Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Laboratory Tests
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howe, J.R. Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2020, 29, xv–xvi. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Leung, G.; Eads, J.R.; Katona, B.W. Gastroenteropancreatic Neuroendocrine Tumors. Gastroenterol. Clin. N. Am. 2022, 51, 625–647. [Google Scholar] [CrossRef]
- Borczuk, A.C. Pulmonary Neuroendocrine Tumors. Surg. Pathol. Clin. 2020, 13, 35–55. [Google Scholar] [CrossRef]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J. Thorac. Oncol. 2020, 15, 1577–1598. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Siddiqi, A. Primary well differentiated neuroendocrine tumor of ovary collides with Mucinous borderline tumor in a postmenopausal female: A report of case and review of literature. Pathologica 2017, 109, 375–378. [Google Scholar]
- Mardi, K.; Negi, L.; Srivastava, S. Well differentiated neuroendocrine tumor of the kidney: Report of a rare case with review of literature. Indian J. Pathol. Microbiol. 2017, 60, 105–107. [Google Scholar] [CrossRef]
- Lubana, S.S.; Singh, N.; Chan, H.C.; Heimann, D. Primary neuroendocrine tumor (carcinoid tumor) of the testis: A case report with review of literature. Am. J. Case Rep. 2015, 16, 328–332. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Ramesh, A.; Chatterjee, A.; Subramaniam, R.M. Neuroendocrine Neoplasms: Epidemiology, Diagnosis, and Management. PET Clin. 2023, 18, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, G.; Jiang, C.; Fan, M.; Yuan, L.; Shi, H.; Lin, R. Epidemiologic trends and survival of early-onset gastroenteropancreatic neuroendocrine neoplasms. Front. Endocrinol. 2023, 14, 1241724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panzuto, F.; Partelli, S.; Campana, D.; de Braud, F.; Spada, F.; Cives, M.; Tafuto, S.; Bertuzzi, A.; Gelsomino, F.; Bergamo, F.; et al. Epidemiology of gastroenteropancreatic neuroendocrine neoplasms: A review and protocol presentation for bridging tumor registry data with the Italian association for neuroendocrine tumors (Itanet) national database. Endocrine 2024, 84, 42–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masui, T.; Ito, T.; Komoto, I.; Uemoto, S.; JNETS Project Study Group. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: A population-based study. BMC Cancer 2020, 20, 1104. [Google Scholar] [CrossRef]
- Rossi, R.E.; Massironi, S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J. Clin. Med. 2022, 11, 3794. [Google Scholar] [CrossRef]
