Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy
Abstract
Simple Summary
Abstract
1. Mortalin Structure and Function
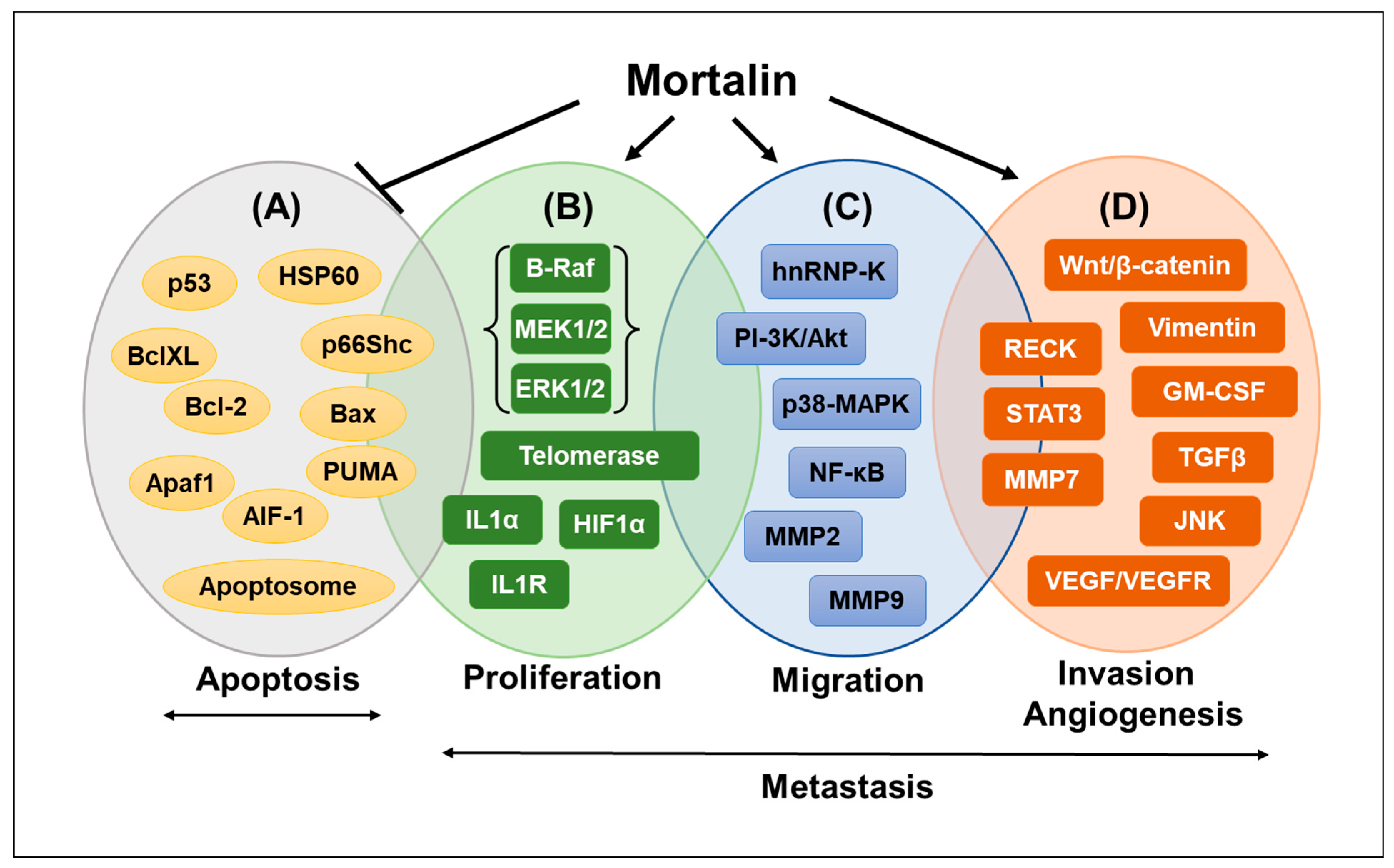
2. Role of Mortalin in Cancer
3. Mortalin Inhibitors for Cancer Therapy
3.1. Inhibitory Peptides
3.1.1. UBX Domain-Containing Protein (UBXN2)
3.1.2. SMR-Derived Peptides
3.2. Inhibitory RNAs
miRNAs (miRNA-200b, mi-RNA-200c and miRNA217)
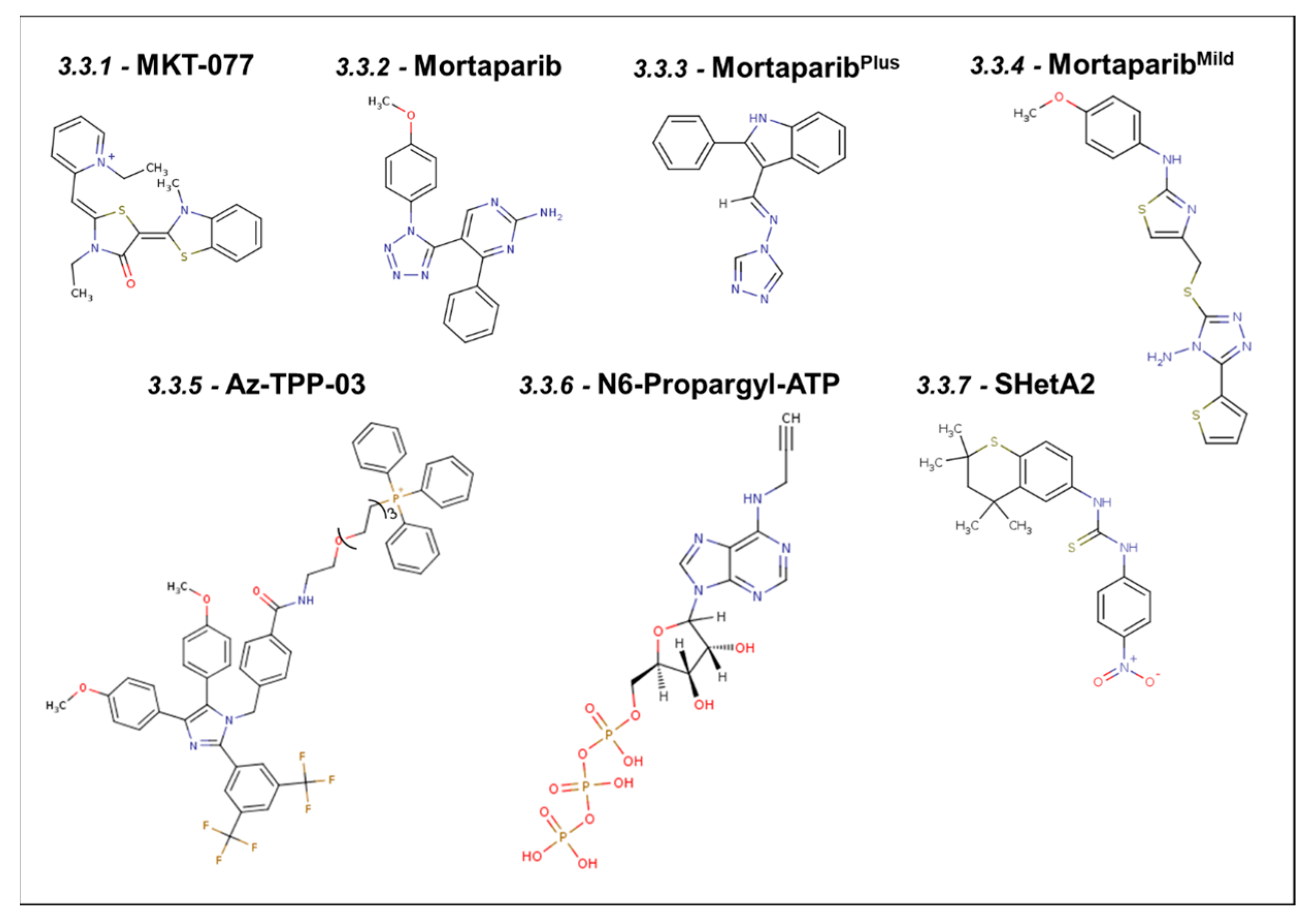
3.3. Synthetic Small Molecule Inhibitors of Mortalin
3.3.1. MKT-077
3.3.2. Mortaparib
3.3.3. MortaparibPlus
3.3.4. MortaparibMild
3.3.5. Apoptozole–Triphenylphosphonium Conjugate (Az-TPP-03)
3.3.6. ADP Analog Inhibitors
3.3.7. SHetA2
4. Natural Small Molecule Inhibitors of Mortalin
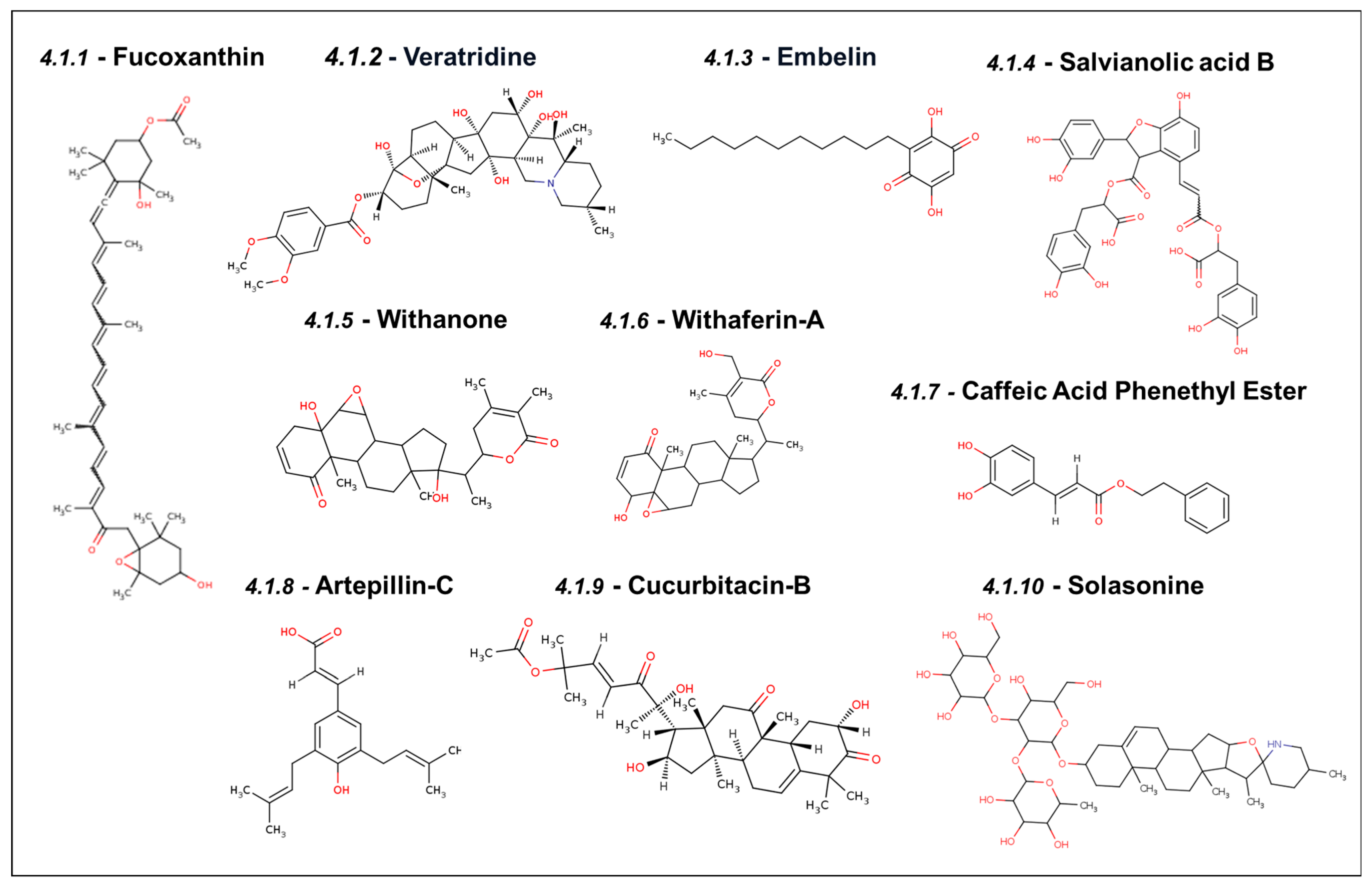
4.1. Experimentally Validated Natural Inhibitors of Mortalin
4.1.1. Fucoxanthin
4.1.2. Veratridine
4.1.3. Embelin
4.1.4. Salvianolic Acid B
4.1.5. Withanone
4.1.6. Withaferin-A
4.1.7. Caffeic Acid Phenethyl Ester
4.1.8. Artepillin-C
4.1.9. Cucurbitacin-B
4.1.10. Solasonine
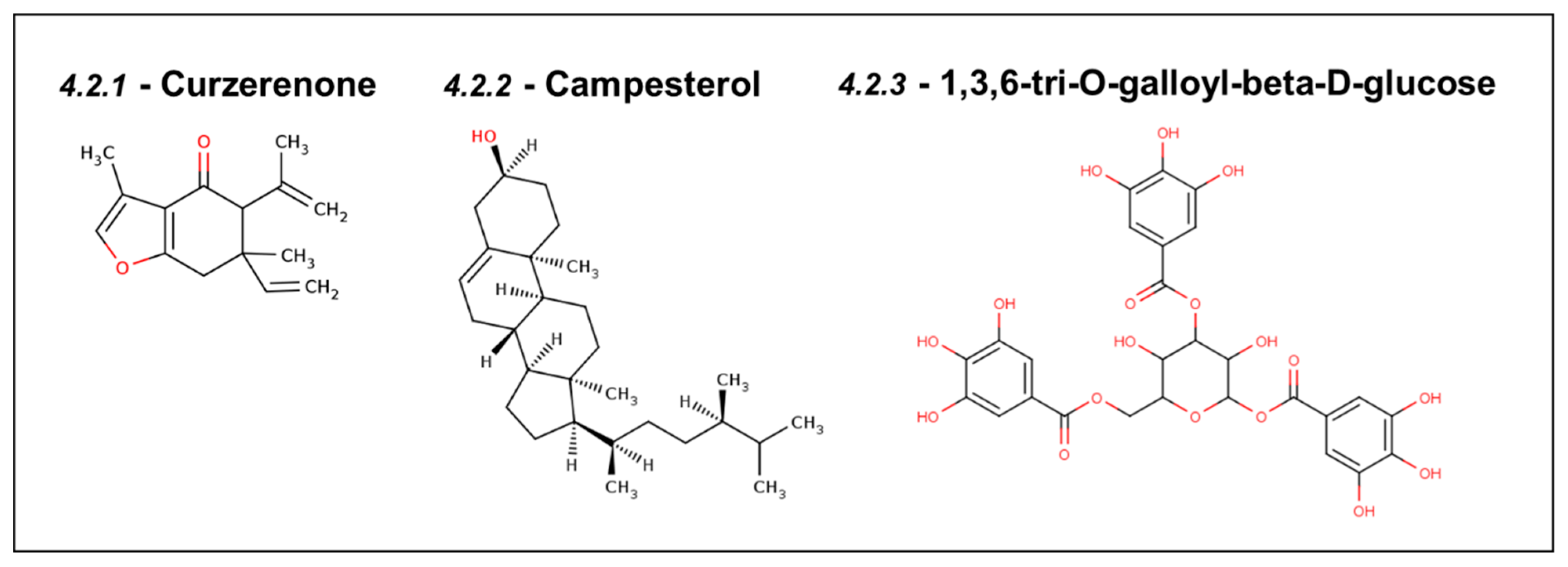
4.2. Natural Inhibitors of Mortalin Predicted Using Computer-Aided Drug Discovery
4.2.1. Curzerenone
4.2.2. Campesterol
4.2.3. DTOM (1,3,6-tri-O-galloyl-beta-D-glucose)
5. Inhibitors of the Hsp70 Family of Proteins—Non-Specific Inhibitors of Mortalin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kaul, S.C.; Ikawa, Y.; Sugimoto, Y. Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J. Biol. Chem. 1993, 268, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Wadhwa, R.; Kawai, R.; Kaul, S.C.; Sifers, R.N.; Bick, R.J.; Smith, J.R.; Pereira-Smith, O.M. Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem. Biophys. Res. Commun. 2000, 275, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Karnezis, A.N.; Murphy, S.P.; Hoang, T.; Freeman, B.C.; Phillips, B.; Morimoto, R.I. Cloning and Subcellular Localization of Human Mitochondrial hsp70 (∗). J. Biol. Chem. 1995, 270, 1705–1710. [Google Scholar] [CrossRef]
- Kaul, S.C.; Wadhwa, R. Mortalin Biology: Life, Stress and Death; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yang, H.; Zhou, X.; Liu, X.; Yang, L.; Chen, Q.; Zhao, D.; Zuo, J.; Liu, W. Mitochondrial dysfunction induced by knockdown of mortalin is rescued by Parkin. Biochem. Biophys. Res. Commun. 2011, 410, 114–120. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef]
- Huifu, H.; Shefrin, S.; Yang, S.; Zhang, Z.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Cucurbitacin-B inhibits cancer cell migration by targeting mortalin and HDM2: Computational and in vitro experimental evidence. J. Biomol. Struct. Dyn. 2024, 42, 2643–2652. [Google Scholar] [CrossRef]
- Widodo, N.; Priyandoko, D.; Shah, N.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by Ashwagandha leaf extract and its component Withanone involves ROS signaling. PLoS ONE 2010, 5, e13536. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, V.; D’Silva, P. Loss of function of mtHsp70 Chaperone variants leads to mitochondrial dysfunction in congenital sideroblastic anemia. Front. Cell Dev. Biol. 2022, 10, 847045. [Google Scholar] [CrossRef] [PubMed]
- Esfahanian, N.; Knoblich, C.D.; Bowman, G.A.; Rezvani, K. Mortalin: Protein partners, biological impacts, pathological roles, and therapeutic opportunities. Front. Cell Dev. Biol. 2023, 11, 1028519. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kaul, Z.; Yoon, A.R.; Liu, Y.; Yaguchi, T.; Na, Y.; Ahn, H.M.; Gao, R.; Choi, I.K.; Yun, C.O.; et al. Identification and functional characterization of nuclear mortalin in human carcinogenesis. J. Biol. Chem. 2014, 289, 24832–24844. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Schilke, B.; Williams, B.; Knieszner, H.; Pukszta, S.; D’Silva, P.; Craig, E.A.; Marszalek, J. Evolution of mitochondrial chaperones utilized in Fe-S cluster biogenesis. Curr. Biol. 2006, 16, 1660–1665. [Google Scholar] [CrossRef]
- D’Eletto, M.; Rossin, F.; Occhigrossi, L.; Farrace, M.G.; Faccenda, D.; Desai, R.; Marchi, S.; Refolo, G.; Falasca, L.; Antonioli, M.; et al. Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 2018, 25, 3573–3581. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Fujioka, H.; Liu, J.; Chen, S.; Zhu, X. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc. Natl. Acad. Sci. USA 2019, 116, 25322–25328. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell. Biol. 2006, 175, 901. [Google Scholar] [CrossRef]
