Development of an MRI Radiomic Machine-Learning Model to Predict Triple-Negative Breast Cancer Based on Fibroglandular Tissue of the Contralateral Unaffected Breast in Breast Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histopathologic Analysis
2.3. Imaging Acquisition
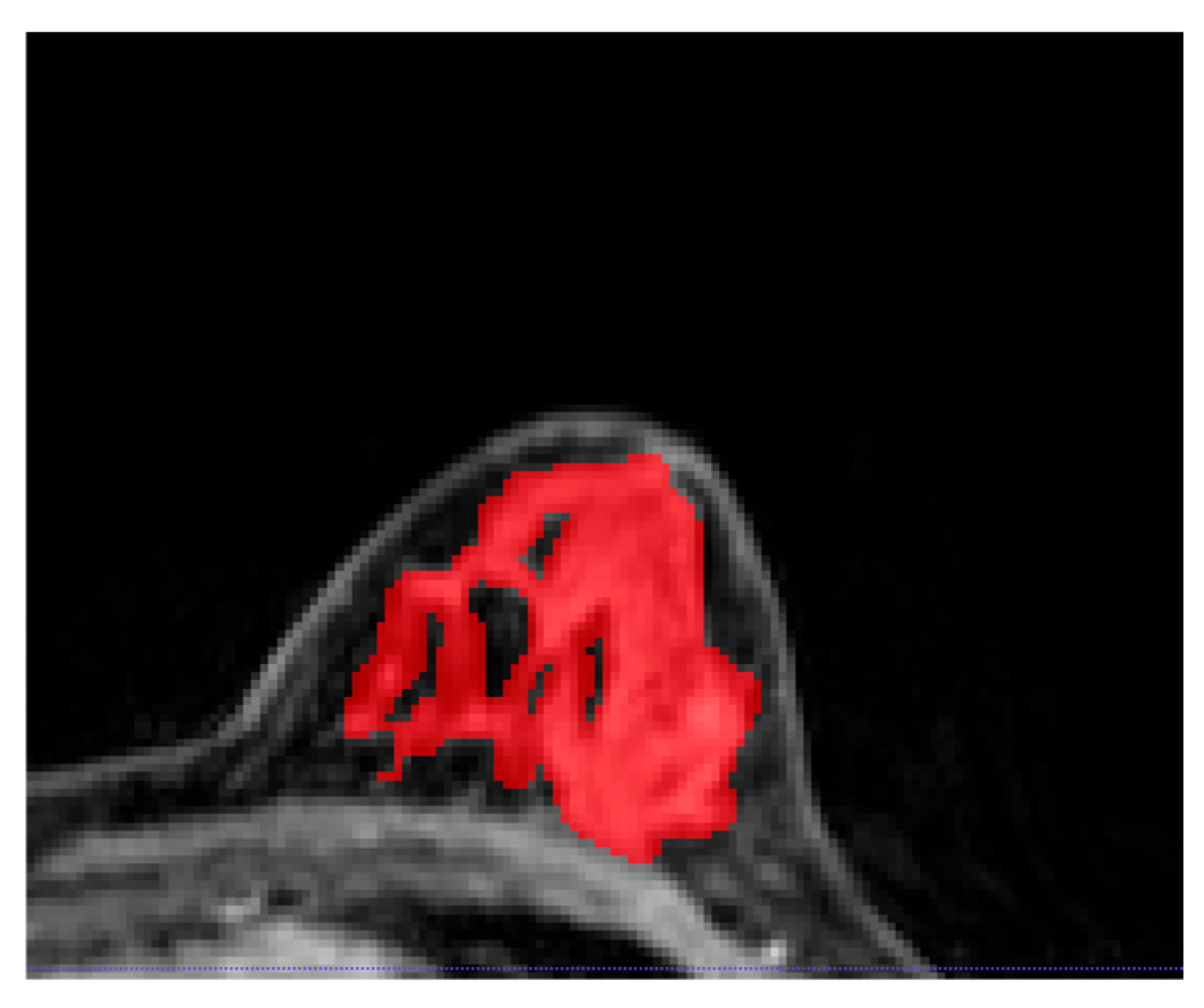
2.4. Image Segmentation
2.5. Radiomics Analysis, Machine Learning, and Prediction Model Building
3. Results
