, Z.; Wang, Z.; Huang, C.; Shi, G.; Ke, A.; Wang, L.; et al. Ten-eleven translocation-2 inactivation restrains IL-10-producing regulatory B cells to enable antitumor immunity in hepatocellular carcinoma. Hepatology 2023, 77, 745–759. [Google Scholar] [CrossRef]
- El Shahawy, A.A.; Gawish, A.A.; Meawed, T.E.; Ahmed, N.M.; Ahmed, A.A.; Abdelhadi, A.A. Interleukin-10 as a marker for response to dendritic cells-dribbles immunotherapy in hepatocellular carcinoma, a mice model. Egypt. J. Immunol. 2024, 31, 123–130. [Google Scholar] [PubMed]
- Shakiba, E.; Ramezani, M.; Sadeghi, M. Evaluation of serum interleukin-10 levels in hepatocellular carcinoma patients: A systematic review and meta-analysis. Clin. Exp. Hepatol. 2018, 4, 35–40. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, M.; Xu, Y.; He, G.; Liu, Q.; Zhu, J.; Zhang, C.; Zhang, X. IL-10 promoter hypomethylation is associated with increased IL-10 expression and poor survival in hepatocellular carcinoma. Transl. Cancer Res. 2019, 8, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Mo, F.K.; Wong, C.S.; Chan, C.M.; Leung, L.K.; Hui, E.P.; Ma, B.B.; Chan, A.T.; Mok, T.S.; Yeo, W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 2012, 118, 3984–3992. [Google Scholar] [CrossRef]
- Chau, G.Y.; Wu, C.W.; Lui, W.Y.; Chang, T.J.; Kao, H.L.; Wu, L.H.; King, K.L.; Loong, C.C.; Hsia, C.Y.; Chi, C.W. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann. Surg. 2000, 231, 552–558. [Google Scholar] [CrossRef]
- Khan, K.A.; Kerbel, R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 2018, 15, 310–324. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, X.; Wei, H.; Sun, R.; Chen, Y.; Tian, Z. Blockade of T-cell receptor with Ig and ITIM domains elicits potent antitumor immunity in naturally occurring HBV-related HCC in mice. Hepatology 2023, 77, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.Y.; Lee, S.H.; Baek, S.W.; Sohn, B.; Jeong, Y.S.; Kang, S.H.; Park, K.; Park, H.; Lee, S.S.; Kaseb, A.O.; et al. Genomic Biomarkers to Predict Response to Atezolizumab Plus Bevacizumab Immunotherapy in Hepatocellular Carcinoma: Insights from the IMbrave150 Trial. Clin. Mol. Hepatol. 2024, 30, 807–823. [Google Scholar] [CrossRef]
- Han, J.W.; Kang, M.W.; Lee, S.K.; Yang, H.; Kim, J.H.; Yoo, J.-S.; Cho, H.S.; Jang, E.J.; Seo, D.H.; Kwon, J.H.; et al. Dynamic Peripheral T-Cell Analysis Identifies On-Treatment Prognostic Biomarkers of Atezolizumab plus Bevacizumab in Hepatocellular Carcinoma. Liver Cancer 2024, 1–3. [Google Scholar] [CrossRef]
- Lee, S.K.; Choi, J.Y.; Jung, E.S.; Kwon, J.H.; Jang, J.W.; Bae, S.H.; Yoon, S.K. An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 5002. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Toan, B.N.; Tan, C.K.; Hasan, I.; Setiawan, L.; Yu, M.L.; Izumi, N.; Huyen, N.N.; Chow, P.K.; Mohamed, R.; et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin. Mol. Hepatol. 2023, 29, 277–292. [Google Scholar] [CrossRef] [PubMed]



| Variables | Total (n = 32) |
|---|---|
| Age, years | 64.2 ± 9.5 |
| Male | 30 (93.8%) |
