Prognostic Value of Myosteatosis and Albumin–Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Population
2.3. Data Collection
2.4. Skeletal Muscle Index (SMI) and Skeletal Muscle Density (SMD)
2.5. TACE Procedure and Treatment Schedule
2.6. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
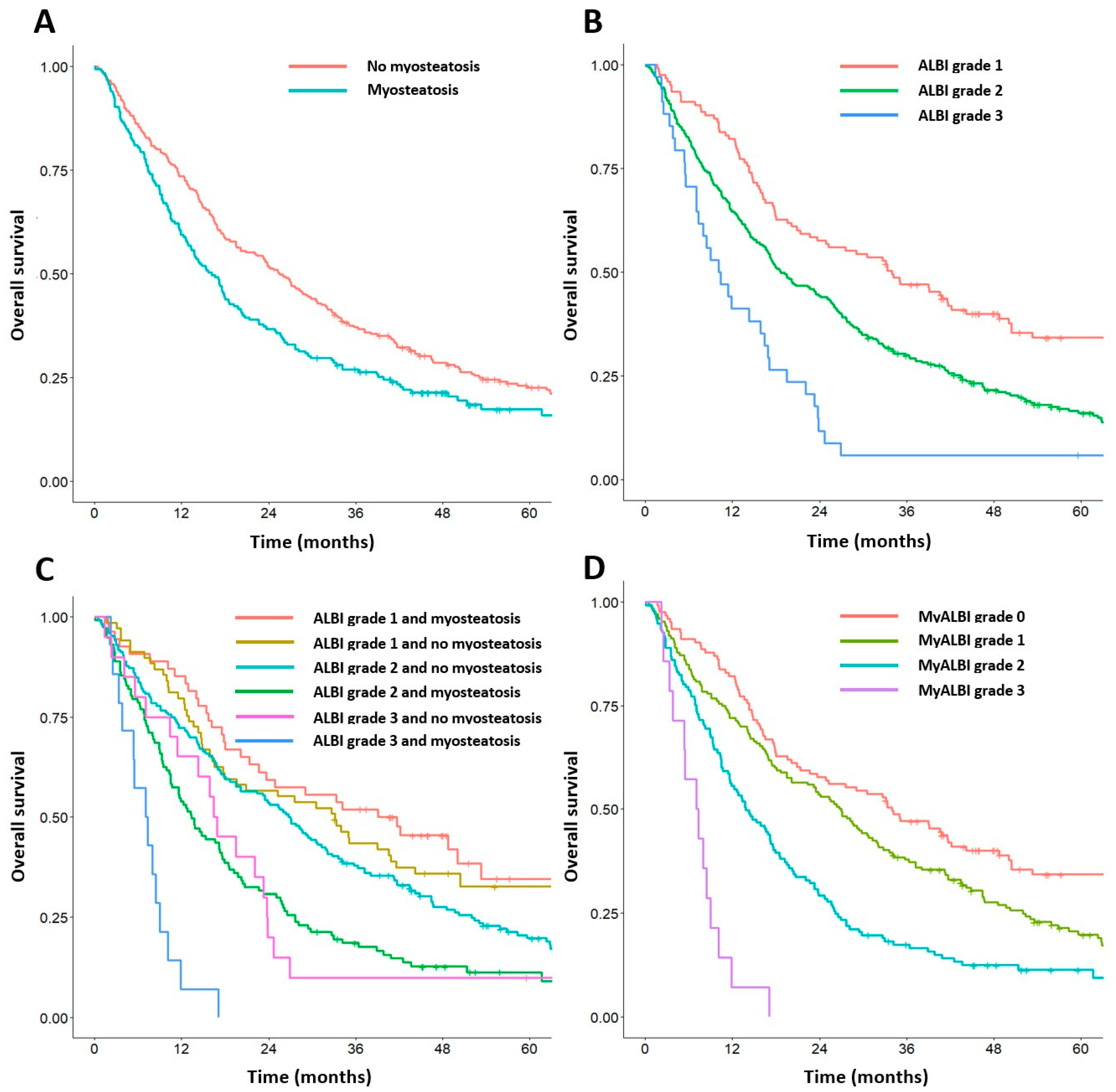
3.2. Overall Survival Analysis
3.3. Correlation Between Myosteatosis and Clinical Parameters
3.4. Establishment of the Prognostic Score Based on Myosteatosis and ALBI Grade
3.5. Cumulative OS Rates According to the Myo-ALBI Grade
3.6. Predictive Nomogram Based on the Myo-ALBI Grade
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Burroughs, A.; Dufour, J.-F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B.; Bolondi, L. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef]
- Ha, Y.; Shim, J.H.; Kim, S.; Kim, K.M.; Lim, Y.; Lee, H.C. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J. Gastroenterol. Hepatol. 2014, 29, 787–793. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach—The ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, T.; Li, H.; Wang, M.; Xu, K.; Zheng, D.; Du, X.; Liu, L. Comparison of albumin-bilirubin grade versus Child-Pugh score in predicting the outcome of transarterial chemoembolization for hepatocellular carcinoma using time-dependent ROC. Ann. Transl. Med. 2020, 8, 538. [Google Scholar] [CrossRef]
- Lu, L.-H.; Zhang, Y.-F.; Mu-Yan, C.; Kan, A.; Zhong, X.-P.; Mei, J.; Ling, Y.-H.; Li, S.-H.; Shi, M.; Wei, W.; et al. Platelet-albumin-bilirubin grade: Risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 1430–1437. [Google Scholar] [CrossRef]
- Kariyama, K.; Nouso, K.; Hiraoka, A.; Wakuta, A.; Oonishi, A.; Kuzuya, T.; Toyoda, H.; Tada, T.; Tsuji, K.; Itobayashi, E.; et al. EZ-ALBI Score for Predicting Hepatocellular Carcinoma Prognosis. Liver Cancer 2020, 9, 734–743. [Google Scholar] [CrossRef]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T. Validation of the ALBI-TAE model and comparison of seven scoring systems for predicting survival outcome in patients with intermediate-stage hepatocellular carcinoma undergoing chemoembolization. Cancer Imaging 2023, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Miljkovic, I.; Zmuda, J.M. Epidemiology of myosteatosis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.; Shachar, S.; Nyrop, K.; Muss, H.; Malpica, L.; Williams, G. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Computed tomography-defined low skeletal muscle index and density in cancer patients: Observations from a systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Ina, N.; Chichareon, P. Association of myosteatosis with treatment response and survival in patients with hepatocellular carcinoma undergoing chemoembolization: A retrospective cohort study. Sci. Rep. 2023, 13, 3978. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Cheewakul, J.; Yanaihap, N.; Yue-roh, T.; Kepan, N.; Thiangsook, W.; Rojanaaumpawan, T.; Ina, N. Automated segmentation of skeletal muscle at the third lumbar vertebra using deep learning in computed tomography images. J. Med. Imaging Radiat. Sci. 2022, 53, S37. [Google Scholar] [CrossRef]
- Antoun, S.; Lanoy, E.; Iacovelli, R.; Albiges-Sauvin, L.; Loriot, Y.; Merad-Taoufik, M.; Fizazi, K.; di Palma, M.; Baracos, V.E.; Escudier, B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013, 119, 3377–3384. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-H.; Choi, M.H.; Lee, I.S.; Hong, T.H.; Lee, M.A. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: A retrospective observational study. BMC Cancer 2021, 21, 77. [Google Scholar] [CrossRef]
- Miljkovic, I.; Kuipers, A.L.; Cvejkus, R.; Bunker, C.H.; Patrick, A.L.; Gordon, C.L.; Zmuda, J.M. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity 2016, 24, 476–482. [Google Scholar] [CrossRef]
- Miljkovic, I.; Cauley, J.A.; Wang, P.Y.; Holton, K.F.; Lee, C.G.; Sheu, Y.; Barrett-Connor, E.; Hoffman, A.R.; Lewis, C.B.; Orwoll, E.S.; et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 2013, 21, 2118–2125. [Google Scholar] [CrossRef]
- Toyoda, H.; Johnson, P.J. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022, 4, 100557. [Google Scholar] [CrossRef] [PubMed]
- Jeng, L.-B.; Chan, W.-L.; Teng, C.-F. Prognostic Significance of Serum Albumin Level and Albumin-Based Mono- and Combination Biomarkers in Patients with Hepatocellular Carcinoma. Cancers 2023, 15, 1005. [Google Scholar] [CrossRef]
- Lee, I.-C.; Chen, Y.-T.; Chao, Y.; Huo, T.-I.; Li, C.-P.; Su, C.-W.; Lin, H.-C.; Lee, F.-Y.; Huang, Y.-H. Determinants of survival after sorafenib failure in patients with BCLC-C hepatocellular carcinoma in real-world practice. Medicine 2015, 94, e688. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.; Fulcher, N.; Seal, B.; Pham, T.; Wang, Y.; Paulson, S.; He, A.R. Clinical outcomes by Child-Pugh Class in patients with advanced hepatocellular carcinoma in a community oncology setting. Hepatic Oncol. 2023, 10, HEP47. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Early experience of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma BCLC-B stage patients classified as beyond up to seven criteria—Multicenter analysis. Hepatol. Res. 2022, 52, 308–316. [Google Scholar] [CrossRef]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Janjindamai, P.; Akkakrisee, S.; Piratvisuth, T.; Geater, A. Decision-Making Scoring System for the Repetition of Conventional Transarterial Chemoembolization in Patients With Inoperable Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2022, 13, e00506. [Google Scholar] [CrossRef]



| Baseline Characteristics | All Cohorts (n = 446) |
|---|---|
| Age (years, mean ± SD) | 62.0 ± 10.9 |
| Male, n (%) | 320 (71.7) |
| Comorbidity, n (%) | |
| Diabetes | 126 (28.3) |
| Hypertension | 124 (27.8) |
| Cardiovascular disease | 29 (6.5) |
| Chronic kidney disease | 17 (3.8) |
| Body composition | |
| BMI (kg/m2, <20.0/20.0–24.9/≥25.0), n (%) | 75 (16.8)/220 (49.3)/151 (33.9) |
| SMI (cm2/m2, median (IQR)) | 37.5 (31.9, 43.44) |
| Sarcopenia, n (%) | 144 (32.3) |
| SMD (HU, median (IQR)) | 44.3 (38.6, 48.7) |
| Myosteatosis, n (%) | 185 (41.5) |
| Etiology, n (%) | |
| Hepatitis B | 213 (47.8) |
| Hepatitis C | 95 (21.3) |
| Alcohol | 49 (11.0) |
| Others | 89 (19.9) |
| Laboratory values | |
| Total bilirubin (mg/dL, median (IQR)) | 0.86 (0.53, 1.34) |
| Albumin (g/dL, mean ± SD) | 3.5 ± 0.5 |
| Platelet (×103/L, median (IQR)) | 121 (77, 194) |
| International normalized ratio, median (IQR) | 1.2 (1.1, 1.3) |
| Alpha-fetoprotein (ng/mL, <20/20–200/>200), n (%) | 189 (42.4)/111 (24.9)/146 (32.7) |
| Tumor characteristics | |
| Maximal tumor diameter (<2/2–5/>5 cm), n (%) | 57 (12.8)/187 (41.9)/202 (45.3) |
| Number of tumors (1/2/3/>3), n (%) | 191 (42.8)/79 (17.7)/58 (13.0)/118 (26.5) |
| BCLC staging (A/B/C), n (%) | 122 (27.4)/303 (67.9)/21 (4.7) |
| Models | |
| Child–Pugh class (A/B7/B8–9), n (%) | 363 (81.4)/47 (10.5)/36 (8.1) |
| ALBI grade (1/2/3), n (%) | 123 (27.6)/ 289 (64.8)/ 34 (7.6) |
| TACE sessions, median (IQR) | 2 (1, 4) |
| Clinicopathological Factors | Reference | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | ||
| Age > 65 years | ≤65 years | 0.99 (0.80–1.22) | 0.925 | ||
| Gender: female | male | 0.97 (0.77–1.22) | 0.819 | ||
| BMI: ≥25.0 kg/m2 | <25.0 kg/m2 | 0.92 (0.74–1.14) | 0.452 | ||
| Diabetes: yes | no | 0.88 (0.70–1.10) | 0.258 | ||
| Hypertension: yes | no | 1.13 (0.90–1.42) | 0.294 | ||
| Cardiovascular disease: yes | no | 1.07 (0.72–1.60) | 0.727 | ||
| Chronic kidney disease: yes | no | 0.69 (0.39–1.22) | 0.198 | ||