- Fiorese, C.J.; Schulz, A.M.; Lin, Y.F.; Rosin, N.; Pellegrino, M.W.; Haynes, C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 2016, 26, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Smyrnias, I. The mitochondrial unfolded protein response and its diverse roles in cellular stress. Int. J. Biochem. Cell Biol. 2021, 133, 105934. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Fishelson, Z. Cancer resistance to complement-dependent cytotoxicity (CDC): Problem-oriented research and development. Mol. Immunol. 2009, 46, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Mazkereth, N.; Rocca, F.; Schubert, J.R.; Geisler, C.; Hillman, Y.; Egner, A.; Fishelson, Z. Complement triggers relocation of Mortalin/GRP75 from mitochondria to the plasma membrane. Immunobiology 2016, 221, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Pilzer, D.; Saar, M.; Koya, K.; Fishelson, Z. Mortalin inhibitors sensitize K562 leukemia cells to complement-dependent cytotoxicity. Int. J. Cancer 2010, 126, 1428–1435. [Google Scholar] [CrossRef]
- Ray, M.S.; Moskovich, O.; Iosefson, O.; Fishelson, Z. Mortalin/GRP75 binds to complement C9 and plays a role in resistance to complement-dependent cytotoxicity. J. Biol. Chem. 2014, 289, 15014–15022. [Google Scholar]
- Resendez, E., Jr.; Attenello, J.W.; Grafsky, A.; Chang, C.S.; Lee, A.S. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Molecular Cell. Biol. 1985, 5, 1212–1219. [Google Scholar]
- Kögel, D.; Schomburg, R.; Copanaki, E.; Prehn, J.H.M. Regulation of gene expression by the amyloid precursor protein: Inhibition of the JNK/c-Jun pathway. Cell Death Differ. 2005, 12, 1–9. [Google Scholar] [CrossRef]
- Jin, J.; Hulette, C.; Wang, Y.; Zhang, T.; Pan, C.; Wadhwa, R.; Zhang, J. Proteomic Identification of a Stress Protein, Mortalin/mthsp70/GRP75: Relevance to Parkinson Disease*S. Mol. Cell Proteom. 2006, 5, 1193–1204. [Google Scholar] [CrossRef]
- Texier, B.; Prime, M.; Atamena, D.; Belenguer, P.; Szelechowski, M. Mortalin/Hspa9 involvement and therapeutic perspective in Parkinson’s disease. Neural Regen. Res. 2023, 18, 293–298. [Google Scholar]
- Franco-Iborra, S.; Vila, M.; Perier, C. Mitochondrial quality control in neurodegenerative diseases: Focus on Parkinson’s disease and Huntington’s disease. Front. Neurosci. 2018, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Cellular chaperones as therapeutic targets in ALS to restore protein homeostasis and improve cellular function. Front. Neurosci. 2017, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Royer-Bertrand, B.; Castillo-Taucher, S.; Moreno-Salinas, R.; Cho, T.J.; Chae, J.H.; Choi, M.; Kim, O.H.; Dikoglu, E.; Campos-Xavier, B.; Girardi, E.; et al. Mutations in the heat-shock protein A9 (HSPA9) gene cause the EVEN-PLUS syndrome of congenital malformations and skeletal dysplasia. Sci. Rep. 2015, 5, 17154. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Abe, K.; Ciesielski, S.J.; Schmidt, P.J.; Campagna, D.R.; Rahimov, F.; Schilke, B.A.; Cuijpers, M.; Rieneck, K.; Lausen, B.; Linenberger, M.L.; et al. Congenital sideroblastic anemia due to mutations in the mitochondrial HSP70 homologue HSPA9. Blood. J. Am. Soc. Hematol. 2015, 126, 2734–2738. [Google Scholar]
- Qu, M.; Zhou, Z.; Xu, S.; Chen, C.; Yu, Z.; Wang, D. Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2011, 1368, 336–345. [Google Scholar] [CrossRef]
- Xu, L.; Voloboueva, L.A.; Ouyang, Y.; Giffard, R.G. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 365–374. [Google Scholar] [CrossRef]
- Ando, K.; Oki, E.; Zhao, Y.; Ikawa-Yoshida, A.; Kitao, H.; Saeki, H.; Kimura, Y.; Ida, S.; Morita, M.; Kusumoto, T.; et al. Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastric Cancer 2014, 17, 255–262. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, B.; Zikeliyar, M.; Wang, J.; Jian, H.; Wu, K.; Zhang, Y.; Ding, J. Elevated Mortalin correlates with poor outcome in hepatocellular carcinoma. Ann. Diagn. Pathol. 2019, 42, 59–63. [Google Scholar] [CrossRef]
- Taurin, S.; Seyrantepe, V.; Orlov, S.N.; Tremblay, T.L.; Thibault, P.; Bennett, M.R.; Hamet, P.; Pshezhetsky, A.V. Proteome analysis and functional expression identify mortalin as an antiapoptotic gene induced by elevation of [Na+] i/[K+] i ratio in cultured vascular smooth muscle cells. Circ. Res. 2002, 91, 915–922. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, Z.; Wu, X.; Zhang, M.; Zhang, S.; Jin, T. Mortalin promotes breast cancer malignancy. Exp. Mol. Pathol. 2021, 118, 104593. [Google Scholar] [CrossRef]
