Overall Survival in Real-World Patients with Unresectable Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab Versus Sorafenib or Lenvatinib as First-Line Therapy: Findings from the National Veterans Health Administration Database
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Study Variables
2.4. Statistical Analysis
2.5. Software
3. Results
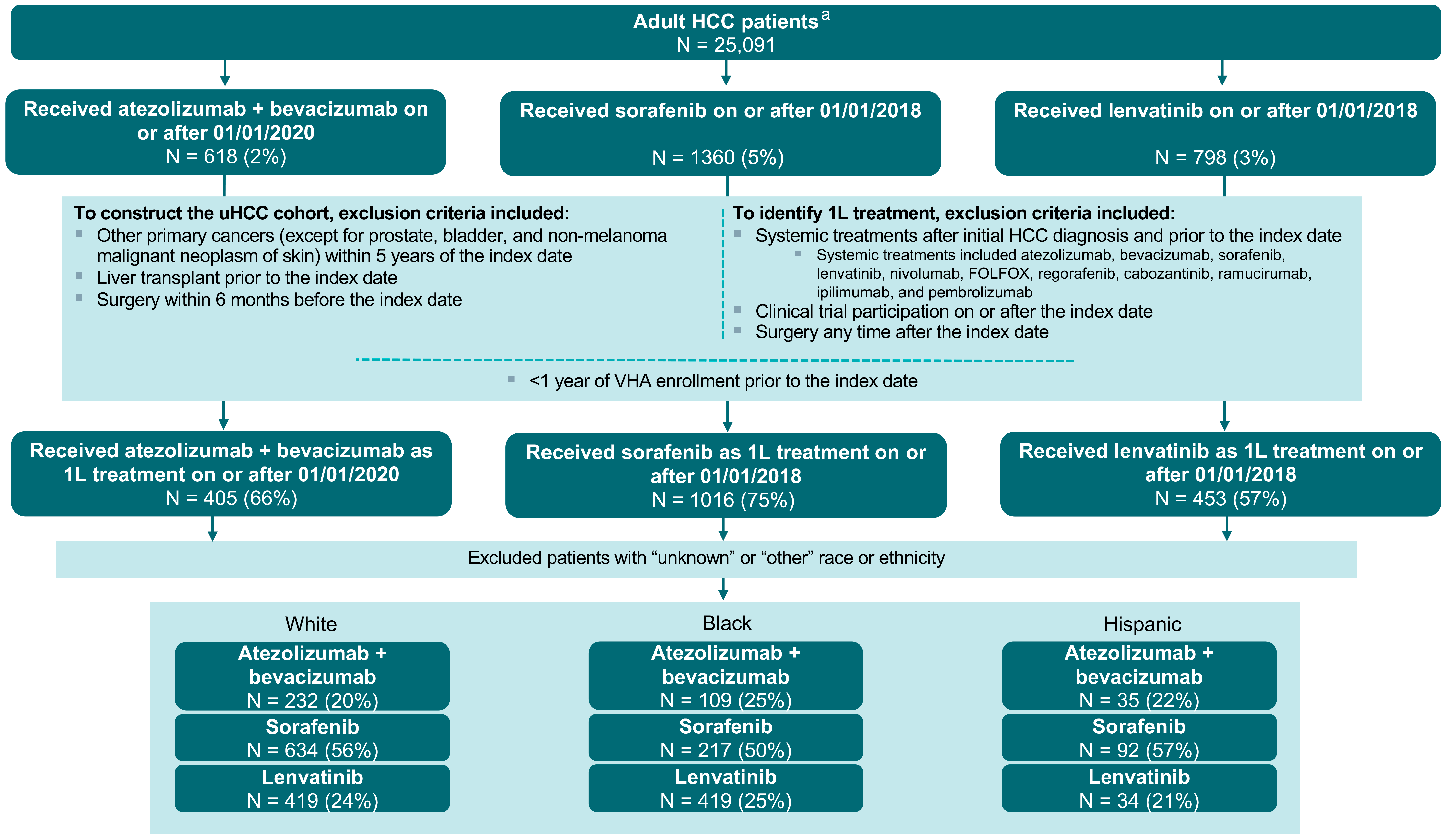
3.1. Sample Selection
3.2. Overall Population of Patients with uHCC
3.2.1. Comparison of Patient Characteristics Across Treatment Groups
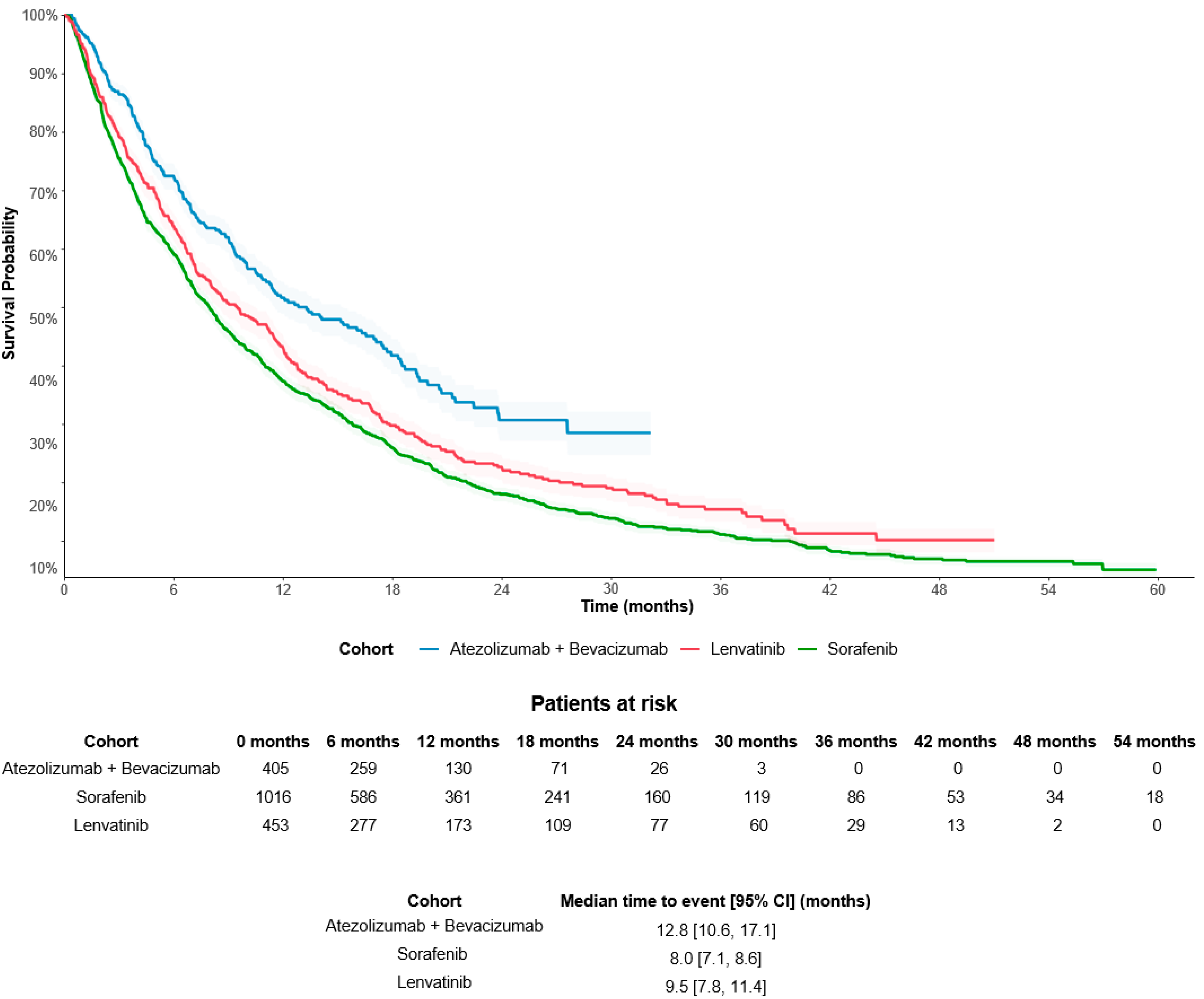
3.2.2. Difference in Overall Survival by Treatment Groups
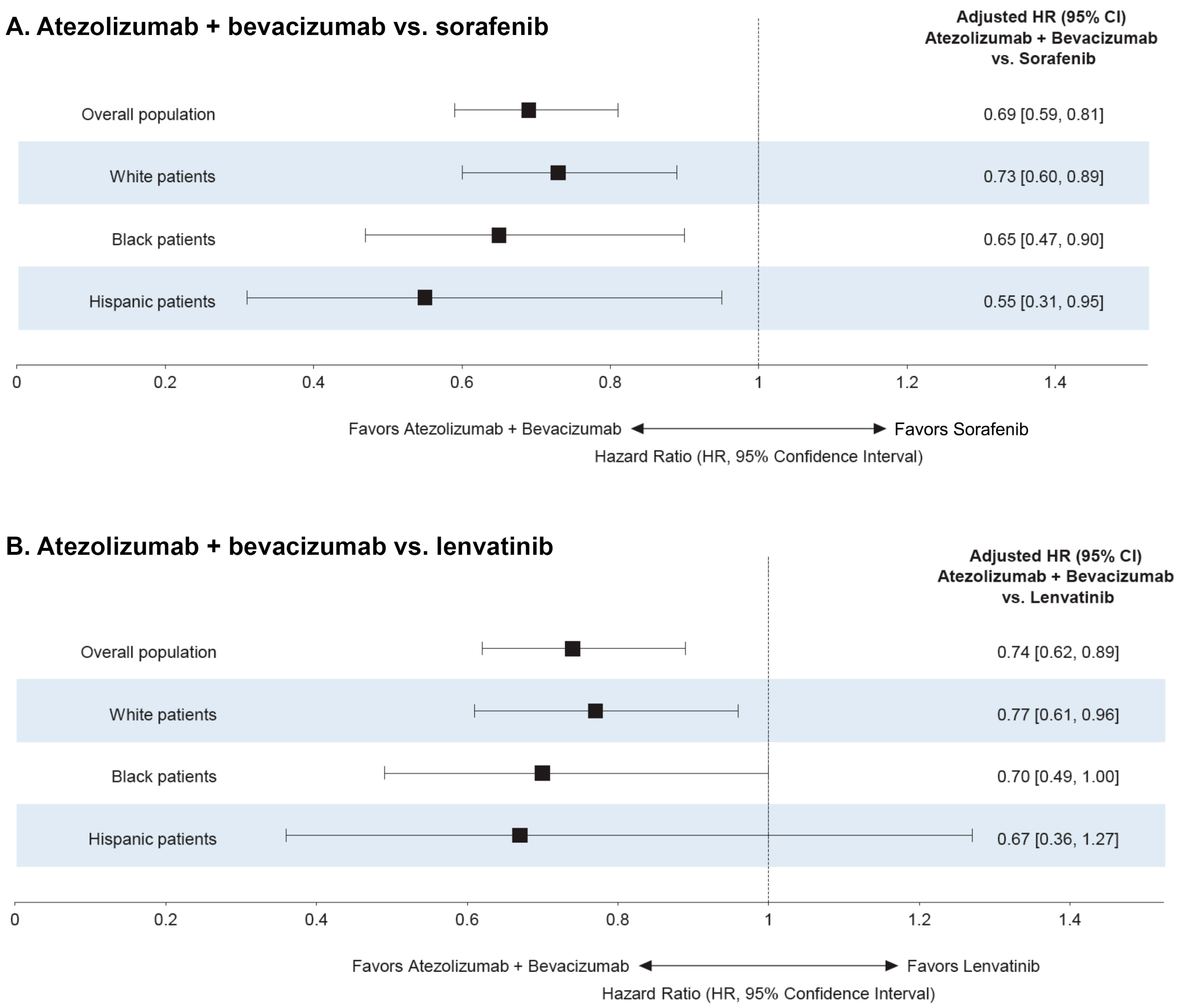
3.3. Analysis by Race and Ethnicity
3.4. Exploratory Subgroup Analysis by Liver Function and Etiology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- National Cancer Institute. Liver Cancer Causes, Risk Factors, and Prevention. Available online: https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors (accessed on 9 April 2024).
