Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
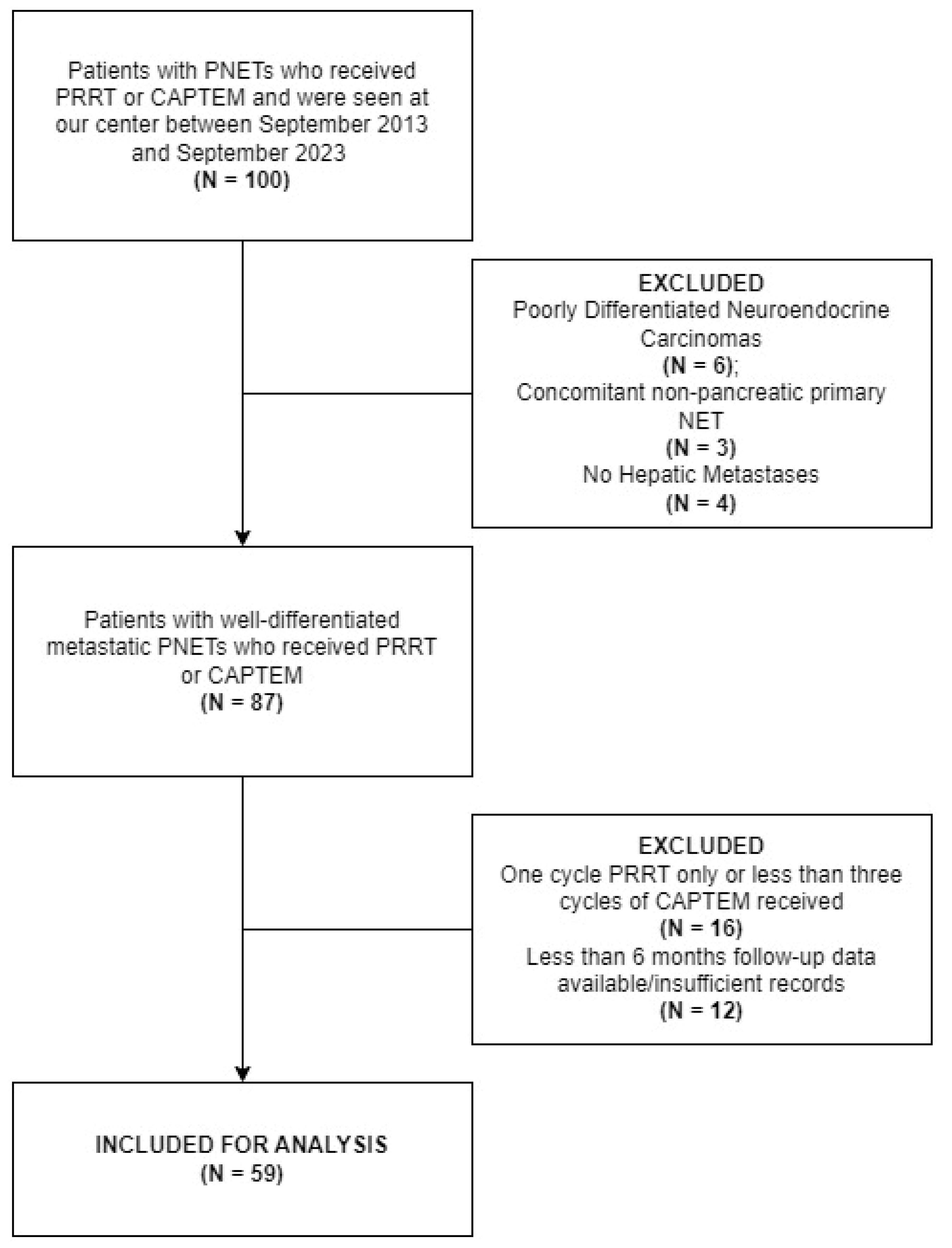
2.1. Subject Selection and Outcomes Evaluation
2.2. Treatment Protocols
2.3. Statistical Analysis
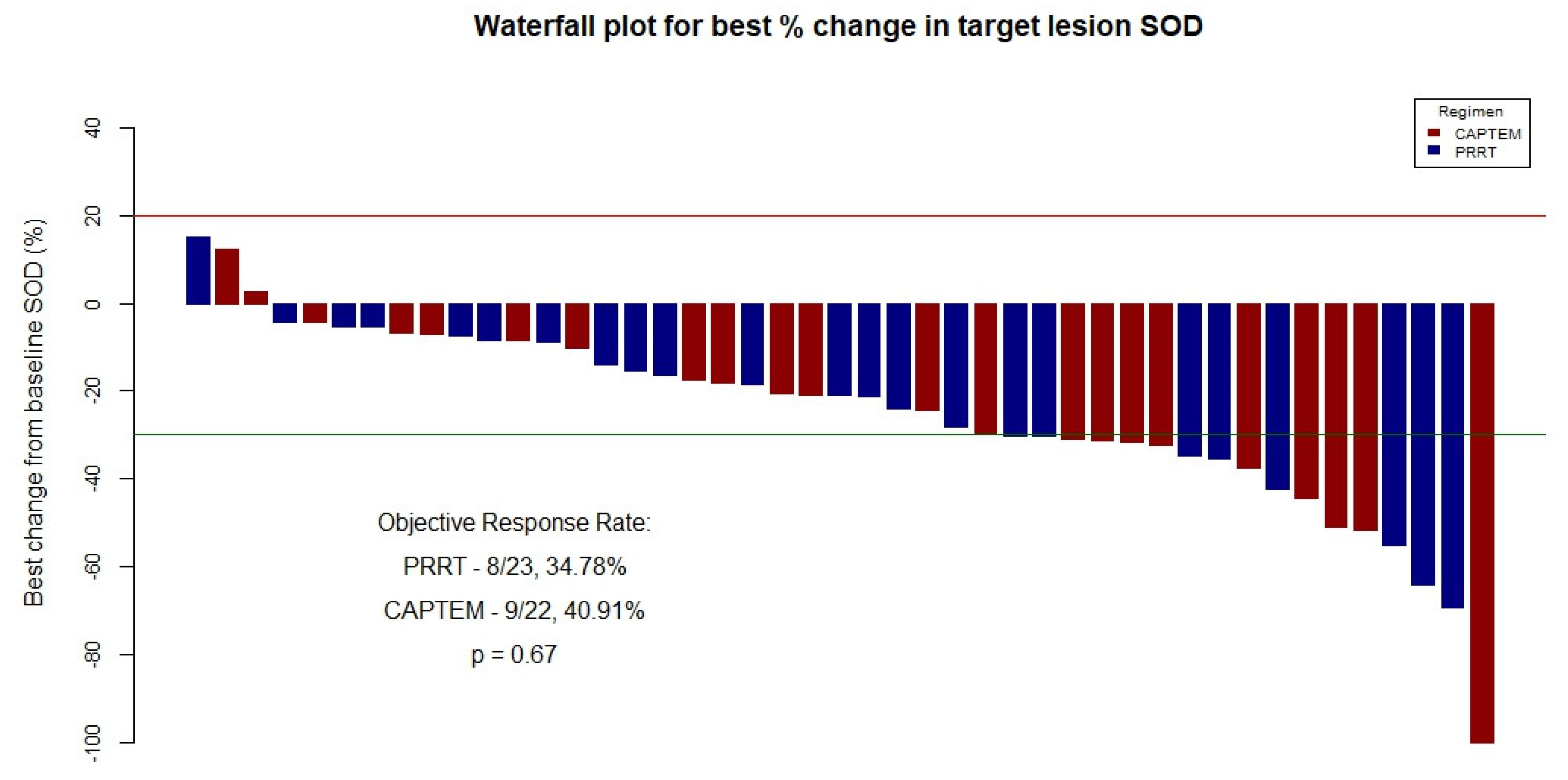
3. Results
3.1. Demographic and Clinicopathologic Features
| CAPTEM | PRRT | p-Value | |||
|---|---|---|---|---|---|
| n | % of available | n | % of available | ||
| Total | 30 | 29 | |||
| Median Age at C1D1 (IQR) | 49.34 (40.48–58.17) | 60.33 (55.79–66.05) | 0.02 * | ||
| SAAB | 0.7 | ||||
| Male | 14 | 46.67% | 15 | 51.72% | |
| Female | 16 | 53.33% | 14 | 48.28% | |
| Grade (N = 54) | 27 | 27 | 0.34 | ||
| 1 or 2 | 19 | 70.37% | 22 | 81.48% | |
| 3 | 8 | 29.63% | 5 | 18.52% | |
| ECOG (N = 58) | 29 | 29 | 0.42 | ||
| 0 | 16 | 55.17% | 19 | 65.52% | |
| 1 or 2 | 13 | 44.83% | 10 | 34.49% | |
| Extrahepatic Metastases | 19 | 63.33% | 20 | 68.97% | 0.65 |
| Bone Metastases | 9 | 30% | 11 | 37.93% | 0.52 |
| Baseline Chromogranin A Levels > 2 ULN (N = 50) | 13/21 | 61.90% | 16/29 | 55.17% | 0.63 |
| Median Ki-67 Index (IQR; N = 52) | 13.30 (5.00–21.00) | 7.90 (3.50–18.50) | 0.13 | ||
| MEN1/DAXX/ATRX Mutation (N = 31) | 9/17 | 52.94% | 10/14 | 71.43% | 0.29 |
| Prior Surgical Intervention for Metastatic Disease | 1 | 3.33% | 14 | 48.28% | <0.001 * |
| Prior Systemic Therapy other than SSA | 8 | 26.67% | 9 | 31.03% | 0.71 |
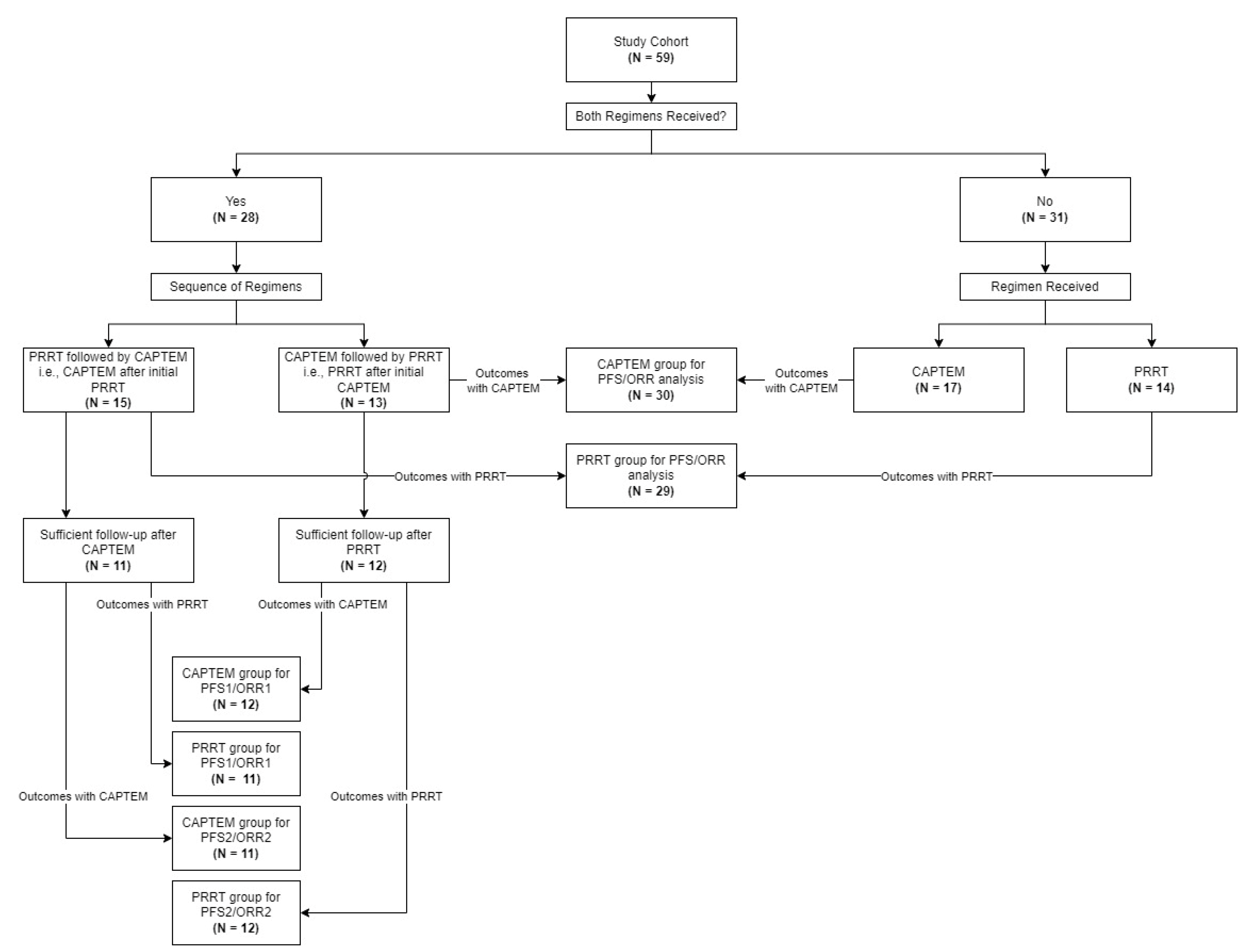
