Holomics and Artificial Intelligence-Driven Precision Oncology for Medullary Thyroid Carcinoma: Addressing Challenges of a Rare and Aggressive Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Management of Medullary Thyroid Carcinoma
2.1. Extent of Thyroidectomy for Medullary Thyroid Carcinoma
2.2. Extent of Cervical Lymphadenectomy
2.3. Management of Locally Advanced or Metastatic Medullary Thyroid Carcinoma
2.4. Postoperative Follow-Up
2.5. Management of Persistent or Recurrent Disease Medullary Thyroid Carcinoma
3. Challenges Associated with the Management of Medullary Thyroid Carcinoma
3.1. Serum Calcitonin
3.2. Hereditary MTC
3.3. Sporadic MTC
3.4. Targeted Therapy
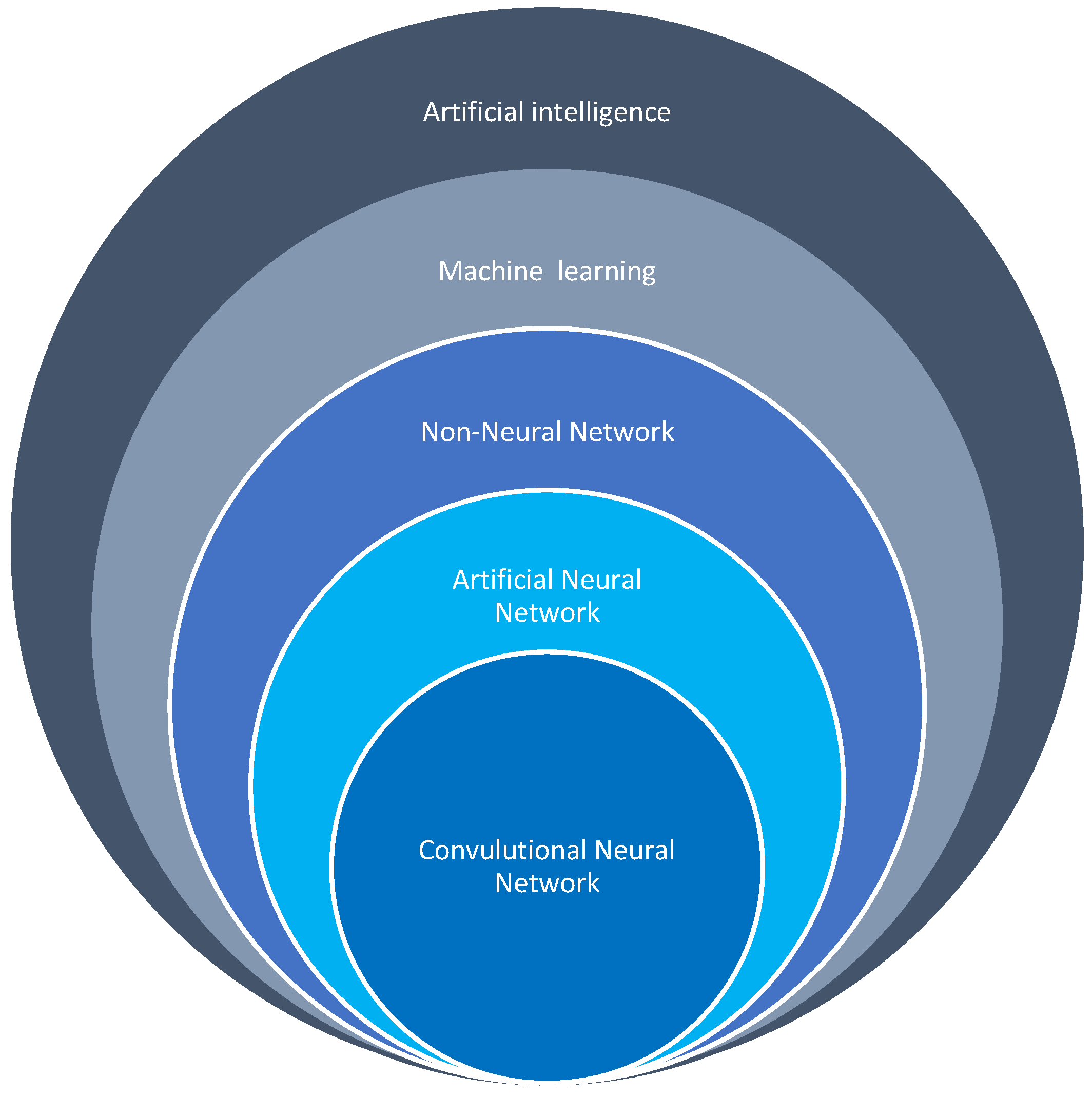
4. Artificial Intelligence in the Healthcare Industry
5. Application of AI during the Investigation of Medullary Thyroid Carcinoma
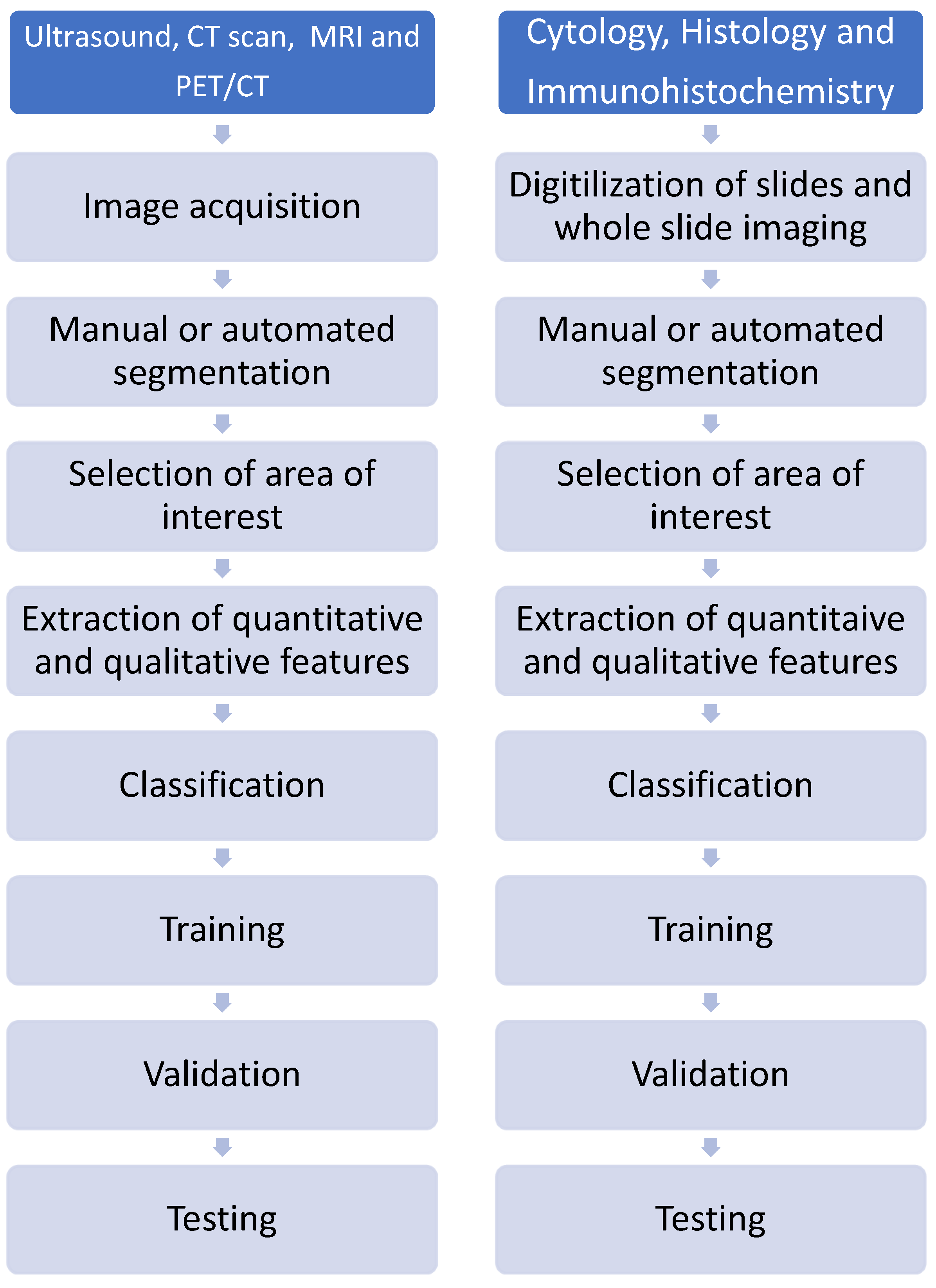
5.1. Radiomics
5.2. Pathomics
5.3. Epigenomics
5.4. Other Omics for the Investigation and Management of Cancer
6. Application of AI in MTC
6.1. Metastatic Workup of Medullary Thyroid Carcinoma
6.2. Risk Stratification of MTC
6.3. Treatment of Locally Advanced and Metastatic MTC
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Siddique, M.; Riaz, S.; Khan, A.I.; Nawaz, M.K.; Bashir, H. Medullary Thyroid Carcinoma: Prognostic Variable And Tumor Markers Affecting Survival. J. Ayub Med. Coll. Abbottaba 2018, 30 (Suppl. 1), S627–S632. [Google Scholar]
- Moo-Young, T.A.; Traugott, A.L.; Moley, J.F. Sporadic and Familial Medullary Thyroid Carcinoma, State of the Art. Surg. Clin. N. Am. 2009, 89, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A.; Pacini, F.; Robinson, B.G.; Santoro, M. Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update. J. Clin. Endocrinol. Metab. 2013, 98, 3149–3164. [Google Scholar] [CrossRef]
- Ricci, C.; Salvemini, A.; Dalmiglio, C.; Castagna, M.G.; Cantara, S. From Circulating Tumor Cells to Mirna: New Challenges in the Diagnosis and Prognosis of Medullary Thyroid Cancer. Cancers 2023, 15, 4009. [Google Scholar] [CrossRef] [PubMed]
- Shakira, W.E.; Boroomand, S.; Kia, S.K.; Hedayati, M. MicroRNAs in thyroid cancer with focus on medullary thyroid carcinoma: Potential therapeutic targets and diagnostic/prognostic markers and web based tools. Oncol. Res. 2024, 32, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kahles, A.; Lehmann, K.-V.; Toussaint, N.C.; Huser, M.; Stark, S.G.; Sachsenberg, T.; Stegle, O.; Kohlbacher, O.; Sander, C. Cancer Genome Atlas Research Network., Ratch, G. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell 2018, 34, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef]
- Bonnal, S.C.; Lopez-Oreja, I.; Valcarcel, J. Roles and mechanisms of alternative splicing in cancer—Implications for care. Nat. Rev. Clin. Oncol. 2020, 17, 457–474. [Google Scholar] [CrossRef]
