Comprehensive Analysis of Receptor Status, Histopathological Classifications (B1–B5), and Cumulative Histological Dimensions in Breast Cancer: Predictors of Malignancy and Diagnostic Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Comparative Analysis of Demographic, Lifestyle, and Clinical Factors between Malignant and Benign Breast Lesions
3.2. Logistic Regression Analysis and Performance Evaluation of Predictors for Malignancy in Breast Lesions
3.3. Linear Regression Analysis of Predictors for Cumulative Histological Dimension in Breast Lesions
4. Discussion
4.1. Demographic Factors and Patient History
4.2. Diagnostic Tools, Hormone Receptor Status, and B Classification
4.3. Tumor Size and Extent
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, V.B.; Anastasiu-Popov, D.M.; Eftenoiu, A.E.; Popescu, R.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Puscasiu, D.; Cobec, I.M. Observational Study of Men and Women with Breast Cancer in Terms of Overall Survival. Cancers 2024, 16, 3049. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.M.; Al-Sharif, S.; Ala’ Bereshy, R.; Al-Eitan, S.F.; Banikhaled, S.H.; Al-Qudimat, A.R.; Al-Zoubi, R.M.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef]
- Bidoli, E.; Virdone, S.; Hamdi-Cherif, M.; Toffolutti, F.; Taborelli, M.; Panato, C.; Serraino, D. Worldwide Age at Onset of Female Breast Cancer: A 25-Year Population-Based Cancer Registry Study. Sci. Rep. 2019, 9, 14111. [Google Scholar] [CrossRef]
- Barclay, N.L.; Burn, E.; Delmestri, A.; Duarte-Salles, T.; Golozar, A.; Man, W.Y.; Tan, E.H.; Tietzova, I.; Prieto-Alhambra, D.; Newby, D. Trends in incidence, prevalence, and survival of breast cancer in the United Kingdom from 2000 to 2021. Sci. Rep. 2024, 14, 19069. [Google Scholar] [CrossRef]
- Hendrick, R.E.; Monticciolo, D.L.; Biggs, K.W.; Malak, S.F. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer 2021, 127, 4384–4392. [Google Scholar] [CrossRef]
- Micha, J.P.; Rettenmaier, M.A.; Bohart, R.D.; Goldstein, B.H. Hormone Therapy and Risk of Breast Cancer: Where Are We Now? J. Menopausal Med. 2022, 28, 47. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Behavioral Risk Factors in Breast Cancer: Can Risk Be Modified? Oncologist 2003, 8, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Cobec, I.M.; Popescu, R.; Moatar, A.E.; Verdes, D. Ovarian cancer under the magnifying glass. Rom. J. Mil. Med. 2021, 124, 291. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Cobec, I.M.; Ceauşu, R.A.; Popescu, R.; Tudor, A.; Raica, M. Platelet derived growth factor BB: A “must-have” therapeutic target “redivivus” in ovarian cancer. Cancer Genom. Proteom. 2016, 13, 511–518. [Google Scholar] [CrossRef]
- Vy, V.P.T.; Yao, M.M.S.; Le, N.Q.K.; Chan, W.P. Machine Learning Algorithm for Distinguishing Ductal Carcinoma In Situ from Invasive Breast Cancer. Cancers 2022, 14, 2437. [Google Scholar] [CrossRef]
- Cserni, G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica 2020, 112, 25–41. [Google Scholar] [CrossRef]
- Yang, C.; Lei, C.; Zhang, Y.; Zhang, J.; Ji, F.; Pan, W.; Zhang, L.; Gao, H.; Yang, M.; Li, J.; et al. Comparison of Overall Survival Between Invasive Lobular Breast Carcinoma and Invasive Ductal Breast Carcinoma: A Propensity Score Matching Study Based on SEER Database. Front. Oncol. 2020, 10, 590643. [Google Scholar] [CrossRef]
- Sheikh SEl Rathbone, M.; Chaudhary, K.; Joshi, A.; Lee, J.; Muthukumar, S.; Mylona, E.; Roxanis, I.; Rees, J. Rates and Outcomes of Breast Lesions of Uncertain Malignant Potential (B3) benchmarked against the National Breast Screening Pathology Audit; Improving Performance in a High Volume Screening Unit. Clin. Breast Cancer 2022, 22, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Moore, S. The Importance of Hormone Status in Breast Cancer: A Guide to Hormone Dependent Breast Cancer (HDBC). Available online: https://www.news-medical.net/health/The-Importance-of-Hormone-Status-in-Breast-Cancer-A-Guide-to-Hormone-Dependent-Breast-Cancer-(HDBC).aspx (accessed on 1 September 2024).