- Deocaris, C.; Lu, W.J.; C Kaul, S.; Wadhwa, R. Druggability of mortalin for cancer and neuro-degenerative disorders. Curr. Pharm. Des. 2013, 19, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, A. Abrogating the interaction between p53 and mortalin (Grp75/HSPA9/mtHsp70) for cancer therapy: The story so far. Front. Cell Dev. Biol. 2022, 10, 879632. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Leu, J.L.; Della Pietra, A.C.; George, D.L.; Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003, 33, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef]
- Ichihara, M.; Murakumo, Y.; Takahashi, M. RET and neuroendocrine tumors. Cancer Lett. 2004, 204, 197–211. [Google Scholar] [CrossRef]
- Wu, P.K.; Hong, S.K.; Veeranki, S.; Karkhanis, M.; Starenki, D.; Plaza, J.A.; Park, J.I. A mortalin/HSPA9-mediated switch in tumor-suppressive signaling of Raf/MEK/extracellular signal-regulated kinase. Mol. Cell Biol. 2013, 33, 4051–4067. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Mallini, P.; Lennard, T.; Kirby, J.; Meeson, A. Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat. Rev. 2014, 40, 341–348. [Google Scholar] [CrossRef]
- Pizzatti, L.; Sá, L.A.; de Souza, J.M.; Bisch, P.M.; Abdelhay, E. Altered protein profile in chronic myeloid leukemia chronic phase identified by a comparative proteomic study. Biochim. Biophys. Acta. 2004, 1764, 929–942. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; P Zhou, B. The role of snail in EMT and tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Na, Y.; Kaul, S.C.; Ryu, J.; Lee, J.S.; Ahn, H.M.; Kaul, Z.; Kalra, R.S.; Li, L.; Widodo, N.; Yun, C.O.; et al. Stress chaperone mortalin contributes to epithelial-to-mesenchymal transition and cancer metastasis. Cancer Res. 2016, 76, 2754–2765. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, P.; Kocsis, J.; Saar, M.; Prohászka, Z.; Füst, G.; Fishelson, Z. Elevated levels of mitochondrial mortalin and cytosolic HSP70 in blood as risk factors in patients with colorectal cancer. Int. J. Cancer Res. 2013, 133, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Cui, M.; Wang, X.; Lin, Z. Mortalin contributes to colorectal cancer by promoting proliferation and epithelial–mesenchymal transition. IUBMB Life 2020, 72, 771–781. [Google Scholar] [CrossRef]
- Yun, C.O.; Bhargava, P.; Na, Y.; Lee, J.S.; Ryu, J.; Kaul, S.C.; Wadhwa, R. Relevance of mortalin to cancer cell stemness and cancer therapy. Sci. Rep. 2017, 7, 42016. [Google Scholar] [CrossRef]
- Rai, R.; Kennedy, A.L.; Isingizwe, Z.R.; Javadian, P.; Benbrook, D.M. Similarities and Differences of Hsp70, hsc70, Grp78 and Mortalin as Cancer Biomarkers and Drug Target. Cells 2021, 10, 2996. [Google Scholar] [CrossRef]
- Yoon, A.R.; Wadhwa, R.; Kaul, S.C.; Yun, C.O. Why is mortalin a potential therapeutic target for cancer? Front. Cell Dev. Biol. 2022, 10, 914540. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, M.; Dai, Y.; Shan, W.; Chen, S.; Cai, R.; Yang, H.; Tang, L.; Li, L. Involvement and targeted intervention of mortalin-regulated proteome phosphorylated-modification in hepatocellular carcinoma. Front. Oncol. 2021, 11, 687871. [Google Scholar] [CrossRef]
- Kao, T.Y.; Chiu, Y.C.; Fang, W.C.; Cheng, C.W.; Kuo, C.Y.; Juan, H.F.; Wu, S.H.; Lee, A.Y. Mitochondrial Lon regulates apoptosis through the association with Hsp60–mtHsp70 complex. Cell Death Dis. 2015, 6, e1642. [Google Scholar] [CrossRef]
- Karkhanis, M.; Park, J.I. Sp1 regulates Raf/MEK/ERK-induced p21CIP1 transcription in TP53-mutated cancer cells. Cell. Signal. 2015, 27, 479–486. [Google Scholar] [CrossRef]