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Rev. Cancer 2018, 18, 173–184. [Google Scholar] [CrossRef]
- Wirth, M.; Tate, J.; Capdevila, J.; Hughes, D.T. MEDULLARY THYROID CANCER: Management guidelines update. Endocr. Pract. 2020, 26, 1063–1086. [Google Scholar]
- Sveen, A.; Kilpinen, S.; Ruusulehto, A.; Lothe, R.A.; Skotheim, R.I. Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene 2016, 35, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.-W.; Abdel-Wahab, O. Therapeutic targeting of splicing in cancer. Nat. Med. 2016, 22, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Dreyfuss, G. Splicing in disease: Disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2017, 18, 762–778. [Google Scholar]
- Khan, M.A.; Bianchi, F.; Rizzi, R.; Sakar, M.S.; Ding, X. CRISPR/Cas9-mediated cancer targeting: Applications and challenges. Theranostics 2018, 8, 2758–2772. [Google Scholar]
- Chen, Z.; Mao, Y.; You, T.; Chen, G. Establishment and validation of a nomogram model for predicting distant metastasis in medullary thyroid carcinoma: An analysis of the SEER database based on the AJCC 8th TNM staging system. Front. Endocrinol. 2023, 14, 1119656. [Google Scholar] [CrossRef]
- Ow, P.M. Genomics and Epigenomics of Medullary Thyroid Carcinoma: From Sporadic Disease to Familial Manifestation. Endocr. Pathol. 2022, 32, 35–43. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Poulsen, P.L.; Rasmussen, A.K.; Feldt-Rasmussen, U.; Schytte, S.; Londero, S.C.; Pedersen, H.B.; Hahn, C.H.; et al. Replication of newly proposed TNM staging system for medullary thyroid carcinoma: A nationwide study. Endocr. Connect. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, K.; Li, F.; He, X. Medullary Thyroid Carcinoma with Elevated Serum CEA and Normal Serum Calcitonin after Surgery: A Case Report and Literature Review. Front. Oncol. 2020, 10, 526716. [Google Scholar] [CrossRef]
- Giovanella, L.; Fontana, M.; Keller, F.; Verburg, F.A.; Cerian, L. Clinical performance of calcitonin and procalcitonin Elecsys® immunoassays in patients with medullary thyroid carcinoma. Clin. Chem. Lab. Med. 2020, 59, 743–747. [Google Scholar] [CrossRef]
- Danila, R.; Livadariu, R.; Branisteanu, D. Calcitonin revisited in 2020. Act. Endocrinol. 2019, 15, 544–548. [Google Scholar] [CrossRef]