- Lamberti, G.; Panzuto, F.; Pavel, M.; O’Toole, D.; Ambrosini, V.; Falconi, M.; Garcia-Carbonero, R.; Riechelmann, R.P.; Rindi, G.; Campana, D. Gastric neuroendocrine neoplasms. Nat. Rev. Dis. Primers 2024, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours) [Aktualizacja zaleceń ogólnych dotyczących postępowania diagnostyczno-terapeutycznego w nowotworach neuroendokrynnych układu pokarmowego (rekomendowane przez Polską Sieć Guzów Neuroendokrynnych)]. Endokrynol. Pol. 2022, 73, 387–454. [Google Scholar] [CrossRef] [PubMed]
- Broder, M.S.; Beenhouwer, D.; Strosberg, J.R.; Neary, M.P.; Cherepanov, D. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: A systematic literature review. World J. Gastroenterol. 2015, 21, 1945–1955. [Google Scholar] [CrossRef]
- Godara, A.; Siddiqui, N.S.; Byrne, M.M.; Saif, M.W. The safety of lanreotide for neuroendocrine tumor. Expert Opin. Drug Saf. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides, 1.8.F.-D.O.P.A. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2150–2151. [Google Scholar] [CrossRef] [PubMed]
- Lugat, A.; Frampas, É.; Touchefeu, Y.; Mirallié, É.; Bras, M.L.; Senellart, H.; Rauscher, A.; Fleury, V.; Campion, L.; Rohmer, V.; et al. Prospective Multicentric Assessment of 68Ga-DOTANOC PET/CT in Grade 1-2 GEP-NET. Cancers 2023, 15, 513. [Google Scholar] [CrossRef]
- Saponjski, J.; Macut, D.; Sobic-Saranovic, D.; Ognjanovic, S.; Bozic Antic, I.; Pavlovic, D.; Artiko, V. Somatostatin receptor scintigraphy in the follow up of neuroendocrine neoplasms of appendix. World J. Clin. Cases 2020, 8, 3697–3707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calomino, N.; Poto, G.E.; Carbone, L.; Bagnacci, G.; Piccioni, S.; Andreucci, E.; Nenci, L.; Marano, L.; Verre, L.; Petrioli, R.; et al. Neuroendocrine tumors’ patients treated with somatostatin analogue could complicate with emergency cholecystectomy. Ann. Ital. Chir. 2023, 94, 518–522. [Google Scholar] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763, Erratum in Lancet Oncol. 2022, 23, e59. [Google Scholar] [CrossRef] [PubMed]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Zemczak, A.; Kołodziej, M.; Gut, P.; Łoń, I.; Pawlak, D.; Mikołajczak, R.; Kamiński, G.; Ruchała, M.; Kos-Kudła, B.; et al. Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects—Polish multicenter experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 922–933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tudela-Lerma, M.; Orcajo-Rincón, J.; Ramón-Botella, E.; Álvarez-Luque, A.; González-Leyte, M.; Rotger-Regi, A.; Velasco-Sánchez, E.; Colón-Rodríguez, A. Efficacy and safety of Yttrium-90 radioembolization in the treatment of neuroendocrine liver metastases. Long-term monitoring and impact on survival. Rev. Esp. Med. Nucl. Imagen Mol. Engl. Ed. 2021, 40, 82–90, (In English and Spanish). [Google Scholar] [CrossRef] [PubMed]