- Hackbart, H.; Cui, X.; Lee, J.S. Androgen receptor in breast cancer and its clinical implication. Transl. Breast Cancer Res. 2023, 4, 30. [Google Scholar] [CrossRef]
- Ravaioli, S.; Maltoni, R.; Pasculli, B.; Parrella, P.; Giudetti, A.M.; Vergara, D.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. Androgen receptor in breast cancer: The “5W” questions. Front. Endocrinol. 2022, 13, 977331. [Google Scholar] [CrossRef]
- Sun, H.; Ding, Q.; Sahin, A.A. Immunohistochemistry in the Diagnosis and Classification of Breast Tumors. Arch. Pathol. Lab. Med. 2023, 147, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, V.B.; Vlad, T.; Popescu, R.; Vlad, C.S.; Elisabeta Moatar, A.; Cobec, I.M. Triple-Negative Breast Cancer: Molecular Particularities Still a Challenge. Diagnostics 2024, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Arciero, C.A.; Jiang, R.; Behera, M.; Peng, L.; Li, X. Different Breast Cancer Subtypes Show Different Metastatic Patterns: A Study from A Large Public Database. Asian Pac. J. Cancer Prev. 2020, 21, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, L.; Olafsdottir, E.J.; Barkardottir, R.B.; Bjarnadottir, O.; Jonasson, J.G.; Sigurdsson, S.; Tryggvadottir, L. Estrogen receptor-positive breast cancer and adverse outcome in BRCA2 mutation carriers and young non-carrier patients. NPJ Breast Cancer 2023, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Moisand, A.; Madéry, M.; Boyer, T.; Domblides, C.; Blaye, C.; Larmonier, N. Hormone Receptor Signaling and Breast Cancer Resistance to Anti-Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 15048. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H. Prognostic and predictive parameters in breast pathology: A pathologist’s primer. Mod. Pathol. 2021, 34, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Cobec, I.; Moleriu, L.; Moatar, A.; Rempen, A. First clinical experience with CDK4/6 inhibitors in breast cancer therapy. Exp. Ther. Med. 2021, 21, 522. [Google Scholar] [CrossRef]