- Matrone, A.; Gambale, C.; Prete, A.; Elisei, R. Sporadic Medullary Thyroid Carcinoma:Towards a Precision Medicine. Front. Endocrinol. 2022, 13, 864253. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; Gambale, C.; Prete, A.; Piaggi, P.; Cappagli, V.; Bottici, V.; Romei, C.; Ciampi Torregrossa, L.; De Napoli, L.; Molinaro, E.; et al. Impact of Advanced Age on the Clinical Presentation and Outcome of Sporadic Medullary Thyroid Carcinoma. Cancers 2020, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Gild, M.L.; Clifton-Bligh, R.J.; Wirth, L.J.; Robinson, B.G. Medullary Thyroid Cancer: Updates and Challenges. Endocr. Rev. 2023, 44, 934–946. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiiyama, T.; Fukushima, M.; Miya, A. Static Prognostic Factors and Appropriate Surgical Designs for Patients with Medullary Thyroid Carcinoma: The Second Report from a Single-Institution Study in Japan. World J. Surg. 2018, 42, 3954–3966. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Cavedon, E.; Watutantrige-Fernando, S.; Barollo, S.; Bertazza, L.; Manso, J.; Iacobone, M.; Nacamulli, D.; Galupppini, F.; Pennelli, G.; et al. Unique Case of a Large Indolent Medullary Thyroid Carcinoma: Time to Reconsider the Medullary Thyroid Adenoma Entity? Eur. Thyroid J. 2019, 8, 108–112. [Google Scholar] [CrossRef]
- Hamdy, O.; Awny, S.; Metwally, I.H. Medullary thyroid cancer: Epidemiological pattern and factors contributing to recurrence and metastasis. Medullary thyroid cancer: Epidemiological pattern and factors contributing to recurrence and metastasis. Ann. R. Coll. Surg. Engl. 2020, 102, 499–503. [Google Scholar] [CrossRef]
- Apaydin, T.; Imre, E.; Yavuz, D. Determinants of remission in a case series of medullary thyroid carcinoma. Turk. J. Med. Sci. 2021, 51, 2050–2056. [Google Scholar] [CrossRef]
- Darabi, S.; Adeyelu, T.; Elliott, A.; Sukari, A.; Hodges, K.; Abdulla, F.; Zuazo, C.E.; Wise-Draper, T.; Wang, T.; Demeure, M.J. Genomic and Transcriptomic Landscape of RET Wild-Type Medullary Thyroid Cancer and Potential Use of Mitogen-Activated Protein Kinase-Targeted Therapy. J. Am. Coll. Surg. 2024; 239, 50–60. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int. J. Mol. Sci. 2021, 22, 1726. [Google Scholar] [CrossRef]
- Galuppini, F.; Censi, S.; Moro, M.; Carraro, S.; Sbaragalia, M.; Iacobone, M.; Fassan, M.; Mian, C.; Pennelli, G. MicroRNA in Medullary Thyroid Carcinoma: A State of the Art Review of the Regulatory Mechanisms and Future Perspectives. Cells 2021, 10, 955. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Li, B.; Zhao, Z.; Liu, Y.; Zhao, Z.; Jing, S.; Wang, G. Identification of Potential lncRNAs and miRNAd as Diagnostic Biomarkers for Papillary Thyroid Carcinoma Based on Machine Learning. Int. J. Endocrinol. 2021, 2021, 3984463. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Z.; Chen, X.; Zhang, G.; Wang, Y.; Pan, L.; Yan, C.; Yang, G.; Zhao, L.; Han, J.; et al. Plasma Lipidomics Profile Reveals Biomarkers for Papillary Thyroid Carcinoma. Front. Cell Dev. Biol. 2021, 9, 682269. [Google Scholar] [CrossRef]