- Teng, M.; Hu, C.; Yang, B.; Xiao, W.; Zhou, Q.; Li, Y.; Li, Z. Salvianolic acid B targets mortalin and inhibits the migration and invasion of hepatocellular carcinoma via the RECK/STAT3 pathway. Cancer Cell Int. 2021, 21, 654. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.; Abdullah, A.; Nelson, M.E.; Wang, H.; Chauhan, S.C.; Newton, S.S.; Rezvani, K. Structural studies of UBXN2A and mortalin interaction and the putative role of silenced UBXN2A in preventing response to chemotherapy. Cell Stress Chaperones 2016, 21, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.; Abdullah, A.; Boudreau, D.A.; Autenried, R.K.; Gupta, B.K.; Wang, X.; Wang, H.; Schlenker, E.H.; Zhang, D.; Telleria, C.; et al. Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells. Cell Death Dis. 2014, 5, e1118. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.B.; Brena, D.; Wu, J.Y.; Roth, W.W.; Owusu, S.; Bond, V.C. Novel secretion modification region (SMR) peptide exhibits anti-metastatic properties in human breast cancer cells. Sci. Rep. 2022, 12, 13204. [Google Scholar] [CrossRef]
- Huang, M.B.; Wu, J.Y.; Lillard, J.; Bond, V.C. SMR peptide antagonizes mortalin promoted release of extracellular vesicles and affects mortalin protection from complement-dependent cytotoxicity in breast cancer cells and leukemia cells. Oncotarget 2019, 10, 5419. [Google Scholar] [CrossRef]
- Revel, M.; Daugan, M.V.; Sautés-Fridman, C.; Fridman, W.H.; Roumenina, L.T. Complement system: Promoter or suppressor of cancer progression? Antibodies 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhou, L.; Tey, S.K.; Ma, A.P.Y.; Yeung, C.L.S.; Ng, T.H.; Wong, S.W.K.; Liu, B.H.M.; Fung, Y.M.E.; Patz, E.F., Jr.; et al. Tumour extracellular vesicle-derived Complement Factor H promotes tumorigenesis and metastasis by inhibiting complement-dependent cytotoxicity of tumour cells. J. Extracell. Vesicles 2020, 10, e12031. [Google Scholar] [CrossRef]
- Hillman, Y.; Mazkereth, N.; Farberov, L.; Shomron, N.; Fishelson, Z. Regulation of complement-dependent cytotoxicity by microRNAs miR-200b, miR-200c, and miR-217. J. Immunol. 2016, 196, 5156–5165. [Google Scholar] [CrossRef]
- Fishelson, Z.; Kirschfink, M. Complement C5b-9 and cancer: Mechanisms of cell damage, cancer counteractions, and approaches for intervention. Front. Immunol. 2019, 10, 752. [Google Scholar] [CrossRef]
- Maruta, H.; Tikoo, A.; Shakri, R.; Shishido, T. The anti-RAS cancer drug MKT-077 is an F-actin cross-linker. Ann. N. Y. Acad. Sci. 1999, 886, 283–284. [Google Scholar] [CrossRef]
- Tikoo, A.; Cutler, H.; Lo, S.H.; Chen, L.B.; Maruta, H. Treatment of Ras-induced cancers by the F-actin cappers tensin and chaetoglobosin K, in combination with the caspase-1 inhibitor N1445. Cancer J. Sci. Am. 1999, 5, 293–300. [Google Scholar] [PubMed]
- Chiba, Y.; Kubota, T.; Watanabe, M.; Otani, Y.; Teramoto, T.; Matsumoto, Y.; Koya, K.; Kitajima, M. Selective antitumor activity of MKT-077, a delocalized lipophilic cation, on normal cells and cancer cells in vitro. J. Surg. Oncol. 1998, 69, 105–110. [Google Scholar] [CrossRef]
- Moll, U.M.; Ostermeyer, A.G.; Haladay, R.; Winkfield, B.; Frazier, M.; Zambetti, G. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol. Cell Biol. 1996, 16, 1126–1137. [Google Scholar] [CrossRef]
- Ostermeyer, A.G.; Runko, E.; Winkfield, B.; Ahn, B.; Moll, U.M. Cytoplasmically sequestered wild-type p53 protein in neuroblastoma is relocated to the nucleus by a C-terminal peptide. Proc. Natl. Acad. Sci. USA 1996, 93, 15190–15194. [Google Scholar] [CrossRef]
- Koya, K.; Li, Y.; Wang, H.; Ukai, T.; Tatsuta, N.; Kawakami, M.; Shishido, T.; Chen, L.B. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996, 56, 538–543. [Google Scholar] [PubMed]
- Propper, D.J.; Braybrooke, J.P.; Taylor, D.J.; Lodi, R.; Styles, P.; Cramer, J.A.; Collins, W.C.J.; Levitt, N.C.; Talbot, D.C.; Ganesan, T.S.; et al. Phase I trial of the selective mitochondrial toxin MKT 077 in chemo-resistant solid tumours. Ann. Oncol. 1999, 10, 923–927. [Google Scholar] [CrossRef]
- Putri, J.F.; Bhargava, P.; Dhanjal, J.K.; Yaguchi, T.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Mortaparib, a novel dual inhibitor of mortalin and PARP1, is a potential drug candidate for ovarian and cervical cancers. J. Exp. Clin. Cancer Res. 2019, 38, 499. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Elwakeel, A.; Sari, A.N.; Dhanjal, J.K.; Meidinna, H.N.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Mutant p53(L194F) Harboring Luminal-A Breast Cancer Cells Are Refractory to Apoptosis and Cell Cycle Arrest in Response to Mortaparib(Plus), a Multimodal Small Molecule Inhibitor. Cancers 2021, 13, 3043. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.N.; Elwakeel, A.; Dhanjal, J.K.; Kumar, V.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Identification and characterization of mortaparibPlus—A novel triazole derivative that targets mortalin-p53 interaction and inhibits cancer-cell proliferation by wild-type p53-dependent and-independent mechanisms. Cancers 2021, 13, 835. [Google Scholar] [CrossRef]