- Balogh, J.; Victor III, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Leowattana, W.; Leowattana, T.; Leowattana, P. Systemic treatment for unresectable hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 1551–1568. [Google Scholar] [CrossRef]
- Akce, M.; El-Rayes, B.F.; Wajapeyee, N. Combinatorial targeting of immune checkpoints and epigenetic regulators for hepatocellular carcinoma therapy. Oncogene 2023, 42, 1051–1057. [Google Scholar] [CrossRef]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Abd El Aziz, M.A.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.-L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA approval summary: Atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin ®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Kudo, M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers 2020, 12, 1089. [Google Scholar] [CrossRef]
- NCCN Hepatocellular Carcinoma (Version 2.2024): National Comprehensive Cancer Network 2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1514 (accessed on 22 July 2024).
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Sacco, R.; Ramai, D.; Tortora, R.; di Costanzo, G.G.; Burlone, M.E.; Pirisi, M.; Federico, P.; Daniele, B.; Silletta, M.; Gallo, P.; et al. Role of etiology in hepatocellular carcinoma patients treated with lenvatinib: A counterfactual event-based mediation analysis. Cancers 2023, 15, 381. [Google Scholar] [CrossRef]
- Meyer, T.; Galani, S.; Lopes, A.; Vogel, A. Aetiology of liver disease and response to immune checkpoint inhibitors: An updated meta-analysis confirms benefit in those with non-viral liver disease. J. Hepatol. 2023, 79, e73–e76. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Tevethia, H.; Kumar, K.; Premkumar, M.; Muttaiah, M.D.; Hiraoka, A.; Hatanaka, T.; Tada, T.; Kumada, T.; Kakizaki, S.; et al. Effectiveness and safety of atezolizumab-bevacizumab in patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis. EClinicalMedicine 2023, 63, 102179. [Google Scholar] [CrossRef]
- Rich, N.E.; Hester, C.; Odewole, M.; Murphy, C.C.; Parikh, N.D.; Marrero, J.A.; Yopp, A.C.; Singal, A.G. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 551–559.e1. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.; Kum, H.-C.; Singal, A.G. Racial and ethnic disparities in hepatocellular carcinoma treatment receipt in the United States: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2024, 33, 463–470.e8. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.L.; Rich, N.E.; Singal, A.G.; Kum, H.C. Racial, ethnic, and socioeconomic disparities in curative treatment receipt and survival in hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 1186–1197. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Jones, P.D.; Medina, H.; Cranford, H.M.; Koru-Sengul, T.; Bungum, T.; Wong, R.; Kobetz, E.N.; McGlynn, K.A. Incidence of Etiology-specific Hepatocellular Carcinoma: Diverging Trends and Significant Heterogeneity by Race and Ethnicity. Clin. Gastroenterol. Hepatol. 2024, 22, 562–571.e8. [Google Scholar] [CrossRef]