- Durma, A.D.; Saracyn, M.; Kołodziej, M.; Jóźwik-Plebanek, K.; Dmochowska, B.; Kapusta, W.; Żmudzki, W.; Mróz, A.; Kos-Kudła, B.; Kamiński, G. Epidemiology of Neuroendocrine Neoplasms and Results of Their Treatment with [177Lu]Lu-DOTA-TATE or [177Lu]Lu-DOTA-TATE and [90Y]Y-DOTA-TATE-A Six-Year Experience in High-Reference Polish Neuroendocrine Neoplasm Center. Cancers 2023, 15, 5466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513, Erratum in N. Engl. J. Med. 2011, 364, 1082. [Google Scholar] [CrossRef] [PubMed]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. 2020, 11, 562505. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Everolimus in the treatment of neuroendocrine tumors: Efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin. Pharmacother. 2018, 19, 909–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Y.; Zhao, Z.; Wang, J.; Lv, W.; Lu, L.; Fu, W.; Li, W. Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis. Medicine 2018, 97, e12784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lacombe, C.; Perrier, M.; Hentic, O.; Brixi, H.; De Rycke, O.; Cros, J.; Rebours, V.; Cadiot, G.; Ruszniewski, P.; de Mestier, L. FOLFOX-bevacizumab chemotherapy in patients with metastatic neuroendocrine tumors. J. Neuroendocrinol. 2023, 35, e13227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saracyn, M.; Durma, A.D.; Bober, B.; Kołodziej, M.; Lubas, A.; Kapusta, W.; Niemczyk, S.; Kamiński, G. Long-Term Complications of Radioligand Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms. Nutrients 2022, 15, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bober, B.; Saracyn, M.; Zaręba, K.; Lubas, A.; Mazurkiewicz, P.; Wilińska, E.; Kamiński, G. Early Complications of Radioisotope Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms—A Preliminary Study. J. Clin. Med. 2022, 11, 919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parghane, R.V.; Mitra, A.; Bannore, T.U.; Rakshit, S.; Banerjee, S.; Basu, S. Initial clinical evaluation of indigenous 90Y-DOTATATE in sequential duo-PRRT approach (177Lu-DOTATATE and 90Y-DOTATATE) in neuroendocrine tumors with large bulky disease: Observation on tolerability, 90Y-DOTATATE post- PRRT imaging characteristics (bremsstrahlung and PETCT) and early adverse effects. World J. Nucl. Med. 2020, 20, 73–81. [Google Scholar] [CrossRef]
- Kozuki, T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn. J. Clin. Oncol. 2016, 46, 291–298. [Google Scholar] [CrossRef]