| Hepatitis B virus carrier: yes | no | 1.05 (0.86–1.29) | 0.635 | ||
| Hepatitis C virus carrier: yes | no | 1.05 (0.82–1.33) | 0.708 | ||
| Non-viral hepatitis: yes | no | 0.89 (0.71–1.12) | 0.311 | ||
| Platelet: <100 (×104 µL) | ≥100 | 0.94 (0.76–1.16) | 0.550 | ||
| INR: >1.2 | ≤1.2 | 1.15 (0.93–1.41) | 0.195 | ||
| Creatinine: >1.2 mg/dL | ≤1.2 mg/dL | 1.24 (0.89–1.74) | 0.205 | ||
| Albumin: <4.0 g/dL | ≥4.0 g/dL | 1.69 (1.27–2.25) | <0.001 | ||
| Bilirubin: >2.0 mg/dL | ≤2.0 mg/dL | 1.37 (1.00–1.89) | 0.054 | ||
| ALBI grade | Grade 1 | ||||
| Grade 2 | 1.78 (1.39–2.29) | <0.001 | 1.96 (1.53–2.53) | <0.001 | |
| Grade 3 | 3.28 (2.17–4.96) | <0.001 | 3.37 (2.22–5.12) | <0.001 | |
| Alpha-fetoprotein: >200 ng/mL | ≤200 ng/mL | 1.68 (1.36–2.09) | <0.001 | 1.47 (1.18–1.83) | <0.001 |
| Up-to-seven criteria: within | beyond | 2.11 (1.71–2.59) | <0.001 | 2.00 (1.61–2.47) | <0.001 |
| Sarcopenia: present | Absent | 1.24 (1.00–1.54) | 0.049 | ||
| Myosteatosis: present | Absent | 1.34 (1.09–1.66) | 0.006 | 1.41 (1.14–1.75) | 0.002 |
| Clinicopathological Factors | Myo-ALBI Grade | ||||
|---|---|---|---|---|---|
| Grade 0 (n = 123) | Grade 1 (n = 172) | Grade 2 (n = 137) | Grade 3 (n = 14) | p-Value | |
| Age, year, mean (SD) | 61.9 (11.7) | 59.1 (9.8) | 65.4 (10.6) | 67.1 (10.7) | <0.001 |
| Sex, n (%) | 0.046 | ||||
| Male | 96 (78.0) | 128 (74.4) | 87 (63.5) | 9 (64.3) | |
| Female | 27 (22.0) | 44 (25.6) | 50 (36.5) | 5 (35.7) | |
| BMI, kg/m2, median (IQR) | 23.1 (20.9, 26.1) | 23.3 (20.8, 25.1) | 24.5 (21.9, 27.3) | 24.0 (22.5, 26.3) | 0.032 |
| Diabetes, n (%) | 0.895 | ||||
| No | 89 (72.4) | 126 (73.3) | 95 (69.3) | 10 (71.4) | |
| Yes | 34 (27.6) | 46 (26.7) | 42 (30.7) | 4 (28.6) | |
| Hypertension, n (%) | <0.001 | ||||
| No | 83 (67.5) | 142 (82.6) | 86 (62.8) | 11 (78.6) | |
| Yes | 40 (32.5) | 30 (17.4) | 51 (37.2) | 3 (21.4) | |
| Cardiovascular disease, n (%) | 0.064 | ||||
| No | 109 (88.6) | 163 (94.8) | 131 (95.6) | 14 (100) | |
| Yes | 14 (11.4) | 9 (5.2) | 6 (4.4) | 0 (0) | |
| Chronic kidney disease, n (%) | 0.498 | ||||
| No | 116 (94.3) | 165 (95.9) | 134 (97.8) | 14 (100) | |
| Yes | 7 (5.7) | 7 (4.1) | 3 (2.2) | 0 (0) | |
| Hepatitis B virus carrier, n (%) | 0.005 | ||||
| No | 62 (50.4) | 73 (42.4) | 85 (62.0) | 9 (64.3) | |
| Yes | 61 (49.6) | 99 (57.6) | 52 (38.0) | 5 (35.7) | |
| Hepatitis C virus carrier, n (%) | 0.167 | ||||
| No | 101 (82.1) | 134 (77.9) | 104 (75.9) | 8 (57.1) | |
| Yes | 22 (17.9) | 38 (22.1) | 33 (24.1) | 6 (42.9) | |