- Kumar, V.; Sari, A.N.; Meidinna, H.N.; Dhanjal, J.K.; Subramani, C.; Basu, B.; Kaul, S.C.; Vrati, S.; Sundar, D.; Wadhwa, R. Computational and in vitro experimental analyses of the Anti-COVID-19 potential of Mortaparib and MortaparibPlus. Biosci. Rep. 2021, 41, BSR20212156. [Google Scholar] [CrossRef] [PubMed]
- Meidinna, H.N.; Shefrin, S.; Sari, A.N.; Zhang, H.; Dhanjal, J.K.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Identification of a new member of Mortaparib class of inhibitors that target mortalin and PARP1. Front. Cell Dev. Biol. 2022, 10, 918970. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, D.M. SHetA2 attack on mortalin and colleagues in cancer therapy and prevention. Front. Cell Dev. Biol. 2022, 10, 848682. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Yang, S.; Meidinna, H.N.; Sari, A.N.; Bhargava, P.; Kaul, S.C. Mixtures of Three Mortaparibs with Enhanced Anticancer, Anti-Migration, and Antistress Activities: Molecular Characterization in p53-Null Cancer Cells. Cancers 2024, 16, 2239. [Google Scholar] [CrossRef]
- Park, S.H.; Baek, K.H.; Shin, I.; Shin, I. Subcellular Hsp70 inhibitors promote cancer cell death via different mechanisms. Cell Chem. Biol. 2018, 25, 1242–1254. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, D.M.; Nammalwar, B.; Long, A.; Matsumoto, H.; Singh, A.; Bunce, R.A.; Berlin, K.D. SHetA2 interference with mortalin binding to p66shc and p53 identified using drug-conjugated magnetic microspheres. Investig. New Drugs 2014, 32, 412–423. [Google Scholar] [CrossRef]
- Ko, S.K.; Kim, S.K.; Share, A.; Lynch, V.M.; Park, J.; Namkung, W.; Van Rossom, W.; Busschaert, N.; Gale, P.A.; Sessler, J.L.; et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat. Chem. 2014, 6, 885–892. [Google Scholar] [CrossRef]
- Dewson, G.; Kluck, R.M. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J. Cell Sci. 2009, 122, 2801–2808. [Google Scholar] [CrossRef]
- Moseng, M.A.; Nix, J.C.; Page, R.C. 2-and N6-functionalized adenosine-5’-diphosphate analogs for the inhibition of Mortalin. FEBS Lett. 2019, 593, 2030–2039. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Lo, S.N.H.; Louie, M.; Rajagopalan, V. SHetA2, a new cancer-preventive drug candidate. Anti-cancer Drugs: Nature, Synthesis and Cell. InTech 2016, 67. [Google Scholar] [CrossRef]
- Nammalwar, B.; Berlin, K.D.; Bunce, R.A. SHetA2—A mini review of a promising anticancer drug. JSM Chem. 2013, 1, 1–6. [Google Scholar]
- Benbrook, D.M. Refining retinoids with heteroatoms. Mini-Rev. Med. Chem. 2002, 2, 277–283. [Google Scholar] [CrossRef]
- Chun, K.H.; Benbrook, D.M.; Berlin, K.D.; Hong, W.K.; Lotan, R. The synthetic heteroarotinoid SHetA2 induces apoptosis in squamous carcinoma cells through a receptor-independent and mitochondria-dependent pathway. Cancer Res. 2003, 63, 3826–3832. [Google Scholar]
- Guruswamy, S.; Lightfoot, S.; Gold, M.A.; Hassan, R.; Berlin, K.D.; Ivey, R.T.; Benbrook, D.M. Effects of retinoids on cancerous phenotype and apoptosis in organotypic cultures of ovarian carcinoma. J. Natl. Cancer Inst. 2001, 93, 516–525. [Google Scholar] [CrossRef]
- Liu, S.; Brown, C.W.; Berlin, K.D.; Dhar, A.; Guruswamy, S.; Brown, D.; Gardner, G.J.; Birrer, M.J.; Benbrook, D.M. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J. Med. Chem. 2004, 47, 999–1007. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Hannafon, B.; Gill, L.; Kelly, W.; Benbrook, D. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol. Cancer Ther. 2007, 6, 1814–1822. [Google Scholar] [CrossRef]
- Liu, T.; Masamha, C.P.; Chengedza, S.; Berlin, K.D.; Lightfoot, S.; He, F.; Benbrook, D.M. Development of flexible-heteroarotinoids for kidney cancer. Mol. Cancer Ther. 2009, 8, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, X.; Yue, P.; Benbrook, D.M.; Berlin, K.D.; Khuri, F.R.; Sun, S.Y. Involvement of c-FLIP and survivin down-regulation in flexible heteroarotinoid-induced apoptosis and enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol. Cancer Ther. 2008, 7, 3556–3565. [Google Scholar] [CrossRef]