- Pinto, E.; Meneghel, P.; Farinati, F.; Russo, F.P.; Pelizzaro, F.; Gambato, M. Efficacy of immunotherapy in hepatocellular carcinoma: Does liver disease etiology have a role? Dig. Liver Dis. 2023, 56, 579–588. [Google Scholar] [CrossRef]
- Jan, J.; Osho, A.; Murphy, C.C.; Mazure, C.M.; Singal, A.G.; Rich, N.E. Gender, age, racial and ethnic disparities in clinical trial enrollment for primary liver cancer. Gastroenterol. 2022, 163, 14–20.e2. [Google Scholar] [CrossRef]
- Kaplan, D.E.; Dai, F.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Knott, A.; Pedrosa, M.; Pocha, C.; Mehta, R.; et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score from a National Electronic Healthcare Database. Clin. Gastroenterol. Hepatol. 2015, 13, e1–e6. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Alkadimi, M.; Lucero, K.; Boyle, L.D.; Fierro, M.E.; Franklin, K.; Nooruddin, Z.; Mader, M. Real-world data: Clinical characteristics and outcomes of patients with advanced hepatocellular carcinoma treated with atezolizumab plus bevacizumab in Veteran Health Administration. J. Clin. Oncol. 2023, 41, 2651. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur. J. Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef]
- Jost-Brinkmann, F.; Demir, M.; Wree, A.; Luedde, T.; Loosen, S.H.; Müller, T.; Tacke, F.; Roderburg, C.; Mohr, R. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: Results from a German real-world cohort. Aliment. Pharmacol. Ther. 2023, 57, 1313–1325. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1399–1410. [Google Scholar] [CrossRef]
- Asemota, J.; Oladunjoye, O.; Babalola, A.; Nwosu, U.; Liu, P.S.; Oladunjoye, A.O.; Castro-Webb, N.; Miksad, R.A. Comparison of Hepatocellular Carcinoma in Hispanic and Non-Hispanic Patients. Cureus 2021, 13, e14884. [Google Scholar] [CrossRef]
- Thylur, R.P.; Roy, S.K.; Shrivastava, A.; LaVeist, T.A.; Shankar, S.; Srivastava, R.K. Assessment of risk factors, and racial and ethnic differences in hepatocellular carcinoma. JGH Open 2020, 4, 351–359. [Google Scholar] [CrossRef]
- Mathur, A.K.; Osborne, N.H.; Lynch, R.J.; Ghaferi, A.A.; Dimick, J.B.; Sonnenday, C.J. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch. Surg. 2010, 145, 1158–1163. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.L.; Rich, N.E.; Singal, A.G.; Kum, H.-C. Racial, ethnic, and socioeconomic disparities in treatment delay among patients with hepatocellular carcinoma in the United States. Clin. Gastroenterol. Hepatol. 2023, 21, 1281–1292.e10. [Google Scholar] [CrossRef]
- Schoenberger, H.; Rich, N.E.; Jones, P.; Yekkaluri, S.; Yopp, A.; Singal, A.G. Racial and ethnic disparities in barriers to care in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 1094–1096.e2. [Google Scholar] [CrossRef]
- Wong, R.J.; Corley, D.A. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig. Dis. Sci. 2009, 54, 2031–2039. [Google Scholar] [CrossRef][Green Version]