- Miller, T.P.; Fisher, B.T.; Getz, K.D.; Sack, L.; Razzaghi, H.; Seif, A.E.; Bagatell, R.; Adamson, P.C.; Aplenc, R. Unintended consequences of evolution of the Common Terminology Criteria for Adverse Events. Pediatr. Blood Cancer 2019, 66, e27747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunikowska, J.; Pawlak, D.; Bąk, M.I.; Kos-Kudła, B.; Mikołajczak, R.; Królicki, L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with 90Y/177Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: A 10-year study. Ann. Nucl. Med. 2017, 31, 347–356. [Google Scholar] [CrossRef]
- Kennedy, K.R.; Turner, J.H.; MacDonald, W.B.G.; Claringbold, P.G.; Boardman, G.; Ransom, D.T. Long-term survival and toxicity in patients with neuroendocrine tumors treated with 177 Lu-octreotate peptide radionuclide therapy. Cancer 2022, 128, 2182–2192. [Google Scholar] [CrossRef]
- Trautwein, N.F.; Schwenck, J.; Jacoby, J.; Reischl, G.; Fiz, F.; Zender, L.; Dittmann, H.; Hinterleitner, M.; la Fougère, C. Long-term prognostic factors for PRRT in neuroendocrine tumors. Front. Med. 2023, 10, 1169970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, N.; Naraev, B.G.; Engelman, E.G.; Zimmerman, M.B.; Bushnell DLJr OʼDorisio, T.M.; OʼDorisio, M.S.; Menda, Y.; Müller-Brand, J.; Howe, J.R.; Halfdanarson, T.R. Peptide Receptor Radionuclide Therapy Outcomes in a North American Cohort With Metastatic Well-Differentiated Neuroendocrine Tumors. Pancreas 2017, 46, 151–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; de Herder, W.W.; Feelders, R.A.; Krenning, E.P.; Kwekkeboom, D.J. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Endocr. Relat. Cancer 2017, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, J.J.; Krenning, E.P.; de Jong, F.H.; de Rijke, Y.B.; Feelders, R.A.; van Aken, M.O.; de Herder, W.W.; Kwekkeboom, D.J. Effects of therapy with [177Lu-DOTA 0,Tyr 3]octreotate on endocrine function. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1758–1766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef]

| Parameter | Unit | Reference Range |
|---|---|---|
| GFR | mL/min/1.73 m2 | >60 |
| CREA | mg/dL | 0.7–1.2 |
| RBC | mil/µL | 3.5–5.5 |
| WBC | 1000/µL | 4.0–10.0 |
| PLT | 1000/µL | 150.0–400.0 |
| HGB | g/dL | 11.0–18.0 |
| GLU | mmol/L | 3.9–5.6 |
| CgA | ng/mL | 19–100 |
| n = 127 | Before I Course | Before IV Course | CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Mean | SD | Mean | SD | Δ | L | H | p |
| GFR | mL/min/1.73 m2 | 87.32 | 22.47 | 83.20 | 20.79 | −4.12 | −11.61 | 3.37 | 0.277 |
| CREA | mg/dL | 0.90 | 0.26 | 0.93 | 0.26 | 0.03 | 0 | 0.05 | 0.106 |
| RBC | mil/µL | 4.37 | 0.52 | 3.87 | 0.55 | −0.49 | −0.57 | −0.42 | <0.001 |
| WBC | 1000/µL | 6.83 | 2.29 | 5.00 | 2.12 | −1.83 | −21.4 | −1.49 | <0.001 |
| PLT | 1000/µL | 250.25 | 74.98 | 202.69 | 72.44 | −47.57 | −57.77 | −37.37 | <0.001 |