3.1. Clinical–Pathologic Characteristics
3.2. Significant Radiomic Features
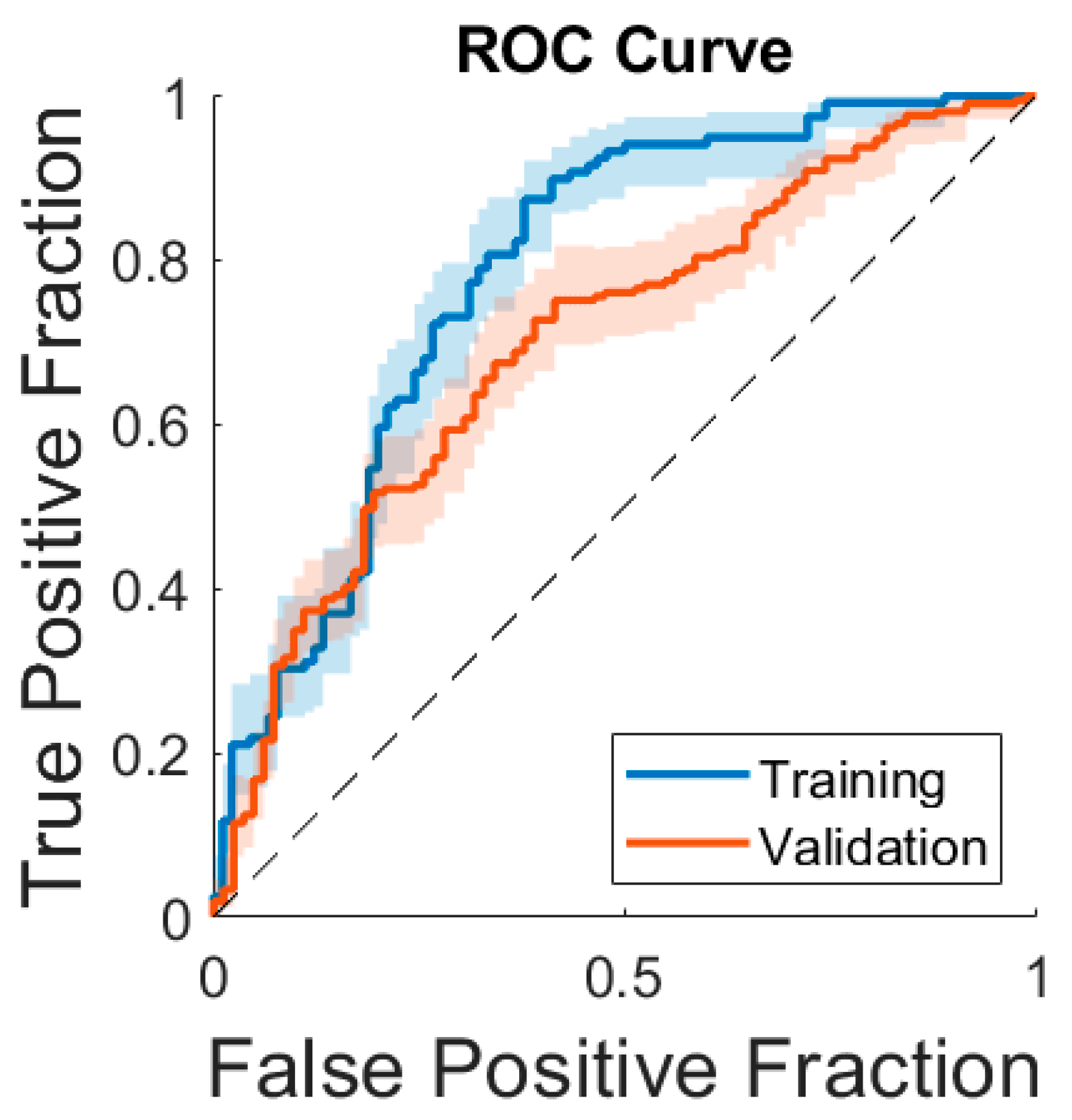
3.3. Model Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Radiology. ACR Practice Parameter for the Performance of Contrast-Enhanced Magnetic Resonance Imaging (MRI) of the Breast. Revised 2023 (Resolution 8). Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/mr-contrast-breast.pdf (accessed on 21 February 2024).