- Espinoza, M.; Muquith, M.; Lim, M.; Zhu, H.; Singal, A.G.; Hsiehchen, D. Disease etiology and outcomes after atezolizumab plus bevacizumab in hepatocellular carcinoma: Post-hoc analysis of IMbrave150. Gastroenterol 2023, 165, 286–288.e4. [Google Scholar] [CrossRef]
- Roche, H.-L. A Study Evaluating Atezolizumab, with or without Bevacizumab, in Patients with Unresectable Hepatocellular Carcinoma and Child-Pugh B7 and B8 Cirrhosis (Kirros) 2023. Available online: https://clinicaltrials.gov/study/NCT06096779 (accessed on 10 July 2024).
- Liu, P.; Xie, S.H.; Hu, S.; Cheng, X.; Gao, T.; Zhang, C.; Song, Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget 2017, 8, 68131–68137. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver. Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]

| By Index Treatment | By Race/Ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A + B | Sorafenib | A + B vs. Sorafenib p-Value | Lenvatinib | A + B vs. Lenvatinib p-Value | White | Black | White vs. Black p-Value | Hispanic | White vs. Hispanic p-Value | |
| Index treatment, n (%) | ||||||||||
| A + B | - | - | - | - | - | 232 (20.4) | 109 (24.9) | <0.05 * | 35 (21.7) | 0.683 |
| Sorafenib | - | - | - | - | - | 634 (55.6) | 217 (49.7) | <0.05 * | 92 (57.1) | 0.715 |
| Lenvatinib | - | - | - | - | - | 274 (24.0) | 111 (25.4) | 0.572 | 34 (21.1) | 0.415 |
| Race/ethnicity, n (%) | ||||||||||
| White, non-Hispanic | 232 (57.3) | 634 (62.4) | 0.074 | 274 (60.5) | 0.341 | - | - | - | - | - |
| Black | 109 (26.9) | 217 (21.4) | <0.05 * | 111 (24.5) | 0.420 | - | - | - | - | - |
| Hispanic | 35 (8.6) | 92 (9.1) | 0.805 | 34 (7.5) | 0.541 | - | - | - | - | - |
| Native American | 2 (0.5) | 7 (0.7) | 1.000 | 8 (1.8) | 0.113 | - | - | - | - | - |
| Asian | 2 (0.5) | 5 (0.5) | 1.000 | 2 (0.4) | 1.000 | - | - | - | - | - |
| Other | 4 (1.0) | 5 (0.5) | 0.285 | 3 (0.7) | 0.713 | - | - | - | - | - |
| Unknown | 21 (5.2) | 56 (5.5) | 0.806 | 21 (4.6) | 0.710 | - | - | - | - | - |
| Age in years, mean ± SD [median] | 69.5 ± 5.6 [69.6] | 69.3 ± 6.4 [69.1] | 0.559 | 69.3 ± 6.3 [69.3] | 0.538 | 69.8 ± 6.2 [69.6] | 68.1 ± 5.3 [68.0] | <0.001 *** | 69.7 ± 7.6 [69.9] | 0.808 |
| Male, n (%) | 397 (98.0) | 1006 (99.0) | 0.132 | 450 (99.3) | 0.088 | 1128 (98.9) | 429 (98.2) | 0.217 | 161 (100.0) | 0.381 |
| Geographic regions, n (%) | ||||||||||
| South | 116 (28.6) | 304 (29.9) | 0.633 | 128 (28.3) | 0.900 | 315 (27.6) | 152 (34.8) | <0.01 ** | 44 (27.3) | 0.936 |
| Midwest | 109 (26.9) | 319 (31.4) | 0.096 | 118 (26.0) | 0.774 | 333 (29.2) | 149 (34.1) | 0.059 | 40 (24.8) | 0.252 |
| West | 147 (36.3) | 271 (26.7) | <0.001 *** | 154 (34.0) | 0.481 | 370 (32.5) | 68 (15.6) | <0.001 *** | 67 (41.6) | <0.05 * |
| Northeast | 33 (8.1) | 122 (12.0) | <0.05 * | 53 (11.7) | 0.084 | 122 (10.7) | 68 (15.6) | <0.01 ** | 10 (6.2) | 0.077 |
| Index year distribution, n (%) | ||||||||||
| 2018 | 0 (0.0) | 425 (41.8) | <0.001 *** | 24 (5.3) | <0.001 *** | 270 (23.7) | 98 (22.4) | 0.597 | 50 (31.1) | <0.05 * |
| 2019 | 0 (0.0) | 293 (28.8) | <0.001 *** | 161 (35.5) | <0.001 *** | 278 (24.4) | 112 (25.6) | 0.609 | 33 (20.5) | 0.279 |