- Elliott, M.J.; Shen, S.; Lam, D.L.; Brown, T.; Lawson, M.B.; Iyengar, N.M.; Cescon, D.W. Enhancing Early-Stage Breast Cancer Survivorship: Evidence-Based Strategies, Surveillance Testing, and Imaging Guidelines. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432564. [Google Scholar] [CrossRef]
- Amato, O.; Guarneri, V.; Girardi, F. Epidemiology trends and progress in breast cancer survival: Earlier diagnosis, new therapeutics. Curr. Opin. Oncol. 2023, 35, 612–619. [Google Scholar] [CrossRef]
- Burciu, O.M.; Sas, I.; Popoiu, T.A.; Merce, A.G.; Moleriu, L.; Cobec, I.M. Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer. Int. J. Mol. Sci. 2024, 25, 8506. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and Racial Disparity in Breast Cancer. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2019; pp. 31–49. [Google Scholar]
- Kresovich, J.K.; Xu, Z.; O’Brien, K.M.; Weinberg, C.R.; Sandler, D.P.; Taylor, J.A. Methylation-based biological age and breast cancer risk. J. Natl. Cancer Inst. 2019, 111, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Hankinson, S.E.; Rosner, B.; Holmes, M.D.; Willett, W.C. Physical activity and risk of breast cancer among postmenopausal women. Arch. Intern. Med. 2010, 170, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Liu, Y.; Fan, Y.; Wang, L.; Jiang, E. Association of Healthy Diet and Physical Activity With Breast Cancer: Lifestyle Interventions and Oncology Education. Front. Public Health 2022, 10, 797794. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Hess, K.; Candelaria, R.P.; Eghtedari, M.; Adrada, B.E.; Sneige, N.; Fornage, B.D. Comparison of the accuracy of US-guided biopsy of breast masses performed with 14-gauge, 16-gauge and 18-gauge automated cutting needle biopsy devices, and review of the literature. Eur. Radiol. 2017, 27, 2928–2933. [Google Scholar] [CrossRef]