- Wernli, K.J.; DeMartini, W.B.; Ichikawa, L.; Lehman, C.D.; Onega, T.; Kerlikowske, K.; Henderson, L.M.; Geller, B.M.; Hofmann, M.; Yankaskas, B.C. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern. Med. 2014, 174, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, E.M.; Drohan, B.; Bosinoff, P.; Semine, A.; Jellinek, M.; Cronin, C.; Millham, F.; Dowd, D.; Rourke, T.; Block, C.; et al. Which risk model to use? Clinical implications of the ACS MRI screening guidelines. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J. Natl. Cancer Inst. 2011, 103, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute, DCCPS. Cancer Stat Facts: Female Breast Cancer Subtypes. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 21 February 2024).
- Stead, L.A.; Lash, T.L.; Sobieraj, J.E.; Chi, D.D.; Westrup, J.L.; Charlot, M.; Blanchard, R.A.; Lee, J.C.; King, T.C.; Rosenberg, C.L. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009, 11, R18. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Prossnitz, E.R.; Royce, M.; Nibbe, A. Temporal trends in breast cancer survival by race and ethnicity: A population-based cohort study. PLoS ONE 2019, 14, e0224064. [Google Scholar] [CrossRef]
- Dietze, E.C.; Sistrunk, C.; Miranda-Carboni, G.; O’Regan, R.; Seewaldt, V.L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 2015, 15, 248–254. [Google Scholar] [CrossRef]
- Roberts, E.; Howell, S.; Evans, D.G. Polygenic risk scores and breast cancer risk prediction. Breast 2023, 67, 71–77. [Google Scholar] [CrossRef]
- Shimelis, H.; LaDuca, H.; Hu, C.; Hart, S.N.; Na, J.; Thomas, A.; Akinhanmi, M.; Moore, R.M.; Brauch, H.; Cox, A.; et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J. Natl. Cancer Inst. 2018, 110, 855–862. [Google Scholar] [CrossRef]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet 2019, 104, 21–34. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Leithner, D.; Mayerhoefer, M.E.; Martinez, D.F.; Jochelson, M.S.; Morris, E.A.; Thakur, S.B.; Pinker, K. Non-Invasive Assessment of Breast Cancer Molecular Subtypes with Multiparametric Magnetic Resonance Imaging Radiomics. J. Clin. Med. 2020, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.H.; Lin, Y.; Chan, S.; Zhou, J.; Chow, D.; Chang, P.; Kwong, T.; Yeh, D.C.; Wang, X.; et al. Prediction of breast cancer molecular subtypes on DCE-MRI using convolutional neural network with transfer learning between two centers. Eur. Radiol. 2021, 31, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Bai, L.; Jia, H.; Lin, G. Noninvasive assessment of breast cancer molecular subtypes on multiparametric MRI using convolutional neural network with transfer learning. Thorac. Cancer 2022, 13, 3183–3191. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef]
- Besson, F.L.; Henry, T.; Meyer, C.; Chevance, V.; Roblot, V.; Blanchet, E.; Arnould, V.; Grimon, G.; Chekroun, M.; Mabille, L.; et al. Rapid Contour-based Segmentation for (18)F-FDG PET Imaging of Lung Tumors by Using ITK-SNAP: Comparison to Expert-based Segmentation. Radiology 2018, 288, 277–284. [Google Scholar] [CrossRef]