- Orsini, F.; Migliaccio, E.; Moroni, M.; Contursi, C.; Raker, V.A.; Piccini, D.; Martin-Padura, I.; Pelliccia, G.; Trinei, M.; Bono, M.; et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J. Biol. Chem. 2004, 279, 25689–25695. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. J. Evid. Based Complement. Altern. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef]
- Abdullah, A.; Sane, S.; Branick, K.A.; Freeling, J.L.; Wang, H.; Zhang, D.; Rezvani, K. A plant alkaloid, veratridine, potentiates cancer chemosensitivity by UBXN2A-dependent inhibition of an oncoprotein, mortalin-2. Oncotarget 2015, 6, 23561. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Shymatak, M.; Chatterjee, S.; Wai, L.K.; Arumugam, G. Inhibition of Angiogenesis and nitric oxide synthase (NOS), by embelin & vilangin using in vitro, in vivo & in silico studies. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 543. [Google Scholar]
- Tang, Q.; Zhong, H.; Xie, F.; Xie, J.; Chen, H.; Yao, G. Expression of miR-106b-25 induced by salvianolic acid B inhibits epithelial-to-mesenchymal transition in HK-2 cells. Eur. J. Pharmacol. 2014, 741, 97–103. [Google Scholar] [CrossRef]
- Kaul, S.C.; Wadhwa, R. Science of Ashwagandha: Preventive and Therapeutic Potentials, 1st ed.; Springer International Publishing: Cham, Switerland, 2017; p. 508. [Google Scholar]
- Sari, A.N.; Dhanjal, J.K.; Elwakeel, A.; Kumar, V.; Meidinna, H.N.; Zhang, H.; Ishida, Y.; Terao, K.; Sundar, D.; Kaul, S.C.; et al. A low dose combination of withaferin A and caffeic acid phenethyl ester possesses anti-metastatic potential in vitro: Molecular targets and mechanisms. Cancers 2022, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.Q.; Tran, T.H.V.; Pham, Q.L.; Gairin, J.E. In silico analysis of the binding properties of solasonine to mortalin and p53, and in vitro pharmacological studies of its apoptotic and cytotoxic effects on human HepG2 and Hep3b hepatocellular carcinoma cells. Fundam. Clin. Pharmacol. 2019, 33, 385–396. [Google Scholar] [CrossRef]
- Ahmed Hamdi, O.A.; Syed Abdul Rahman, S.N.; Awang, K.; Abdul Wahab, N.; Looi, C.Y.; Thomas, N.F.; Abd Malek, S.N. Cytotoxic constituents from the rhizomes of Curcuma zedoaria. Sci. World J. 2014, 2014, 321943. [Google Scholar] [CrossRef]
- Fitriana, N.; Khairunnisa, F.A.; Rifa’i, M.; Widodo. Potential compound of Curcuma xanthorrhiza and Curcuma zedoaria as Mortalin inhibitor to control cancer cell growth through computational study. IOP Conf. Ser. Earth Environ. Sci. 2019, 391, 012032. [Google Scholar] [CrossRef]
- Hartati, F.K.; Djauhari, A.B. Potential of black rice (Oryza sativa L.) as anticancer through mortalin-p53 complex inhibitors. Biointerface Res. Appl. Chem 2020, 10, 6174–6181. [Google Scholar]
- Nagpal, N.; Goyal, S.; Dhanjal, J.K.; Ye, L.; Kaul, S.C.; Wadhwa, R.; Chaturvedi, R.; Grover, A. Molecular dynamics-based identification of novel natural mortalin–p53 abrogators as anticancer agents. J. Recept. Signal Transduct. 2017, 37, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Liu, W.; Shao, Y.; Chen, L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp. Biol. Med. 2014, 239, 638–645. [Google Scholar] [CrossRef]
- Tang, X.; Tan, L.; Shi, K.; Peng, J.; Xiao, Y.; Li, W.; Chen, L.; Yang, Q.; Qian, Z. Gold nanorods together with HSP inhibitor-VER-155008 micelles for colon cancer mild-temperature photothermal therapy. Acta Pharm. Sin. B 2018, 8, 587–601. [Google Scholar] [CrossRef]
- Fewell, S.W.; Day, B.W.; Brodsky, J.L. Identification of an inhibitor of hsc70-mediated protein translocation and ATP hydrolysis. J. Biol. Chem. 2001, 276, 910–914. [Google Scholar] [CrossRef]
- Prince, T.; Ackerman, A.; Cavanaugh, A.; Schreiter, B.; Juengst, B.; Andolino, C.; Danella, J.; Chernin, M.; Williams, H. Dual targeting of HSP70 does not induce the heat shock response and synergistically reduces cell viability in muscle invasive bladder cancer. Oncotarget 2018, 9, 32702. [Google Scholar] [CrossRef]
- Huryn, D.M.; Brodsky, J.L.; Brummond, K.M.; Chambers, P.G.; Eyer, B.; Ireland, A.W.; Kawasumi, M.; LaPorte, M.G.; Lloyd, K.; Manteau, B.; et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc. Natl. Acad. Sci. USA 2011, 108, 6757–6762. [Google Scholar] [CrossRef] [PubMed]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.J.; Pimkina, J.; Frank, A.; Murphy, M.E.; George, D.L. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 2009, 36, 15–27. [Google Scholar] [CrossRef]