| 2020 | 86 (21.2) | 169 (16.6) | <0.05 * | 129 (28.5) | <0.05 * | 244 (21.4) | 84 (19.2) | 0.339 | 32 (19.9) | 0.657 |
| 2021 | 175 (43.2) | 75 (7.4) | <0.001 *** | 87 (19.2) | <0.001 *** | 200 (17.5) | 87 (19.9) | 0.276 | 24 (14.9) | 0.407 |
| 2022 | 144 (35.6) | 54 (5.3) | <0.001 *** | 52 (11.5) | <0.001 *** | 148 (13.0) | 56 (12.8) | 0.929 | 22 (13.7) | 0.810 |
| BMI, mean ± SD [median] | 28.1 ± 5.7 [27.3] | 27.9 ± 5.6 [27.4] | 0.540 | 27.4 ± 5.9 [26.7] | 0.093 | 28.3 ± 5.8 [27.7] | 26.4 ± 5.4 [25.7] | <0.001 *** | 28.5 ± 5.1 [28.0] | 0.674 |
| Extrahepatic metastases | 114 (28.1) | 147 (14.5) | <0.001 *** | 86 (19.0) | <0.01 ** | 227 (19.9) | 70 (16.0) | 0.077 | 28 (17.4) | 0.451 |
| Liver condition-viral a | 274 (67.7) | 652 (64.2) | 0.214 | 303 (66.9) | 0.811 | 657 (57.6) | 393 (89.9) | <0.001 *** | 91 (56.5) | 0.790 |
| Hepatitis B virus | 32 (7.9) | 44 (4.3) | <0.01 ** | 20 (4.4) | <0.05 * | 43 (3.8) | 38 (8.7) | <0.001 *** | 7 (4.3) | 0.722 |
| Hepatitis C virus | 261 (64.4) | 638 (62.8) | 0.560 | 297 (65.6) | 0.732 | 641 (56.2) | 382 (87.4) | <0.001 *** | 89 (55.3) | 0.820 |
| Liver condition- non-viral | 48 (11.9) | 130 (12.8) | 0.628 | 54 (11.9) | 0.975 | 171 (15.0) | 13 (3.0) | <0.001 *** | 31 (19.3) | 0.163 |
| Cirrhosis | 296 (73.1) | 710 (69.9) | 0.230 | 314 (69.3) | 0.224 | 802 (70.4) | 313 (71.6) | 0.619 | 113 (70.2) | 0.966 |
| Child–Pugh score, n (%) | ||||||||||
| A (5–6 points) | 345 (85.2) | 681 (67.0) | <0.001 *** | 348 (76.8) | <0.01 ** | 820 (71.9) | 335 (76.7) | 0.058 | 126 (78.3) | 0.091 |
| A5 | 220 (54.3) | 356 (35.0) | <0.001 *** | 214 (47.2) | <0.05 * | 480 (42.1) | 176 (40.3) | 0.509 | 77 (47.8) | 0.170 |
| A6 | 125 (30.9) | 325 (32.0) | 0.681 | 134 (29.6) | 0.683 | 340 (29.8) | 159 (36.4) | <0.05 * | 49 (30.4) | 0.874 |
| B (7–9 points) | 56 (13.8) | 319 (31.4) | <0.001 *** | 101 (22.3) | <0.01 ** | 302 (26.5) | 99 (22.7) | 0.117 | 35 (21.7) | 0.198 |
| B7 | 37 (9.1) | 199 (19.6) | <0.001 *** | 70 (15.5) | <0.01 ** | 193 (16.9) | 68 (15.6) | 0.513 | 18 (11.2) | 0.064 |
| B8 | 15 (3.7) | 94 (9.3) | <0.001 *** | 22 (4.9) | 0.407 | 84 (7.4) | 26 (5.9) | 0.322 | 11 (6.8) | 0.807 |
| B9 | 4 (1.0) | 26 (2.6) | 0.063 | 9 (2.0) | 0.232 | 25 (2.2) | 5 (1.1) | 0.172 | 6 (3.7) | 0.262 |
| C (10–15 points) | 4 (1.0) | 16 (1.6) | 0.396 | 4 (0.9) | 1.000 | 18 (1.6) | 3 (0.7) | 0.166 | 0 (0.0) | 0.152 |
| C10 | 3 (0.7) | 12 (1.2) | 0.576 | 0 (0.0) | 0.105 | 11 (1.0) | 2 (0.5) | 0.534 | 0 (0.0) | 0.378 |
| C11 | 1 (0.2) | 4 (0.4) | 1.000 | 4 (0.9) | 0.377 | 7 (0.6) | 1 (0.2) | 0.457 | 0 (0.0) | 1.000 |
| Ascites, n (%) | ||||||||||
| No evidence of ascites | 380 (93.8) | 863 (84.9) | <0.001 *** | 411 (90.7) | 0.091 | 986 (86.5) | 406 (92.9) | <0.001 *** | 144 (89.4) | 0.300 |
| Mild | 21 (5.2) | 130 (12.8) | <0.001 *** | 37 (8.2) | 0.082 | 134 (11.8) | 24 (5.5) | <0.001 *** | 15 (9.3) | 0.363 |
| Severe | 4 (1.0) | 23 (2.3) | 0.112 | 5 (1.1) | 1.000 | 20 (1.8) | 7 (1.6) | 0.834 | 2 (1.2) | 1.000 |
| Encephalopathy, n (%) | ||||||||||