3.2. Treatments Received and Group Comparison
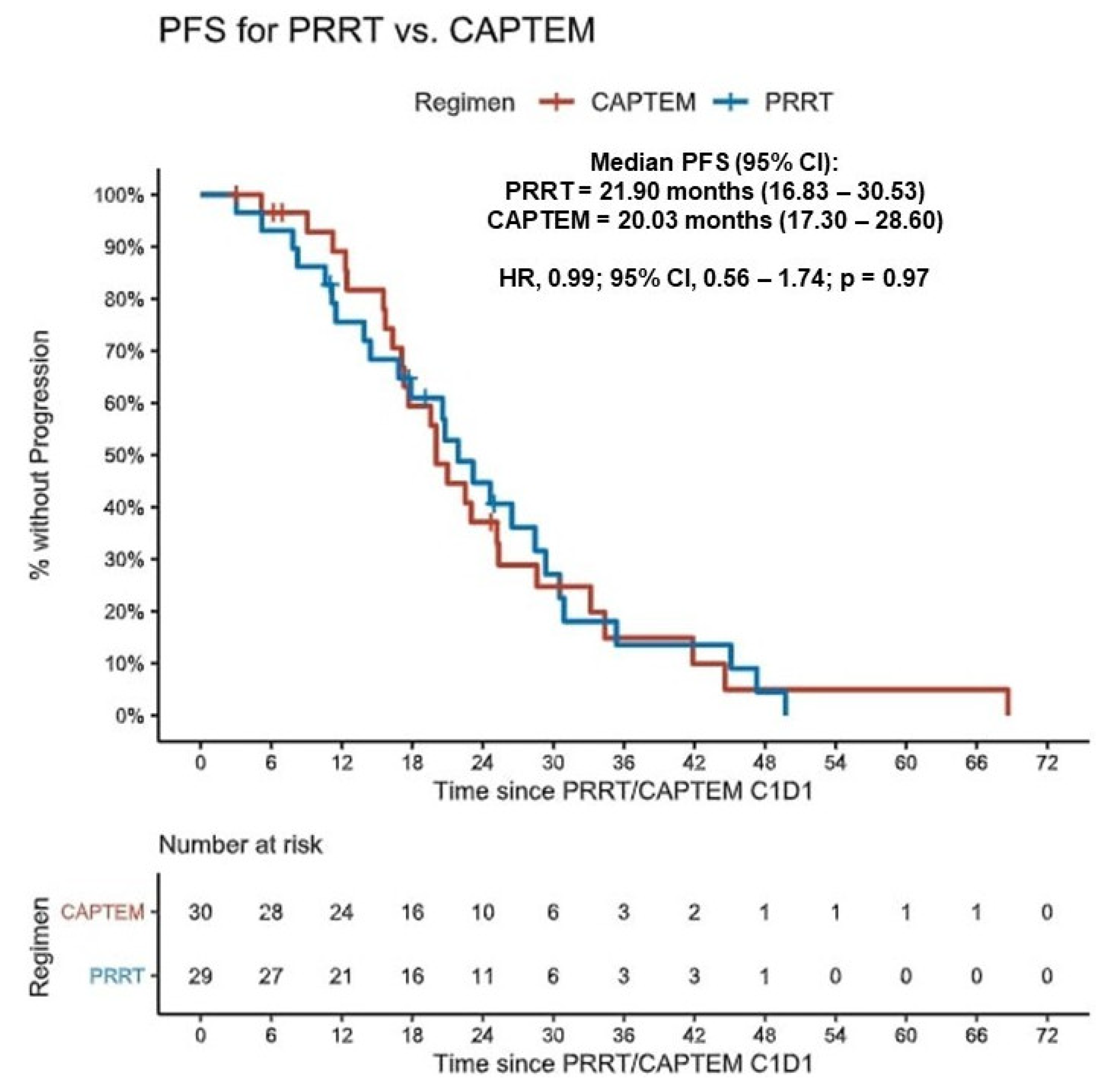
3.3. Primary Outcomes Evaluation–Entire Cohort
3.4. PFS1, PFS2, ORR1, and ORR2 Analysis—Cohort with Both Treatments Received
3.5. Overall Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.R.; Harris, C.; Baeg, K.J.; Aronson, A.; Wisnivesky, J.P.; Kim, M.K. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2212–2217.e1. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Mazza, G.L.; Mi, L.; Oliver, T.; Starr, J.; Gudmundsdottir, H.; Cleary, S.P.; Hobday, T.; Halfdanarson, T.R. Survival and Incidence Patterns of Pancreatic Neuroendocrine Tumors Over the Last 2 Decades: A SEER Database Analysis. Oncologist 2022, 27, 573–578. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Li, D.; Rock, A.; Kessler, J.; Ballena, R.; Hyder, S.; Mo, C.; Chang, S.; Singh, G. Understanding the Management and Treatment of Well-Differentiated Pancreatic Neuroendocrine Tumors: A Clinician’s Guide to a Complex Illness. JCO Oncol. Pract. 2020, 16, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863. [Google Scholar] [CrossRef]
- Riechelmann, R.P.; Taboada, R.G.; de Jesus, V.H.F.; Iglesia, M.; Trikalinos, N.A. Therapy Sequencing in Patients With Advanced Neuroendocrine Neoplasms. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389278. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment With the Radiolabeled Somatostatin Analog [177Lu-DOTA0,Tyr3]Octreotate: Toxicity, Efficacy, and Survival. JCO 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, W.A.; Bodei, L.; Mueller-Brand, J.; de Herder, W.W.; Kvols, L.K.; Kwekkeboom, D.J. GEP–NETs UPDATE: Radionuclide Therapy in Neuroendocrine Tumors. Eur. J. Endocrinol. 2015, 172, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]Octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Kim, S.-J.; Pak, K.; Koo, P.J.; Kwak, J.J.; Chang, S. The Efficacy of 177Lu-Labelled Peptide Receptor Radionuclide Therapy in Patients with Neuroendocrine Tumours: A Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Mitjavila, M.; Jimenez-Fonseca, P.; Belló, P.; Pubul, V.; Percovich, J.C.; Garcia-Burillo, A.; Hernando, J.; Arbizu, J.; Rodeño, E.; Estorch, M.; et al. Efficacy of [177Lu]Lu-DOTATATE in Metastatic Neuroendocrine Neoplasms of Different Locations: Data from the SEPTRALU Study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2486–2500. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus Long-Acting Octreotide versus High-dose Long-Acting Octreotide for the Treatment of Newly Diagnosed, Advanced Grade 2–3, Well-Differentiated, Gastroenteropancreatic Neuroendocrine Tumours (NETTER-2): An Open-Label, Randomised, Phase 3 Study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A Review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, J.R.; Rajdev, P.; Mukherji, S.K. Temozolomide (Temodar). AJNR Am. J. Neuroradiol. 