- Opsahl, E.M.; Akslen, L.A.; Schlichting, E.; Aas, T.; Brauckhoff, K.; Hagen, A.I.; Rosenlud, A.F.; Sgstad, E.; Groholt, K.K.; Maehle, L.; et al. Trends in Diagnostics, Surgical Treatment, and Prognostic Factors for Outcomes in Medullary Thyroid Carcinoma in Norway: A Nationwide Population-Based Study. Eur. Thyroid J. 2019, 8, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. Medullary Thyroid Carcinoma: Why is Specialization Mandatory? Visc. Med. 2018, 34, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, B.H. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinal. Metab. 2021, 36, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, R.; Basnayake, O.; Jayarajah, U.; Seneviratne, S. Management of medullary carcinoma of the thyroid: A review. J. Int. Med. Res. 2022, 50, 3000605221110698. [Google Scholar] [CrossRef]
- Wu, X.; Li, B.; Zheng, C. Clinical Characteristics, Surgical Management, and Prognostic Factors of Medullary Thyroid Carcinoma: A Retrospective, Single-Center Study. Technol. Cancer Res. Treat. 2022, 21, 21153330338221078435. [Google Scholar] [CrossRef]
- Khan, S.A.; Aziz, A.; Esbhani, U.A.; Masood, M.Q. Medullary Thyroid Cancer: An Experience from a Tertiary Care Hospital of a Developing Country. Indian J. Endocrinol. Metab. 2022, 26, 68–72. [Google Scholar] [CrossRef]
- Luo, Z.; Hong, Y.; Yan, C.; Ye, Q.; Wang, Y.; Haung, P. Nomogram for preoperative estimation risk of cervical lymph node metastasis in medullary thyroid carcinoma. Front. Oncol. 2022, 12, 883429. [Google Scholar] [CrossRef]
- Beukhof, C.M.; Brabander, T.; van Nederveen, F.H.; van Velthuysun, M.F.; de Rijike, Y.B.; Hofland, L.J.; Franssen, G.J.H.; Froberg, L.A.C.; Kam, B.L.R.; Visser, W.E.; et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: Predictors and pitfalls. BMC Cancer 2019, 19, 325. [Google Scholar] [CrossRef]
- Meng, K.; Luo, H.; Chen, H.; Guo, H.; Xia, W. Prognosis value of numbers of metastatic lymph node in medullary thyroid carcinoma population-based study using the SEER 18 database. Medicine 2019, 98, e13884. [Google Scholar] [CrossRef]
- Grossrubatscher, E.; Fanciulli, G.; Pes, L.; Sesti, F.; Dolci, C.; de Cicco, F.; Colao, A.; Faggiano, A.; Nike Group. Advances in the Management of Medullary Thyroid: Focus on Peptide Receptor Radionuclide Therapy. J. Clin. Med. 2020, 9, 3507. [Google Scholar] [CrossRef] [PubMed]
- Haung, S.; Zhong, J.; Zhang, Z.; Chen, R.; Li, J.; Sun, J.; Chen, H. Prognosis of radiotherapy in medullary thyroid carcinoma patients without distant metastasis. Transl. Cancer Res. 2021, 10, 4714–4726. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, B.; Riss, P.; Scheuba, C.; Raderer, M. How I treat medullary thyroid cancer. ESMO Open 2021, 6, 100183. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.E.; Oliver, J.R.; Rotsides, J.M.; Shao, Q.; Patel, K.N.; Morris, L.G.T.; Givi, B. Nodal disease burden and outcome of medullary thyroid carcinoma. Head. Neck 2021, 43, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kukulska, A.; Krajewska, J.; Kolosza, Z.; Grzadziel, A.; Gajek, M.; Paliczka-Cieslik, E.; Sygula, D.; Ficek, K.; Kluczewska-Galka, A.; Jarzab, B. Stereotactic radiotherapy is useful treatment option for patients with medullary thyroid cancer. BMC Endocr. Disord. 2021, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yu, P.-C.; Lei, B.-W.; Li, C.-W.; Zhang, Y.; Tan, L.-C.; Shi, R.-L.; Wang, J.; Ma, B.; Xu, W.-B.; et al. Association Between Programmed Death-Ligand 1 Expression and Clinicopathological Characteristics, Structural Recurrence, and Biochemical Recurrence/Persistent Disease in Medullary Thyroid Carcinoma. Thyroid 2019, 29, 1269–1278. [Google Scholar] [CrossRef]
- Araque, K.A.; Gubbi, S.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar] [CrossRef]
- Oczko-Wojciechowska, M.; Czarniecka, A.; Gawlik, T.; Jarzab, B.; Krajewska, J. Current status of the prognostic molecular markers in medullary thyroid carcinoma. Endocr. Connect. 2020, 9, R251–R263. [Google Scholar] [CrossRef]
- Golingan, H.; Hunis, B.; Golding, A.C.; Bimston, D.N.; Harel, R.M. Neoadjuvant Lenvatinib In Advanced Unresectable Medullary Thyroid Carcinoma: A Case Report. AACE Clin. Case Rep. 2019, 6, e73–e78. [Google Scholar] [CrossRef]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. American Thyroid Association Task Force on Medullary Thyroid Carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Filleti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Nerwbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Colombo, C.; Sun, H.; Kim, H.Y.; Pino, A.; De Leo, S.; Gazzano, G.; Persani, L.; Dionigi, G.; Fugazzola, L. Unilateral Surgery for Medullary Thyroid Carcinoma: Seeking for Clinical Practice Guidelines. Front. Endocrinol. 2022, 13, 875875. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W. A High Frequency of Lobectomy Instead of Total Thyroidectomy to Treat Medullary Thyroid Cancer in Korea: Data from the Korean National Health Insurance Service. Endocrinol. Metab. 2020, 35, 784–785. [Google Scholar] [CrossRef] [PubMed]
- Raffel, A.; Cupisti, K.; Krausch, M.; Wolf, A.; Schulte, K.M.; Roher, H.D. Incidentally Found Medullary Thyroid Cancer: Treatment Rationale for Small Tumors. World J. Surg. 2004, 28, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, M.R.; Mazza, E.I.; Mian, C.; Boschin, I.M. Medullary thyroid carcinoma. Expert Rev. Anticancer Ther. 2023, 23, 943–957. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.-A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J. Clin. Oncol. 2021, 40, 96–104. [Google Scholar] [CrossRef]