- Leu, J.I.J.; Pimkina, J.; Pandey, P.; Murphy, M.E.; George, D.L. HSP70 inhibition by the small-molecule 2-phenylethynesulfonamide impairs protein clearance pathways in tumor cells. Mol. Cancer Res. 2011, 9, 936–947. [Google Scholar] [CrossRef]
- Colvin, T.A.; Gabai, V.L.; Gong, J.; Calderwood, S.K.; Li, H.; Gummuluru, S.; Matchuk, O.N.; Smirnova, S.G.; Orlova, N.V.; Zamulaeva, I.A.; et al. Hsp70–Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014, 74, 4731–4740. [Google Scholar] [CrossRef] [PubMed]
- Koren, J., III; Miyata, Y.; Kiray, J.; O’Leary, J.C., III; Nguyen, L.; Guo, J.; Blair, L.J.; Li, X.; Jinwal, U.K.; Cheng, J.Q.; et al. Rhodacyanine derivative selectively targets cancer cells and overcomes tamoxifen resistance. PLoS ONE 2012, 7, e35566. [Google Scholar]
- Li, X.; Srinivasan, S.R.; Connarn, J.; Ahmad, A.; Young, Z.T.; Kabza, A.M.; Zuiderweg, E.R.; Sun, D.; Gestwicki, J.E. Analogues of the allosteric heat shock protein 70 (Hsp70) inhibitor, MKT-077, as anti-cancer agents. ACS Med. Chem. Lett. 2013, 4, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Yaglom, J.A.; Wang, Y.; Li, A.; Li, Z.; Monti, S.; Alexandrov, I.; Lu, X.; Sherman, M.Y. Cancer cell responses to Hsp70 inhibitor JG-98: Comparison with Hsp90 inhibitors and finding synergistic drug combinations. Sci. Rep. 2018, 8, 3010. [Google Scholar]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of methylene blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef]
- Wang, A.M.; Morishima, Y.; Clapp, K.M.; Peng, H.M.; Pratt, W.B.; Gestwicki, J.E.; Osawa, Y.; Lieberman, A.P. Inhibition of hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J. Biol. Chem. 2010, 285, 15714–15723. [Google Scholar] [CrossRef]
- Thomas, S.; Sharma, N.; Gonzalez, R.; Pao, P.W.; Hofman, F.M.; Chen, T.C.; Louie, S.G.; Pirrung, M.C.; Schönthal, A.H. Repositioning of verrucosidin, a purported inhibitor of chaperone protein GRP78, as an inhibitor of mitochondrial electron transport chain complex I. PLoS ONE 2013, 8, e65695. [Google Scholar] [CrossRef]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P.F. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br. J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef]
- Yuan, Z.P.; Chen, L.J.; Fan, L.Y.; Tang, M.H.; Yang, G.L.; Yang, H.S.; Du, X.B.; Wang, G.Q.; Yao, W.X.; Zhao, Q.M.; et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol. Ther. 2015, 23, 128. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, Y.; Kwon, B.M.; Han, D.C. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 2013, 288, 28713–28726. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Sui, J.; Fu, Q.; Lu, Z.; Piao, Z.; Jin, T.; Zhang, M. Mortalin promotes the evolution of androgen-independent prostate cancer through Wnt/beta-catenin signaling pathway. Cancer Cell Int. 2024, 24, 203. [Google Scholar] [CrossRef] [PubMed]
- Shankaranarayana, A.H.; Meduri, B.; Pujar, G.V.; Hariharapura, R.C.; Sethu, A.K.; Singh, M.; Bidye, D. Restoration of p53 functions by suppression of mortalin-p53 sequestration: An emerging target in cancer therapy. Future Med. Chem. 2023, 15, 2087–2112. [Google Scholar] [CrossRef] [PubMed]
- Avraham, M.; Sinkovits, G.; Hurler, L.; Prohászka, Z.; Fishelson, Z. Circulating mortalin in blood and activation of the alternative complement pathway as risk indicators in COVID-19 infection. Front. Immunol. 2024, 15, 1337215. [Google Scholar] [CrossRef]
- Londono, C.; Osorio, C.; Gama, V.; Alzate, O. Mortalin, apoptosis, and neurodegeneration. Biomolecules 2012, 2, 143–164. [Google Scholar] [CrossRef]
- Flachbartová, Z.; Kovacech, B. Mortalin—A multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration. Acta Virol. 2013, 57, 3–15. [Google Scholar] [CrossRef]
- Priyanka; Seth, P. Insights into the Role of Mortalin in Alzheimer’s Disease, Parkinson’s Disease, and HIV-1-Associated Neurocognitive Disorders. Front. Cell Dev. Biol. 2022, 10, 903031. [Google Scholar] [CrossRef]


| ADP Analog | Structure | Kiapp(µM) | Log IC50 |
|---|---|---|---|
| ADP | 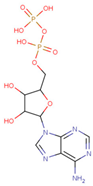 | 352.90 ± 36.21 | 2.613 ± 0.065 |
| 2-Cl ADP | 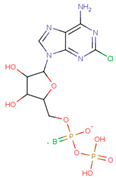 | 45.05 ± 2.42 | 1.752 ± 0.037 |
| 6-Bn ADP | 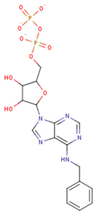 | 86.51 ± 10.26 | 2.074 ± 0.084 |
| 6-PheEt ADP | 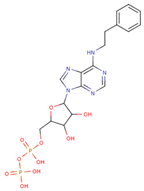 | 97.29 ± 9.96 | 2.238 ± 0.041 |
| 6-Fu ADP |  | 564.60 ± 49.63 | 2.747 ± 0.071 |