2010, 31, 1383–1384. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Inzani, F.; Petrone, G.; Rindi, G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol. Metab. Clin. N. Am. 2018, 47, 463–470. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Aalbersberg, E.A.; Huizing, D.M.; Walraven, I.; Kulkarni, H.R.; Singh, A.; Stokkel, M.P.; Baum, R.P. Parameters to Predict Progression-Free and Overall Survival After Peptide Receptor Radionuclide Therapy: A Multivariate Analysis in 782 Patients. J. Nucl. Med. 2019, 60, 1259–1265. [Google Scholar] [CrossRef]
- Chambers, A.J.; Pasieka, J.L.; Dixon, E.; Rorstad, O. The Palliative Benefit of Aggressive Surgical Intervention for Both Hepatic and Mesenteric Metastases from Neuroendocrine Tumors. Surgery 2008, 144, 645–651; discussion 651–653. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical Management of Hepatic Neuroendocrine Tumor Metastasis: Results from an International Multi-Institutional Analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Graff-Baker, A.N.; Sauer, D.A.; Pommier, S.J.; Pommier, R.F. Expanded Criteria for Carcinoid Liver Debulking: Maintaining Survival and Increasing the Number of Eligible Patients. Surgery 2014, 156, 1369–1377. [Google Scholar] [CrossRef]