| HGB | g/dL | 12.92 | 1.61 | 12.15 | 1.55 | −0.76 | −0.98 | −0.55 | <0.001 |
| GLU | mmol/L | 6.29 | 2.36 | 6.48 | 2.40 | 0.19 | −0.09 | 0.046 | 0.185 |
| Parameters | Units | Median | IQR | Median | IQR | Δ | NA | NA | p |
| CgA | ng/mL | 124.2 | 308.5 | 82.5 | 244.3 | −41.7 | NA | NA | <0.001 |
| n = 44 | Course I | Course IV | CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Mean Δ | SD | Mean Δ | SD | Δ | L | H | p |
| GFR | mL/min/1.73 m2 | 82.14 | 21.52 | 82.57 | 21.5 | 0.43 | −1.88 | 2.74 | 0.708 |
| CREA | mg/dL | 0.96 | 0.26 | 0.96 | 0.24 | 0 | −0.03 | 0.03 | 0.812 |
| RBC | mil/µL | 4.36 | 0.50 | 3.81 | 0.54 | −0.56 | −0.66 | −0.45 | <0.001 |
| WBC | 1000/µL | 6.52 | 1.92 | 4.67 | 1.95 | −1.85 | −2.27 | −1.44 | <0.001 |
| PLT | 1000/µL | 222.34 | 59.79 | 184.61 | 67.83 | −37.73 | −55.82 | −19.64 | <0.001 |
| HGB | g/dL | 12.96 | 1.25 | 12.01 | 1.41 | −0.95 | −1.25 | −0.66 | <0.001 |
| GLU | mmol/L | 5.98 | 1.82 | 6.13 | 1.39 | 0.14 | −0.52 | 0.80 | 0.660 |
| Parameters | Units | Median Δ | IQR | Median Δ | IQR | Δ | x | x | p |
| CgA | ng/mL | 137.7 | 624.5 | 117.8 | 563.6 | −19.9 | NA | NA | 0.035 |
| n = 44 | Course IV | Follow Up | CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Mean | SD | Mean | SD | Δ | L | H | p |
| GFR | mL/min/1.73 m2 | 82.57 | 21.5 | 77.00 | 21.96 | −5.57 | −9.48 | −1.66 | 0.006 |
| CREA | mg/dL | 0.96 | 0.24 | 1.05 | 0.37 | 0.09 | 0.03 | 0.16 | 0.008 |
| RBC | mil/µL | 3.81 | 0.54 | 4.05 | 1.17 | 0.24 | −0.11 | 0.59 | 0.175 |
| WBC | 1000/µL | 4.67 | 1.95 | 5.18 | 2.20 | 0.51 | −0.13 | 1.15 | 0.112 |
| PLT | 1000/µL | 184.61 | 67.83 | 183.23 | 85.45 | −1.39 | −20.58 | 17.81 | 0.885 |
| HGB | g/dL | 12.01 | 1.41 | 11.82 | 1.23 | −0.19 | −0.54 | −0.16 | 0.284 |
| GLU | mmol/L | 6.13 | 1.39 | 6.68 | 1.80 | 0.55 | 0.14 | 0.97 | 0.011 |
| Parameters | Units | Median Δ | IQR | Median Δ | IQR | Δ | x | x | p |
| CgA | ng/mL | 117.8 | 563.6 | 181.0 | 1375.7 | 63.2 | NA | NA | 0.321 |
| n = 44. | Course I | Follow Up | CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Mean | SD | Mean | SD | Δ | L | H | p |
| GFR | mL/min/1.73 m2 | 82.14 | 21.52 | 77.00 | 21.96 | −5.14 | −9.49 | −0.79 | 0.022 |
| CREA | mg/dL | 0.96 | 0.26 | 1.05 | 0.37 | 0.09 | 0.01 | 0.17 | 0.038 |
| RBC | mil/µL | 4.36 | 0.50 | 4.05 | 1.17 | −0.31 | −0.69 | 0.03 | 0.072 |
| WBC | 1000/µL | 6.52 | 1.92 | 5.18 | 2.20 | −1.34 | −2.03 | −0.65 | <0.001 |
| PLT | 1000/µL | 222.34 | 59.79 | 183.23 | 85.45 | −39.11 | −63.90 | −14.32 | 0.003 |
| HGB | g/dL | 12.96 | 1.25 | 11.82 | 1.23 | −1.14 | −1.61 | −0.68 | <0.001 |
| GLU | mmol/L | 5.98 | 1.82 | 6.68 | 1.80 | 0.70 | 0.02 | 1.37 | 0.044 |