| Platelet, ×103/mm3, median (IQR) | 186 (138.5, 233) | 89.5 (68.8, 148.8) | 114 (75, 171) | 97 (65.8, 140.2) | <0.001 |
| INR, median (IQR) | 1.1 (1, 1.1) | 1.2 (1.2, 1.4) | 1.2 (1.1, 1.3) | 1.3 (1.3, 1.4) | <0.001 |
| Creatinine, median (IQR) | 0.9 (0.8, 1.1) | 0.9 (0.7, 1) | 0.9 (0.7, 1.1) | 0.8 (0.7, 1.1) | 0.298 |
| Albumin, ng/mL, median (IQR) | 4.1 (3.9, 4.3) | 3.4 (3.1, 3.6) | 3.2 (2.9, 3.5) | 2.6 (2.5, 2.7) | <0.001 |
| Bilirubin, mg/dL, median (IQR) | 0.5 (0.4, 0.6) | 1 (0.7, 1.4) | 1 (0.7, 1.6) | 2.1 (1.4, 2.7) | <0.001 |
| Alpha-fetoprotein, ng/mL, n (%) | 0.261 | ||||
| >200 | 85 (69.1) | 116 (67.4) | 93 (67.9) | 6 (42.9) | |
| ≤200 | 38 (30.9) | 56 (32.6) | 44 (32.1) | 8 (57.1) | |
| Size of largest tumor, cm, median (IQR) | 5.4 (3.2, 9.2) | 3.8 (2.6, 6.7) | 4.7 (3.1, 9) | 5.1 (3.9, 9) | 0.011 |
| Tumor number, n | <0.001 | ||||
| ≤3 | 106 (86.2) | 124 (72.1) | 90 (65.7) | 8 (57.1) | |
| >3 | 17 (13.8) | 48 (27.9) | 47 (34.3) | 6 (42.9) | |
| Sarcopenia, n (%) | <0.001 | ||||
| Absent | 80 (65.0) | 140 (81.4) | 76 (55.5) | 6 (42.9) | |
| Present | 43 (35.0) | 32 (18.6) | 61 (44.5) | 8 (57.1) | |
| Clinicopathological Factors | Multivariate Analysis | |
|---|---|---|
| Hazard Ratio (95% CI) | p-Value | |
| Myo-ALBI grade | <0.001 | |
| Grade 0 | Reference | |
| Grade 1 | 1.63 (1.24–2.15) | |
| Grade 2 | 2.44 (1.83–3.24) | |
| Grade 3 | 5.93 (3.28–10.72) | |
| Alpha-fetoprotein | 0.001 | |
| ≤200 ng/mL | Reference | |
| >200 ng/mL | 1.45 (1.16–1.81) | |
| Up-to-seven criteria | <0.001 | |
| Within | Reference | |
| Beyond | 1.96 (1.58–2.43) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Ina, N. Prognostic Value of Myosteatosis and Albumin–Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization. Cancers 2024, 16, 3503. https://doi.org/10.3390/cancers16203503
Bannangkoon K, Hongsakul K, Tubtawee T, Ina N. Prognostic Value of Myosteatosis and Albumin–Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization. Cancers. 2024; 16(20):3503. https://doi.org/10.3390/cancers16203503
Chicago/Turabian StyleBannangkoon, Kittipitch, Keerati Hongsakul, Teeravut Tubtawee, and Natee Ina. 2024. "Prognostic Value of Myosteatosis and Albumin–Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization" Cancers 16, no. 20: 3503. https://doi.org/10.3390/cancers16203503
APA StyleBannangkoon, K., Hongsakul, K., Tubtawee, T., & Ina, N. (2024). Prognostic Value of Myosteatosis and Albumin–Bilirubin Grade for Survival in Hepatocellular Carcinoma Post Chemoembolization. Cancers, 16(20), 3503. https://doi.org/10.3390/cancers16203503