- Ejaz, A.; Reames, B.N.; Maithel, S.; Poultsides, G.A.; Bauer, T.W.; Fields, R.C.; Weiss, M.J.; Marques, H.P.; Aldrighetti, L.; Pawlik, T.M. Cytoreductive Debulking Surgery among Patients with Neuroendocrine Liver Metastasis: A Multi-Institutional Analysis. HPB 2018, 20, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ünal, Ç.; Azizy, A.; Karabulut, S.; Taştekin, D.; Akyıldız, A.; Yaşar, S.; Yalçın, Ş.; Çoban, E.; Evrensel, T.; Kalkan, Z.; et al. Efficacy of Capecitabine and Temozolomide Regimen in Neuroendocrine Tumors: Data From the Turkish Oncology Group. Oncologist 2023, 28, 875–884. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Hentic, O.; Rebours, V.; Zappa, M.; Gille, N.; Theou-Anton, N.; Vernerey, D.; Maire, F.; Lévy, P.; Bedossa, P.; et al. MGMT Expression Predicts Response to Temozolomide in Pancreatic Neuroendocrine Tumors. Endocr.-Relat. Cancer 2016, 23, 625–633. [Google Scholar] [CrossRef]
- Brighi, N.; Lamberti, G.; Andrini, E.; Mosconi, C.; Manuzzi, L.; Donati, G.; Lisotti, A.; Campana, D. Prospective Evaluation of MGMT-Promoter Methylation Status and Correlations with Outcomes to Temozolomide-Based Chemotherapy in Well-Differentiated Neuroendocrine Tumors. Curr. Oncol. 2023, 30, 1381–1394. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Rubin, D.; Yao, J.C.; Kulke, M.H.; et al. A Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients with Advanced Pancreatic Neuroendocrine Tumors: Final Analysis of Efficacy and Evaluation of MGMT (ECOG-ACRIN E2211). JCO 2022, 40, 4004. [Google Scholar] [CrossRef]