- Kong, Y.C.; Bhoo-Pathy, N.; O’Rorke, M.; Subramaniam, S.; Bhoo-Pathy, N.T.; See, M.H.; Jamaris, S.; Teoh, K.H.; Bustam, A.Z.; Looi, L.M.; et al. The association between methods of biopsy and survival following breast cancer: A hospital registry based cohort study. Medicine 2020, 99, e19093. [Google Scholar] [CrossRef]
- Wiggs, A.G.; Chandler, J.K.; Aktas, A.; Sumner, S.J.; Stewart, D.A. The Effects of Diet and Exercise on Endogenous Estrogens and Subsequent Breast Cancer Risk in Postmenopausal Women. Front. Endocrinol. 2021, 12, 732255. [Google Scholar] [CrossRef]
- LeBlanc, G.; Lee, I.; Carretta, H.; Luo, Y.; Sinha, D.; Rust, G. Rural-Urban Differences in Breast Cancer Stage at Diagnosis. Women’s Health Rep. 2022, 3, 207–214. [Google Scholar] [CrossRef]
- Kenzik, K.M.; Rocque, G.B.; Landier, W.; Bhatia, S. Urban versus rural residence and outcomes in older patients with breast cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Buzick, J.; Grewal, U.S.; Fleege, N.M.G. Rural-urban disparities in breast cancer-related mortality in the United States (1999–2020). Health Care Access Equity Disparities 2023, 19, 11. [Google Scholar] [CrossRef]
- Belachew, E.B.; Desta, A.F.; Deneke, D.B.; Fenta, B.D.; Alem, A.T.; Abafogi, A.K.; Lukas, F.Y.; Bezabih, M.; Sewasew, D.T.; Kantelhardt, E.J.; et al. Clinicopathological Features of Invasive Breast Cancer: A Five-Year Retrospective Study in Southern and South-Western Ethiopia. Medicines 2023, 10, 30. [Google Scholar] [CrossRef]
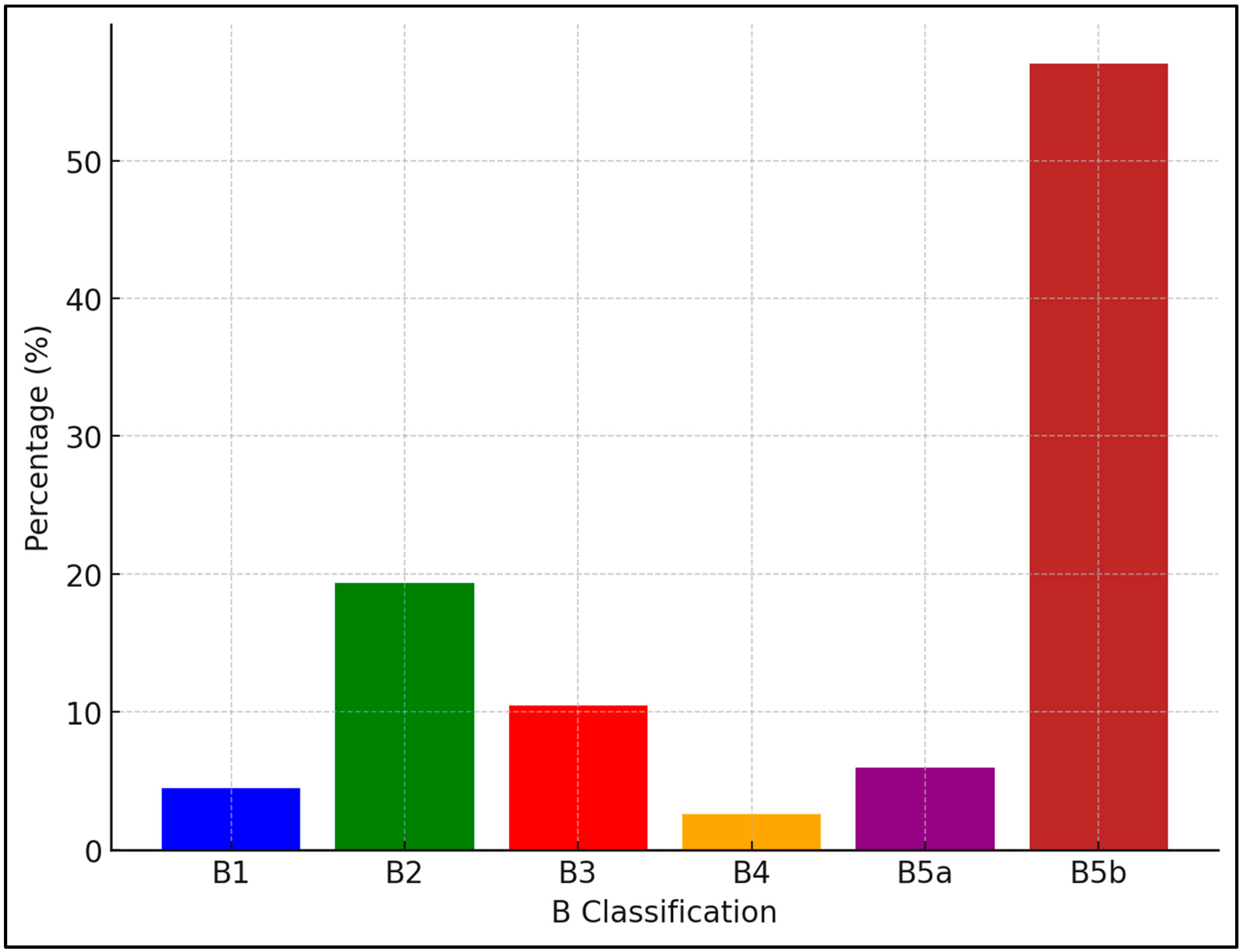

| Variable | Malignant | Benign | W | p-Value |
|---|---|---|---|---|
| Age (years) | 60.00 (55.00–65.00) | 56.00 (52.00–63.00) | 38,793.00 | <0.001 |
| BMI (kg/m2) | 29.27 (25.71–32.87) | 28.73 (25.77–32.00) | 48,144.50 | 0.22 |