| 2-MeS ADP | 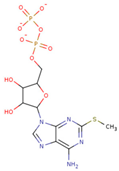 | 523.20 ± 98.35 | 2.955 ± 0.169 |
| 6-(3-MeBn)ADP |  | 675.30 ± 99.35 | 3.154 ± 0.124 |
| 6-cHe ADP | 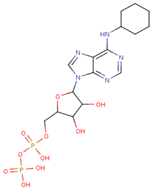 | 733.10 ± 82.07 | 3.203 ± 0.082 |
| Inhibitor | Mode of Action | Reference |
|---|---|---|
| Small Compound Inhibitors | ||
| VER-155008 | Derived from adenosine, it competitively inhibits the ATPase activity of Hsp70. It also regulates allosterically the interaction between SBD and NBD. | [115] |
| Gold Nanorods -formulated MPEG-AuNR -VER-155008 | VER-155008, along with gold nanorods, increases the photosensitivity of tumor cells, and VER-155008 micelles with gold nanorods enhance the inhibition of Hsp70. | [116] |
| NSC 630668-R/1 | R/1 is known to inhibit the DnaJ-mediated ATP hydrolysis activity of Hsp70. It is also known to block the Hsp70-stimulated translocation of its pre-protein into the microsomal vesicles derived from yeast. | [117] |
| MAL3-101 | MAL3-101 inhibits Hsp70 allosterically by inhibiting ATPase activity of Hsp70. | [118] |
| DMT3132 | DMT3132 is a MAL3-101 analog that inhibits Hsp70 by inhibiting its ATPase activity. | [119] |
| DMT3024 | DMT3024 is known to affect the ATPase activity of Hsp70. | [119] |
| MAL2-11B | Inhibits Hsp70 ATPase activity by suppressing the J domain. | [120] |
| Pifithrin-μ (2-phenylethynesulfonamide) | Interacts with the SBD and disrupts interactions with partners such as Hsp40, p53, and p62. | [121,122] |
| YM-1 | An MKT-077 derivative with better cytosolic localization; inhibits BAG3 interaction with Hsp70. | [123,124] |
| JG-83 | An MKT-077 analog; binds at a similar site and hinders interactions with cochaperones and other protein partners. | [125] |
| JG-84 | An MKT-077 derivative that interacts with the allosteric DnaK, NBD of Hsp70, thereby disrupting ATP hydrolysis and affecting the chaperone function. | [125] |
| JG-98 | Abrogation of Hsp70 and the Bcl-2 associated anthanogene3 (Bag3) protein–protein interaction. Also inhibits Mortalin function. | [123,126] |
| Methylene blue | Inhibits the chaperone function of the Hsp70 protein by binding with the glucocorticoid receptor. | [127,128] |
| Natural Inhibitors | ||
| 15-deoxyspergualin (DSG) | Inhibits the ATPase domain of Hsp70 by interacting with the EEVD domain targeting the ABD. | [120] |
| Piericidin A | Suppresses GRP78. | [129] |
| Verrucosidin | Suppresses GRP78. It also inhibits the electron transport chain complex I. | [129] |
| Epigallocatechin-3-gallate (EGCG) | Blocks the ATPase domain of GRP78, thereby repressing it. | [130] |
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | Inhibits the activation of Hsp70 at transcription stage via preventing Hsf1 activation. Also inhibits Mortalin function. | [120] |
| Nano-formulated Quercitin (Q-PEGL) | Quercetin on liposomal pegylation improves the bioavailability and solubility of quercetin. The exact mechanism of quercetin activity is unknown. | [131] |
| Kahweol | Attenuates expression of Hsp70, although the mechanism is unknown. | [132] |
| Cantharidin | Inhibits interaction of Hsp70 promoter and Hsf1, consequently blocking Hsf1-mediated expression of Hsp70. | [133] |
| Docosahexaenoic acid (DHA) | Downregulation of GRP78 protein by modulating upstream pERK1/2 and activation of caspase-4-mediated apoptosis. | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushal, S.; Gupta, S.; Shefrin, S.; Vora, D.S.; Kaul, S.C.; Sundar, D.; Wadhwa, R.; Dhanjal, J.K. Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy. Cancers 2024, 16, 3470. https://doi.org/10.3390/cancers16203470
Kaushal S, Gupta S, Shefrin S, Vora DS, Kaul SC, Sundar D, Wadhwa R, Dhanjal JK. Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy. Cancers. 2024; 16(20):3470. https://doi.org/10.3390/cancers16203470
Chicago/Turabian StyleKaushal, Shruti, Samriddhi Gupta, Seyad Shefrin, Dhvani Sandip Vora, Sunil C. Kaul, Durai Sundar, Renu Wadhwa, and Jaspreet Kaur Dhanjal. 2024. "Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy" Cancers 16, no. 20: 3470. https://doi.org/10.3390/cancers16203470
APA StyleKaushal, S., Gupta, S., Shefrin, S., Vora, D. S., Kaul, S. C., Sundar, D., Wadhwa, R., & Dhanjal, J. K. (2024). Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy. Cancers, 16(20), 3470. https://doi.org/10.3390/cancers16203470