- Lakiza, O.; Lutze, J.; Vogle, A.; Williams, J.; Abukdheir, A.; Miller, P.; Liao, C.-Y.; Pitroda, S.P.; Martinez, C.; Olivas, A.; et al. Loss of MEN1 Function Impairs DNA Repair Capability of Pancreatic Neuroendocrine Tumors. Endocr. Relat. Cancer 2022, 29, 225–239. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, J.; Wang, X.; Ji, W.; Guan, X. DAXX, as a Tumor Suppressor, Impacts DNA Damage Repair and Sensitizes BRCA-Proficient TNBC Cells to PARP Inhibitors. Neoplasia 2019, 21, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Liu, L.; Shen, J.; Hao, H.; Zhang, H.; Lei, L.; Liu, F.; Wang, Z. Enhanced UV Resistance Role of Death Domain–Associated Protein in Human MDA-MB-231 Breast Cancer Cells by Regulation of G2 DNA Damage Checkpoint. Cell Transplant. 2020, 29, 0963689720920277. [Google Scholar] [CrossRef] [PubMed]
- Gulve, N.; Su, C.; Deng, Z.; Soldan, S.S.; Vladimirova, O.; Wickramasinghe, J.; Zheng, H.; Kossenkov, A.V.; Lieberman, P.M. DAXX-ATRX Regulation of P53 Chromatin Binding and DNA Damage Response. Nat. Commun. 2022, 13, 5033. [Google Scholar] [CrossRef] [PubMed]
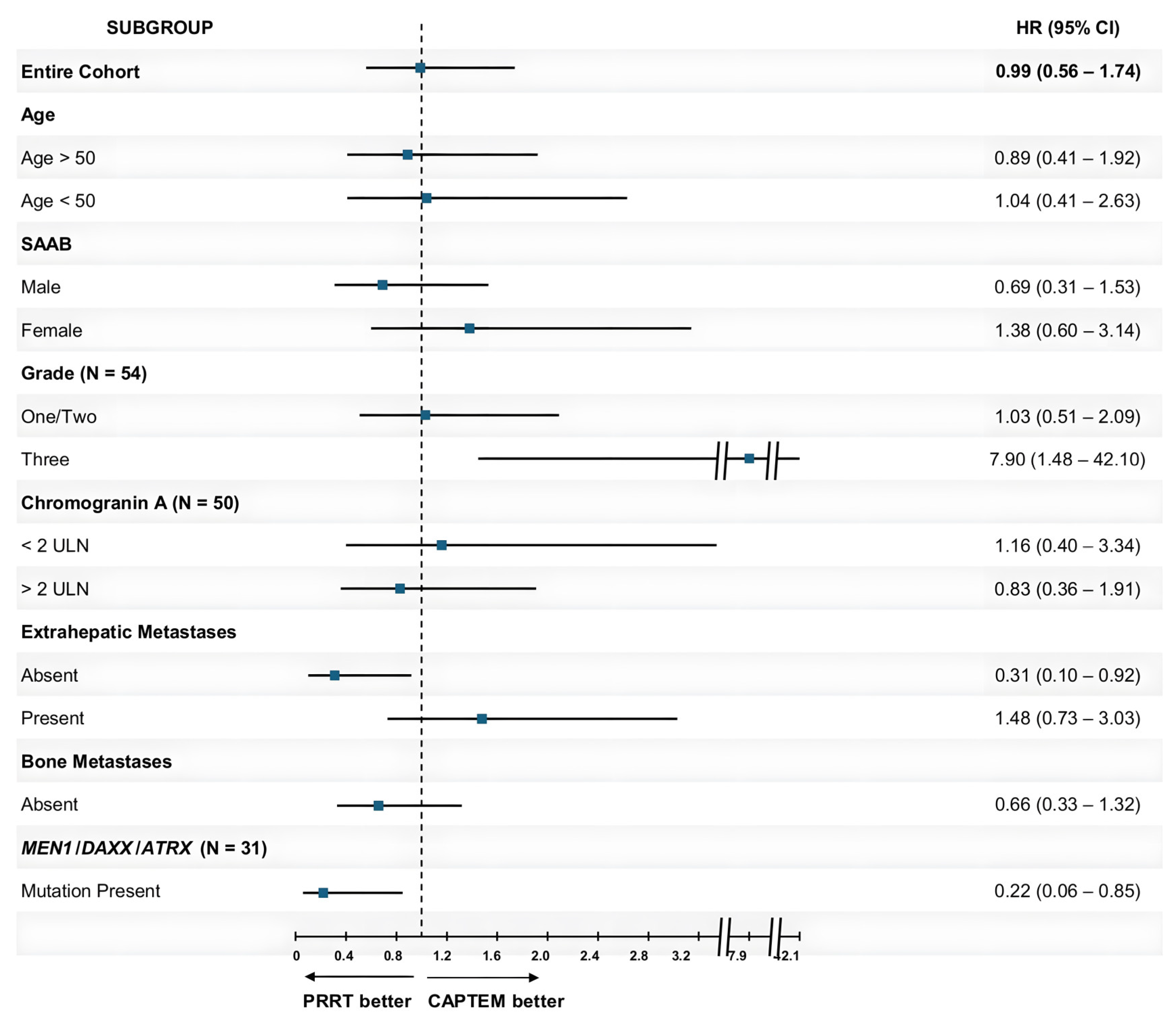
| Subgroup | PRRT Number | mPFS in Months with PRRT (95% CI) | CAPTEM Number | mPFS in Months with CAPTEM (95% CI) | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Entire Cohort | 29 | 21.90 (16.83–30.53) | 30 | 20.03 (17.30–28.60) | 0.99 (0.56–1.74) | 0.97 |