| Cause (n,%) | |
| HBV | 24 (75.0%) |
| Others | 8 (25.0%) |
| AST, IU/mL | 46.5 [31.5; 66.5] |
| ALT, IU/mL | 21.5 [13.0; 36.5] |
| T.bil, mg/dL | 1.0 ± 0.5 |
| Alb, g/dL | 3.7 [3.2; 4.0] |
| PLT, 103/μL | 156.0 ± 81.8 |
| INR | 1.1 [ 1.0; 1.2] |
| Cr, mg/dL | 0.7 [0.7; 0.9] |
| CP class A | 32 (100.0%) |
| AFP, ng/mL | 77.9 [6.0; 2381.3] |
| PIVKA, mAU/mL | 1085.2 [220.6; 8170.3] |
| Tumor size, cm | 5.8 [3.0; 10.5] |
| Multiple intrahepatic HCC | 28 (87.5%) |
| PVTT | 22 (68.8%) |
| Extrahepatic metastasis | 17 (53.1%) |
| Variables | Univariate | Multivariable 1 | Multivariable 2 | Multivariable 3 | ||||
|---|---|---|---|---|---|---|---|---|
| sHR | p-Value | Adjusted sHR (95% CI) | p-Value | Adjusted sHR (95% CI) | p-Value | Adjusted sHR (95% CI) | p-Value | |
| Age (≥65) | 0.49 | 0.200 | ||||||
| Sex (Male) | >0.9 | |||||||
| HBV (vs. others) | 1.12 | 0.800 | ||||||
| Total bilirubin (≥2 mg/dL) | 1.40 | 0.700 | ||||||
| Albumin (≥3 mg/dL) | 0.26 | 0.087 | ||||||
| Platelet (<100 × 103/μL) | 2.60 | 0.066 | ||||||
| CTP class A (vs. class B) | 2.76 | 0.055 | ||||||
| Presence of PVTT | 2.98 | 0.094 | ||||||
| AFP (≥100 ng/mL) | 2.35 | 0.110 | ||||||
| PIVKA-II (≥1000 mAU/mL) | 1.54 | 0.400 | ||||||
| Intrahepatic tumor size (≥5 cm) | 6.59 | 0.014 | 7.21 (1.49–34.9) | 0.014 | 7.24 (1.54–34.1) | 0.012 | 12.1 (2.03–72.1) | 0.006 |
| Multiple intrahepatic tumors | 1.06 | >0.9 | ||||||
| Presence of extrahepatic metastasis | 1.07 | >0.9 | ||||||
| Increased IL-10 levels (≥2.15) | 0.22 | 0.011 | 0.19 (0.05–0.72) | 0.014 | Not included | Not included | ||
| Increased TNF-α levels (≥2.95) | 0.25 | 0.010 | Not included | 0.23 (0.08–0.70) | 0.009 | Not included | ||
| Increased IL-10 and TNF-α levels | 0.14 | 0.011 | Not included | Not included | 0.07 (0.01–0.46) | 0.005 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.K.; Nam, S.W.; Han, J.W.; Kwon, J.H. An Early Increase in IL-10 and TNF-α Levels Following Atezolizumab Plus Bevacizumab Treatment Predicts Survival in Advanced Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Cancers 2024, 16, 3543. https://doi.org/10.3390/cancers16203543
Lee SK, Nam SW, Han JW, Kwon JH. An Early Increase in IL-10 and TNF-α Levels Following Atezolizumab Plus Bevacizumab Treatment Predicts Survival in Advanced Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Cancers. 2024; 16(20):3543. https://doi.org/10.3390/cancers16203543
Chicago/Turabian StyleLee, Soon Kyu, Soon Woo Nam, Ji Won Han, and Jung Hyun Kwon. 2024. "An Early Increase in IL-10 and TNF-α Levels Following Atezolizumab Plus Bevacizumab Treatment Predicts Survival in Advanced Hepatocellular Carcinoma Patients: A Prospective Cohort Study" Cancers 16, no. 20: 3543. https://doi.org/10.3390/cancers16203543
APA StyleLee, S. K., Nam, S. W., Han, J. W., & Kwon, J. H. (2024). An Early Increase in IL-10 and TNF-α Levels Following Atezolizumab Plus Bevacizumab Treatment Predicts Survival in Advanced Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Cancers, 16(20), 3543. https://doi.org/10.3390/cancers16203543