| Menarche (years) | 14.00 (13.00–14.00) | 14.00 (13.00–14.75) | 53,030.50 | 0.41 |
| First birth (years) | 22.00 (21.00–25.00) | 22.00 (20.00–24.00) | 47,515.50 | 0.13 |
| No. births | 3.00 (2.00–3.00) | 3.00 (2.00–3.00) | 50,463.50 | 0.77 |
| Variable | Category | Malignant | Benign | p-Value | |
|---|---|---|---|---|---|
| Demographic and Lifestyle Factors | Living Environment | Urban | 307 (71%) | 127 (29%) | 0.07 |
| Rural | 162 (64%) | 91 (36%) | |||
| Region | NE | 148 (72%) | 58 (28%) | 0.23 | |
| NW | 229 (67%) | 112 (33%) | |||
| SE | 18 (55%) | 15 (45%) | |||
| W | 74 (69%) | 33 (31%) | |||
| Vulnerability | Yes | 170 (64%) | 97 (36%) | 0.04 | |
| No | 299 (71%) | 121 (29%) | |||
| Breastfeeding | Yes | 432 (68%) | 205 (32%) | 0.37 | |
| No | 37 (74%) | 13 (26%) | |||
| Breastfeeding period | <1 month | 23 (77%) | 7 (23%) | 0.18 | |
| 1–6 months | 226 (70%) | 99 (30%) | |||
| 6–12 months | 126 (63%) | 75 (37%) | |||
| >12 months | 94 (72%) | 37 (28%) | |||
| Daily Physical Activity | Yes | 123 (60%) | 82 (40%) | 0.002 | |
| No | 346 (72%) | 136 (28%) | |||
| Alcohol Consumption | Yes | 35 (73%) | 13 (27%) | 0.47 | |
| No | 434 (68%) | 205 (32%) | |||
| Red Meat Consumption | Yes | 358 (67%) | 175 (33%) | 0.25 | |
| No | 111 (72%) | 43 (28%) | |||
| Smoking | Yes | 79 (70%) | 34 (30%) | 0.68 | |
| No | 390 (68%) | 184 (32%) | |||
| Medical and Reproductive History | Hormonal Treatment during Menopause | Yes | 24 (63%) | 14 (37%) | 0.49 |
| No | 445 (69%) | 204 (31%) | |||
| FMH Cancer | Yes | 36 (73%) | 13 (27%) | 0.42 | |
| No | 433 (68%) | 205 (32%) | |||
| Menopause Status | No | 44 (56%) | 34 (44%) | 0.10 | |
| Early | 78 (70%) | 33 (30%) | |||
| Normal | 332 (70%) | 142 (30%) | |||
| Late | 15 (62%) | 9 (38%) | |||
| Prior Breast Examination | Yes | 193 (67%) | 94 (33%) | 0.63 | |
| No | 276 (69%) | 124 (31%) | |||
| Prior Breast Intervention | Yes | 28 (78%) | 8 (22%) | 0.21 | |