| Age | ||||||
| Age > 50 | 22 | 23.17 (17.87–35.37) | 14 | 20.03 (16.33–NR) | 0.89 (0.41–1.92) | 0.77 |
| Age < 50 | 7 | 20.77 (13.90–NR) | 16 | 21.27 (17.10–44.60) | 1.04 (0.41–2.63) | 0.93 |
| SAAB | ||||||
| Male | 15 | 23.17 (20.57–NR) | 14 | 18.43 (15.70–NR) | 0.69 (0.31–1.53) | 0.36 |
| Female | 14 | 16.83 (11.5–NR) | 16 | 23.00 (17.67–NR) | 1.38 (0.60–3.14) | 0.76 |
| Grade (N = 54) | ||||||
| One/Two | 22 | 26.47 (20.77–35.37) | 19 | 23.00 (20.03–NR) | 1.03 (0.51–2.09) | 0.92 |
| Three | 5 | 7.83 (5.20–NR) | 8 | 16.33 (11.23–NR) | 7.9 (1.48–42.10) | 0.02 |
| Baseline Chromogranin A Levels (N = 50) | ||||||
| <2 ULN | 13 | 26.47 (11.50–NR) | 8 | 20.03 (17.67–NR) | 1.16 (0.40–3.34) | 0.79 |
| >2 ULN | 16 | 21.90 (14.43–NR) | 13 | 20.03 (16.33–NR) | 0.83 (0.36–1.91) | 0.65 |
| Extrahepatic Metastases | ||||||
| Absent | 9 | 26.47 (20.77–NR) | 11 | 17.67 (15.70–NR) | 0.31 (0.10–0.92) | 0.03 |
| Present | 20 | 20.57 (14.43–NR) | 19 | 25.20 (17.30–44.60) | 1.48 (0.73–3.03) | 0.28 |
| Bone Metastases | ||||||
| Absent | 18 | 24.63 (20.77–45.10) | 21 | 20.03 (17.30–52.33) | 0.66 (0.33–1.32) | 0.24 |
| Present | 11 | 17.87 (10.60–NR) | 9 | 28.60 (16.33–NR) | 2.84 (0.85–9.52) | 0.09 |
| MEN1/DAXX/ATRX Mutation (N = 31) | ||||||