| Parameters | Units | Median Δ | IQR | Median Δ | IQR | Δ | x | x | p |
| CgA | ng/mL | 137.7 | 624.5 | 181.0 | 1375.7 | 43.3 | NA | NA | 0.658 |
| n = 127 (100%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Decrease | Increase | No Change |
|---|---|---|---|---|---|---|---|---|
| Kidney Function n = 67 (52.75%) | 49 (38.58%) | 18 (14.17%) | 0 | 0 | 0 | 39 | 18 | 10 |
| Leukopenia n = 42 (33.07%) | 31 (24.41%) | 11 (8.66%) | 0 | 0 | 0 | 37 | 5 | 0 |
| Platelets Count n = 27 (21.25%) | 24 (18.89%) | 3 (2.36%) | 0 | 0 | 0 | 27 | 0 | 0 |
| Anemia n = 15 (11.8%) | 14 (11.02%) | 1 (0.78%) | 0 | 0 | 0 | 12 | 3 | 0 |
| n = 44 (100%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Kidney Function n = 30 (68.18%) | 21 (47.72%) | 8 (18.18%) | 1 (2.27%) | 0 | 0 |
| Leukopenia n = 14 (31.81%) | 9 (20.45%) | 5 (11.36%) | 0 | 0 | 0 |
| Platelets Count n = 19 (43.18%) | 18 (40.90%) | 1 (2.27%) | 0 | 0 | 0 |
| Anemia n = 20 (45.45%) | 16 (36.362%) | 4 (9.09%) | 0 | 0 | 0 |
| Characteristic | 12 | 24 | 36 | 48 | 60 | 72 |
|---|---|---|---|---|---|---|
| Overall | 99.0 (97.1, 100.0) | 94.3 (89.5, 99.3) | 86.0 (77.5, 95.5) | 83.5 (74.1, 94.1) | 74.0 (60.0, 91.2) | 63.4 (43.9, 91.6) |
| Characteristic | 12 | 24 | 36 | 48 | 60 | 72 |
|---|---|---|---|---|---|---|
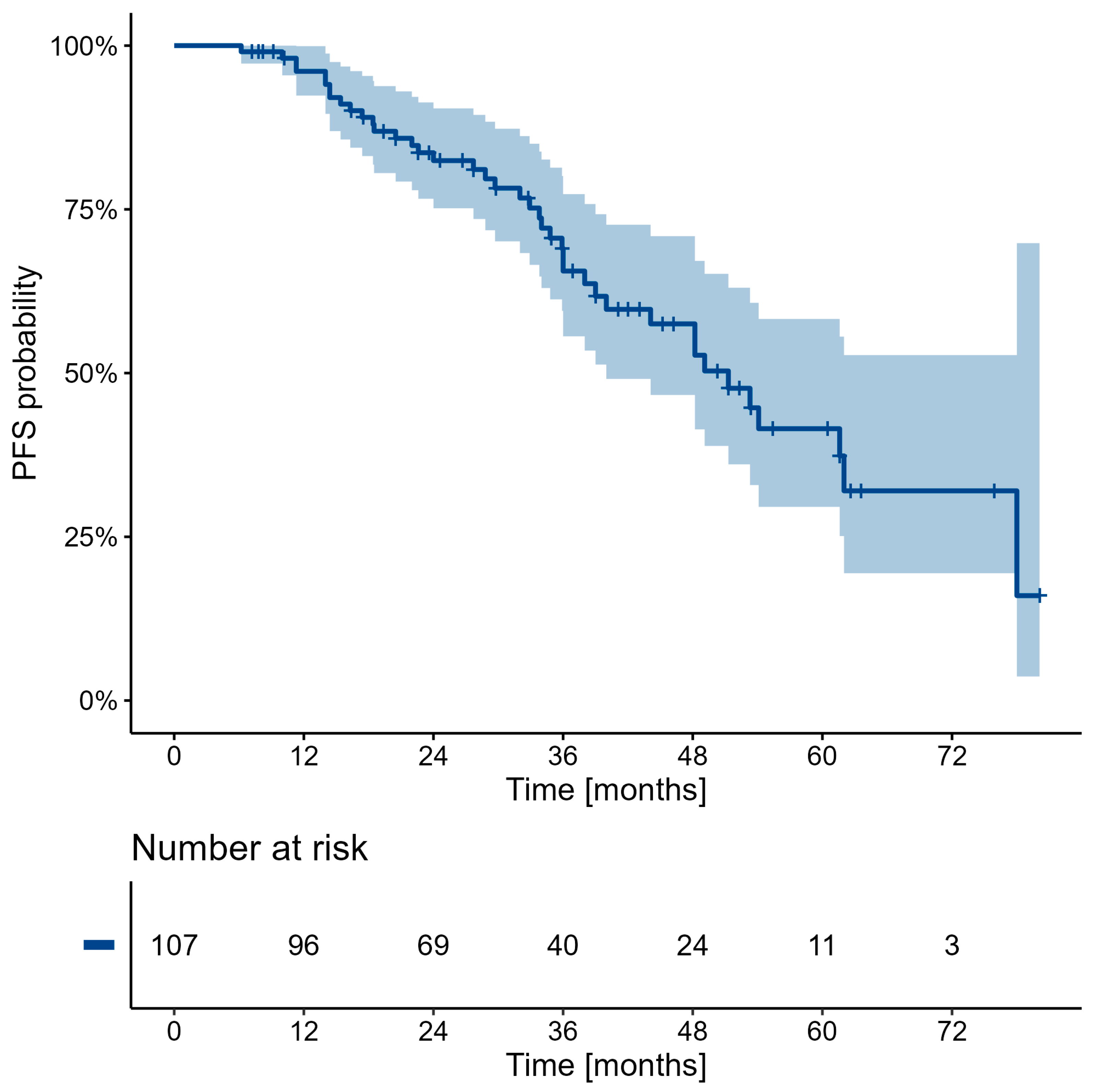
| Overall | 96.1 (92.4, 99.9) | 82.4 (75.2, 90.4) | 65.6 (55.6, 77.3) | 57.5 (46.7, 70.9) | 41.5 (29.6, 58.3) | 32.0 (19.4, 52.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durma, A.D.; Saracyn, M.; Kołodziej, M.; Jóźwik-Plebanek, K.; Brodowska-Kania, D.; Dmochowska, B.; Mróz, A.; Kos-Kudła, B.; Kamiński, G. Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study. Cancers 2024, 16, 3509. https://doi.org/10.3390/cancers16203509
Durma AD, Saracyn M, Kołodziej M, Jóźwik-Plebanek K, Brodowska-Kania D, Dmochowska B, Mróz A, Kos-Kudła B, Kamiński G. Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study. Cancers. 2024; 16(20):3509. https://doi.org/10.3390/cancers16203509
Chicago/Turabian StyleDurma, Adam Daniel, Marek Saracyn, Maciej Kołodziej, Katarzyna Jóźwik-Plebanek, Dorota Brodowska-Kania, Beata Dmochowska, Adrianna Mróz, Beata Kos-Kudła, and Grzegorz Kamiński. 2024. "Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study" Cancers 16, no. 20: 3509. https://doi.org/10.3390/cancers16203509
APA StyleDurma, A. D., Saracyn, M., Kołodziej, M., Jóźwik-Plebanek, K., Brodowska-Kania, D., Dmochowska, B., Mróz, A., Kos-Kudła, B., & Kamiński, G. (2024). Adverse Events of Radioligand Therapy in Patients with Progressive Neuroendocrine Neoplasms: The Biggest Eastern European Prospective Study. Cancers, 16(20), 3509. https://doi.org/10.3390/cancers16203509