- Deasy, J.O.; Blanco, A.I.; Clark, V.H. CERR: A computational environment for radiotherapy research. Med. Phys. 2003, 30, 979–985. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Ballman, K.; Polley, M.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.S.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.C.; Henley, S.J.; Miller, J.W.; Massetti, G.; Thomas, C.C. Patterns and Trends in Age-Specific Black-White Differences in Breast Cancer Incidence and Mortality—United States, 1999–2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Mohammed, S.A.; Shields, A.E. Understanding and effectively addressing breast cancer in African American women: Unpacking the social context. Cancer 2016, 122, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A. Breast Cancer Disparities: Socioeconomic Factors versus Biology. Ann. Surg. Oncol. 2017, 24, 2869–2875. [Google Scholar] [CrossRef]
- Leithner, D.; Horvat, J.V.; Marino, M.A.; Bernard-Davila, B.; Jochelson, M.S.; Ochoa-Albiztegui, R.E.; Martinez, D.F.; Morris, E.A.; Thakur, S.; Pinker, K. Radiomic signatures with contrast-enhanced magnetic resonance imaging for the assessment of breast cancer receptor status and molecular subtypes: Initial results. Breast Cancer Res. 2019, 21, 106. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, N.; Liu, M.; Shi, Y.; Ma, H.; Dong, J.; Zhang, X.; Duan, S.; Wang, B.; Xie, H. Radiomic analysis on magnetic resonance diffusion weighted image in distinguishing triple-negative breast cancer from other subtypes: A feasibility study. Clin. Imaging 2021, 72, 136–141. [Google Scholar] [CrossRef]
- Sha, Y.S.; Chen, J.F. MRI-based radiomics for the diagnosis of triple-negative breast cancer: A meta-analysis. Clin. Radiol. 2022, 77, 655–663. [Google Scholar] [CrossRef]
- Demircioglu, A.; Grueneisen, J.; Ingenwerth, M.; Hoffmann, O.; Pinker-Domenig, K.; Morris, E.; Haubold, J.; Forsting, M.; Nensa, F.; Umutlu, L. A rapid volume of interest-based approach of radiomics analysis of breast MRI for tumor decoding and phenotyping of breast cancer. PLoS ONE 2020, 15, e0234871. [Google Scholar] [CrossRef]
- Wu, M.; Zhong, X.; Peng, Q.; Xu, M.; Huang, S.; Yuan, J.; Ma, J.; Tan, T. Prediction of molecular subtypes of breast cancer using BI-RADS features based on a “white box” machine learning approach in a multi-modal imaging setting. Eur. J. Radiol. 2019, 114, 175–184. [Google Scholar] [CrossRef]
- Wang, J.; Kato, F.; Oyama-Manabe, N.; Li, R.; Cui, Y.; Tha, K.K.; Yamashita, H.; Kudo, K.; Shirato, H. Identifying Triple-Negative Breast Cancer Using Background Parenchymal Enhancement Heterogeneity on Dynamic Contrast-Enhanced MRI: A Pilot Radiomics Study. PLoS ONE 2015, 10, e0143308. [Google Scholar] [CrossRef]
- Ma, M.; Gan, L.; Jiang, Y.; Qin, N.; Li, C.; Zhang, Y.; Wang, X. Radiomics Analysis Based on Automatic Image Segmentation of DCE-MRI for Predicting Triple-Negative and Nontriple-Negative Breast Cancer. Comput. Math. Methods Med. 2021, 2021, 2140465. [Google Scholar] [CrossRef] [PubMed]
- Yala, A.; Mikhael, P.G.; Strand, F.; Lin, G.; Satuluru, S.; Kim, T.; Banerjee, I.; Gichoya, J.; Trivedi, H.; Lehman, C.D.; et al. Multi-Institutional Validation of a Mammography-Based Breast Cancer Risk Model. J. Clin. Oncol. 2022, 40, 1732–1740. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, S.; Zhang, L.; Chen, Q.; Liu, S.; Zhang, B. Artificial Intelligence Risk Model (Mirai) Delivers Robust Generalization and Outperforms Tyrer-Cuzick Guidelines in Breast Cancer Screening. J. Clin. Oncol. 2022, 40, 2280–2281. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Brooks, J.D.; Bernstein, J.L.; Reiner, A.S.; Pike, M.C.; Morris, E.A. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011, 260, 50–60. [Google Scholar] [CrossRef]