| No | 441 (68%) | 210 (32%) | |||
| Diagnostic and Biopsy Techniques | Re-biopsy | Yes | 2 (6%) | 33 (94%) | <0.001 |
| No | 467 (72%) | 184 (28%) | |||
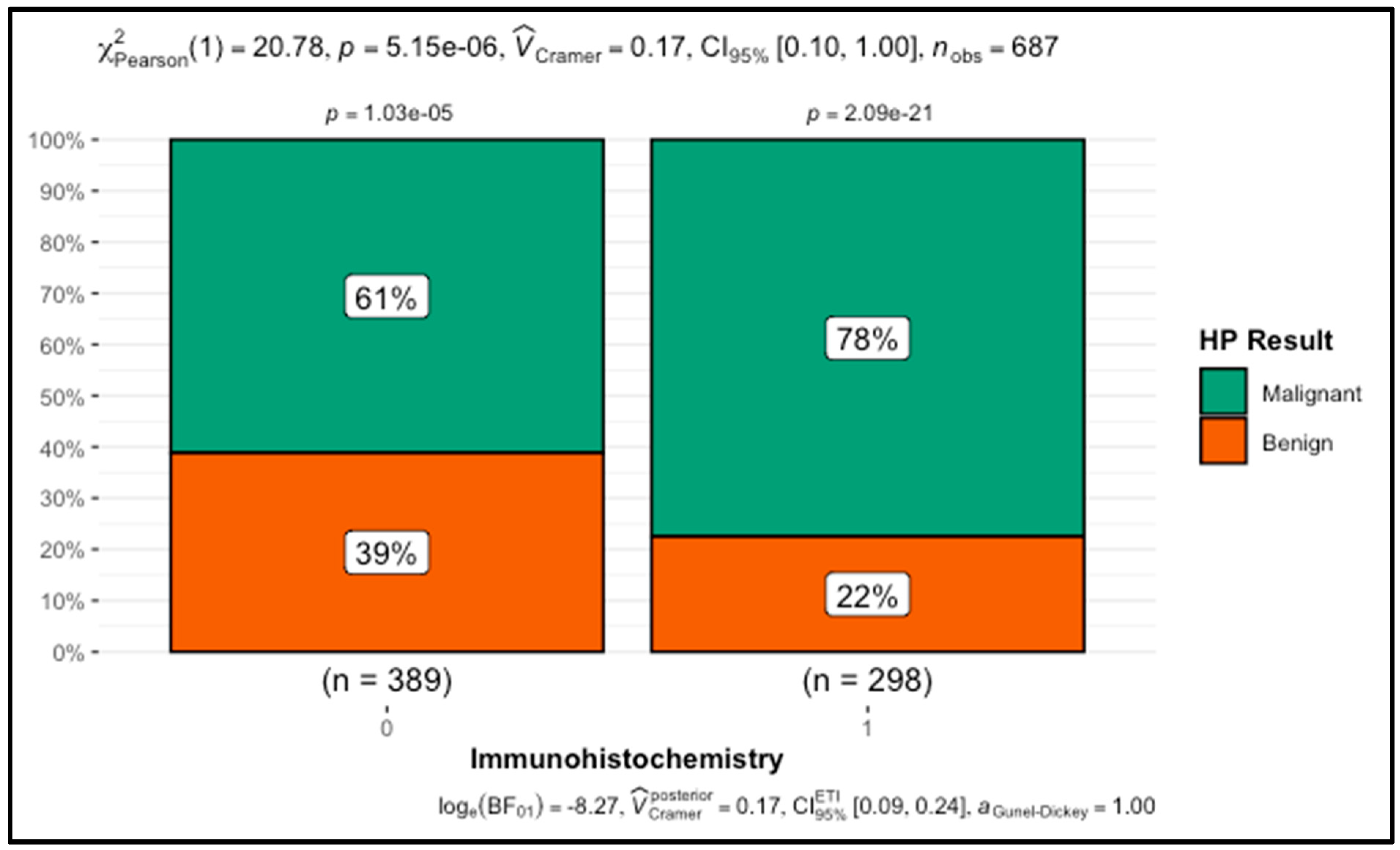
| Immunohistochemistry | Yes | 231 (78%) | 67 (22%) | <0.001 | |
| No | 238 (61%) | 151 (39%) | |||
| Biopsy Pistol Type | Single-use automatic | 68 (64%) | 38 (36%) | 0.47 | |
| Reusable automatic | 388 (69%) | 172 (31%) | |||
| Semi-automatic | 13 (62%) | 8 (38%) | |||
| Needle Thickness | 9-gauge | 11 (29%) | 27 (71%) | <0.001 | |
| 12-gauge | 22 (61%) | 14 (39%) | |||
| 14-gauge | 435 (71%) | 177 (29%) | |||
| 16-gauge | 1 (100%) | 0 (0%) | |||
| System Type | Ultrasound-guided | 447 (72%) | 177 (28%) | <0.001 | |
| Mammography-guided | 22 (35%) | 41 (65%) | |||
| Vacuum Biopsy | Yes | 19 (33%) | 38 (67%) | <0.001 | |
| No | 450 (71%) | 180 (29%) | |||
| Surgical Biopsy | Yes | 38 (60%) | 25 (40%) | 0.15 | |
| No | 431 (69%) | 193 (31%) |
| Receptor | Type | B1 | B2 | B3 | B4 | B5a | B5b | p-Value |
|---|---|---|---|---|---|---|---|---|
| ER | + | 0 (0%) | 4 (1%) | 14 (4%) | 3 (1%) | 21 (5%) | 348 (89%) | <0.001 |
| − | 31 (10%) | 129 (43%) | 58 (20%) | 15 (5%) | 20 (7%) | 44 (15%) | ||
| PR | + | 0 (0%) | 4 (1%) | 12 (3%) | 2 (1%) | 15 (4%) | 324 (91%) | <0.001 |
| − | 31 (9%) | 129 (39%) | 60 (18%) | 16 (5%) | 26 (8%) | 68 (21%) | ||
| AR | + | 0 (0%) | 4 (9%) | 8 (17%) | 0 (0%) | 0 (0%) | 34 (74%) | <0.001 |
| − | 31 (5%) | 129 (20%) | 64 (10%) | 18 (3%) | 41 (6%) | 358 (56%) |
| Predictors | OR | CI | p-Value |
|---|---|---|---|
| ER [+] | 15.79 | 6.09–50.71 | <0.001 |
| PR [+] | 4.83 | 1.38–15.23 | 0.009 |
| Age | 1.06 | 1.02–1.11 | 0.005 |
| Nagelkerke = 0.533 | |||
| Predictors | Estimates | CI | p-Value |
|---|---|---|---|
| Histopathological result [+] | 3.82 | 1.20–6.43 | 0.004 |
| Living Environment [Urban] | −12.18 | −20.76–−3.60 | 0.005 |