| Absent | 4 | 18.80 (7.83–NR) | 8 | 24.08 (12.33–NR) | 2.4 (0.53–10.87) | 0.26 |
| Present | 10 | 28.43 (17.87–NR) | 9 | 18.67 (15.53–NR) | 0.22 (0.06–0.85) | 0.03 |
| Outcomes | PRRT | CAPTEM |
|---|---|---|
| Objective Response Rate | ||
| ORR1 (N = 18) | 4/10, 40% | 2/8, 25% |
| p-value | 0.56 | |
| ORR2 (N = 17) | 2/9, 22.22% | 3/8, 37.5% |
| p-value | 0.55 | |
| Progression-Free Survival | ||
| PFS1 [mPFS (95% CI)] | 20.57 (13.90–NR) | 17.30 (15.53–NR) |
| p-value | 0.27 | |
| PFS2 [mPFS (95% CI)] | 12.52 (8.17–NR) | 9.57 (8.63–NR) |
| p-value | 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gujarathi, R.; Tobias, J.; Abou Azar, S.; Keutgen, X.M.; Liao, C.-Y. Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors. Cancers 2024, 16, 2993. https://doi.org/10.3390/cancers16172993
Gujarathi R, Tobias J, Abou Azar S, Keutgen XM, Liao C-Y. Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors. Cancers. 2024; 16(17):2993. https://doi.org/10.3390/cancers16172993
Chicago/Turabian StyleGujarathi, Rushabh, Joseph Tobias, Sara Abou Azar, Xavier M. Keutgen, and Chih-Yi Liao. 2024. "Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors" Cancers 16, no. 17: 2993. https://doi.org/10.3390/cancers16172993
APA StyleGujarathi, R., Tobias, J., Abou Azar, S., Keutgen, X. M., & Liao, C.-Y. (2024). Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors. Cancers, 16(17), 2993. https://doi.org/10.3390/cancers16172993