- Niell, B.L.; Abdalah, M.; Stringfield, O.; Raghunand, N.; Ataya, D.; Gillies, R.; Balagurunathan, Y. Quantitative Measures of Background Parenchymal Enhancement Predict Breast Cancer Risk. AJR Am. J. Roentgenol. 2021, 217, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Grimm, L.J.; Ghate, S.V.; Kim, C.E.; Soo, M.S.; Yoon, S.C.; Mazurowski, M.A. Machine learning-based prediction of future breast cancer using algorithmically measured background parenchymal enhancement on high-risk screening MRI. J. Magn. Reson. Imaging 2019, 50, 456–464. [Google Scholar] [CrossRef]
- Wang, H.; van der Velden, B.H.M.; Verburg, E.; Bakker, M.F.; Pijnappel, R.M.; Veldhuis, W.B.; van Gils, C.H.; Gilhuijs, K.G.A. Assessing Quantitative Parenchymal Features at Baseline Dynamic Contrast-enhanced MRI and Cancer Occurrence in Women with Extremely Dense Breasts. Radiology 2023, 308, e222841. [Google Scholar] [CrossRef]

| F1 Score | AUC | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| Training set (n = 250) | 78% [71–86%] | 78% [72–84%] | 81% [74–88%] | 67% [57–76%] | 76% [69–84%] | 72% [63–82%] |
| Validation set (n = 291) | 66% [59–72%] | 71% [63–76%] | 54% [47–60%] | 74% [65–84%] | 84% [78–90%] | 39% [31–47%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Gullo, R.; Ochoa-Albiztegui, R.E.; Chakraborty, J.; Thakur, S.B.; Robson, M.; Jochelson, M.S.; Varela, K.; Resch, D.; Eskreis-Winkler, S.; Pinker, K. Development of an MRI Radiomic Machine-Learning Model to Predict Triple-Negative Breast Cancer Based on Fibroglandular Tissue of the Contralateral Unaffected Breast in Breast Cancer Patients. Cancers 2024, 16, 3480. https://doi.org/10.3390/cancers16203480
Lo Gullo R, Ochoa-Albiztegui RE, Chakraborty J, Thakur SB, Robson M, Jochelson MS, Varela K, Resch D, Eskreis-Winkler S, Pinker K. Development of an MRI Radiomic Machine-Learning Model to Predict Triple-Negative Breast Cancer Based on Fibroglandular Tissue of the Contralateral Unaffected Breast in Breast Cancer Patients. Cancers. 2024; 16(20):3480. https://doi.org/10.3390/cancers16203480
Chicago/Turabian StyleLo Gullo, Roberto, Rosa Elena Ochoa-Albiztegui, Jayasree Chakraborty, Sunitha B. Thakur, Mark Robson, Maxine S. Jochelson, Keitha Varela, Daphne Resch, Sarah Eskreis-Winkler, and Katja Pinker. 2024. "Development of an MRI Radiomic Machine-Learning Model to Predict Triple-Negative Breast Cancer Based on Fibroglandular Tissue of the Contralateral Unaffected Breast in Breast Cancer Patients" Cancers 16, no. 20: 3480. https://doi.org/10.3390/cancers16203480
APA StyleLo Gullo, R., Ochoa-Albiztegui, R. E., Chakraborty, J., Thakur, S. B., Robson, M., Jochelson, M. S., Varela, K., Resch, D., Eskreis-Winkler, S., & Pinker, K. (2024). Development of an MRI Radiomic Machine-Learning Model to Predict Triple-Negative Breast Cancer Based on Fibroglandular Tissue of the Contralateral Unaffected Breast in Breast Cancer Patients. Cancers, 16(20), 3480. https://doi.org/10.3390/cancers16203480