| Region [NW] | −12.15 | −14.92–−9.38 | <0.001 |
| Region [SE] | −10.41 | −16.24–−4.57 | <0.001 |
| Region [W] | −34.76 | −38.49–−31.02 | <0.001 |
| Vulnerability [No] | 12.16 | 3.65–20.66 | 0.005 |
| Breast Examination [Yes] | −2.77 | −5.20–−0.34 | 0.025 |
| No. histological fragments | 2.99 | 2.66–3.31 | <0.001 |
| adjusted = 0.485 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burciu, O.M.; Sas, I.; Merce, A.-G.; Cerbu, S.; Moatar, A.E.; Eftenoiu, A.-E.; Cobec, I.M. Comprehensive Analysis of Receptor Status, Histopathological Classifications (B1–B5), and Cumulative Histological Dimensions in Breast Cancer: Predictors of Malignancy and Diagnostic Implications. Cancers 2024, 16, 3471. https://doi.org/10.3390/cancers16203471
Burciu OM, Sas I, Merce A-G, Cerbu S, Moatar AE, Eftenoiu A-E, Cobec IM. Comprehensive Analysis of Receptor Status, Histopathological Classifications (B1–B5), and Cumulative Histological Dimensions in Breast Cancer: Predictors of Malignancy and Diagnostic Implications. Cancers. 2024; 16(20):3471. https://doi.org/10.3390/cancers16203471
Chicago/Turabian StyleBurciu, Oana Maria, Ioan Sas, Adrian-Grigore Merce, Simona Cerbu, Aurica Elisabeta Moatar, Anca-Elena Eftenoiu, and Ionut Marcel Cobec. 2024. "Comprehensive Analysis of Receptor Status, Histopathological Classifications (B1–B5), and Cumulative Histological Dimensions in Breast Cancer: Predictors of Malignancy and Diagnostic Implications" Cancers 16, no. 20: 3471. https://doi.org/10.3390/cancers16203471
APA StyleBurciu, O. M., Sas, I., Merce, A.-G., Cerbu, S., Moatar, A. E., Eftenoiu, A.-E., & Cobec, I. M. (2024). Comprehensive Analysis of Receptor Status, Histopathological Classifications (B1–B5), and Cumulative Histological Dimensions in Breast Cancer: Predictors of Malignancy and Diagnostic Implications. Cancers, 16(20), 3471. https://doi.org/10.3390/cancers16203471