| No evidence of encephalopathy | 404 (99.8) | 1002 (98.6) | 0.081 | 448 (98.9) | 0.222 | 1130 (99.1) | 432 (98.9) | 0.575 | 158 (98.1) | 0.211 |
| Mild | 1 (0.2) | 13 (1.3) | 0.131 | 5 (1.1) | 0.222 | 9 (0.8) | 5 (1.1) | 0.550 | 3 (1.9) | 0.177 |
| Severe | 0 (0.0) | 1 (0.1) | 1.000 | 0 (0.0) | 1.000 | 1 (0.1) | 0 (0.0) | 1.000 | 0 (0.0) | 1.000 |
| Modified ALBI grade, n (%) | ||||||||||
| Grade 1 | 138 (34.1) | 213 (21.0) | <0.001 *** | 143 (31.6) | 0.435 | 306 (26.8) | 112 (25.6) | 0.625 | 42 (26.1) | 0.839 |
| Grade 2A | 140 (34.6) | 279 (27.5) | <0.01 ** | 130 (28.7) | 0.065 | 326 (28.6) | 132 (30.2) | 0.529 | 55 (34.2) | 0.146 |
| Grade 2B | 106 (26.2) | 401 (39.5) | <0.001 *** | 148 (32.7) | <0.05 * | 391 (34.3) | 155 (35.5) | 0.662 | 56 (34.8) | 0.904 |
| Grade 3 | 21 (5.2) | 123 (12.1) | <0.001 *** | 32 (7.1) | 0.254 | 117 (10.3) | 38 (8.7) | 0.349 | 8 (5.0) | <0.05 * |
| Comorbidities | ||||||||||
| Diabetes mellitus | 189 (46.7) | 435 (42.8) | 0.187 | 209 (46.1) | 0.877 | 490 (43.0) | 181 (41.4) | 0.574 | 97 (60.2) | <0.001 *** |
| Hypertension | 318 (78.5) | 755 (74.3) | 0.096 | 339 (74.8) | 0.203 | 837 (73.4) | 363 (83.1) | <0.001 *** | 117 (72.7) | 0.840 |
| CCI, mean ± SD [median] | 6.6 ± 2.6 [6.0] | 5.8 ± 2.4 [5.0] | <0.001 *** | 5.8 ± 2.4 [6.0] | <0.001 *** | 6.0 ± 2.5 [6.0] | 6.0 ± 2.5 [5.0] | 0.596 | 6.0 ± 2.4 [6.0] | 0.927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, D.E.; Tan, R.; Xiang, C.; Mu, F.; Hernandez, S.; Ogale, S.; Li, J.; Lin, Y.; Shi, L.; Singal, A.G. Overall Survival in Real-World Patients with Unresectable Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab Versus Sorafenib or Lenvatinib as First-Line Therapy: Findings from the National Veterans Health Administration Database. Cancers 2024, 16, 3508. https://doi.org/10.3390/cancers16203508
Kaplan DE, Tan R, Xiang C, Mu F, Hernandez S, Ogale S, Li J, Lin Y, Shi L, Singal AG. Overall Survival in Real-World Patients with Unresectable Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab Versus Sorafenib or Lenvatinib as First-Line Therapy: Findings from the National Veterans Health Administration Database. Cancers. 2024; 16(20):3508. https://doi.org/10.3390/cancers16203508
Chicago/Turabian StyleKaplan, David E., Ruoding Tan, Cheryl Xiang, Fan Mu, Sairy Hernandez, Sarika Ogale, Jiayang Li, Yilu Lin, Lizheng Shi, and Amit G. Singal. 2024. "Overall Survival in Real-World Patients with Unresectable Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab Versus Sorafenib or Lenvatinib as First-Line Therapy: Findings from the National Veterans Health Administration Database" Cancers 16, no. 20: 3508. https://doi.org/10.3390/cancers16203508
APA StyleKaplan, D. E., Tan, R., Xiang, C., Mu, F., Hernandez, S., Ogale, S., Li, J., Lin, Y., Shi, L., & Singal, A. G. (2024). Overall Survival in Real-World Patients with Unresectable Hepatocellular Carcinoma Receiving Atezolizumab Plus Bevacizumab Versus Sorafenib or Lenvatinib as First-Line Therapy: Findings from the National Veterans Health Administration Database. Cancers, 16(20), 3508. https://doi.org/10.3390/cancers16203508