- Tao, Z.; Deng, X.; Ding, Z.; Guo, B.; Fan, Y. Improved survival after primary tumor resection in distant metastasis medullary thyroid carcinoma: A population based cohort study with propensity score matching. Sci Rep. 2024, 14(1), 17260. [Google Scholar] [CrossRef]
- Fleming, J.B.; Lee, J.E.; Bouvet, M.; Schultz, P.N.; Sherman, S.I.; Sellin, R.V.; Friend, K.E.; Burgess, M.A.; Cote, G.J.; Gagel, R.F.; et al. Surgical Strategy for the treatment of medullary thyroid carcinoma. Ann. Surg. 1999, 230, 697–707. [Google Scholar] [CrossRef]
- Kebebew, E.; Greebnspan, F.S.; Clark, O.H.; Woeber, K.A.; Grunwell, J. Extent of disease and practice patterns for medullary thyroid cancer. J. Am. Coll. Surg. 2005, 200, 890–896. [Google Scholar] [CrossRef]
- Kiriakopoulos, A.; Giannakis, P.; Menenakos, E. Calcitonin: Current concepts and differential diagnosis. Therapeutic Adv. Endocrinol. Metabol. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Xiao, J.; Jiang, J.; Chen, W.; Hong, T.; Li, B.; He, X.; Liu, W. Combination of ultrasound and serological tests for detecting occult lateral lymph node metastases in medullary thyroid cancer. Cancer Med. 2023, 12, 11417–11426. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.J.; Zhou, T.; Wu, S.; Xu, M.X.; Sun, S.H. Do medullary thyroid carcinoma patients with high calcitonin require bilateral neck lymph node clearance? A case report. World J. Clin. Cases 2021, 9, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Risk Patterns of Distant Metastases in Follicular, Papillary and Medullary Thyroid Cancer. Horm. Metab. Res. 2022, 54, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Giannelli, J.; Marques, B.; Piccardo, A.; Crescenzi, A.; Deandrea, M. Head-to-head comparison of FNA cytology vs. calcitonin measurements in FNA washout fluid (FNA-CT) to diagnose medullary thyroid carcinoma. A systematic review and meta-analysis. Endocrine 2022, 75, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Modigliani, E.; Cohen, R.; Campos, J.M.; Conte-Devolx, B.; Maes, B.; Boneu, A.; Schlumberger, M.; Bigorgne, J.C.; Dumontier, P.; Leclerc, L.; et al. Prognostic factors for the survival and for biochemical cure in medullary thyroid carcinoma; results in 899 patients. The GETC Study group. Clin. Endocrinol. 1998, 48, 265–273. [Google Scholar] [CrossRef]
- Bartz-Kurycki, M.A.; Oluwo, O.E.; Morrison-Wiseman, L.F. Medullary thyroid carcinoma; recent advances in the identification, treatment, and prognosis. Therap. Adv. Endocrinol. Metaboli. 2021, 12, 20420188211049611. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.; Ehya, H.; et al. Thyroid Carcinoma, version 2.2022. J. Natl. Compr. Can. Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Y.; Shen, C.; Zhang, Y.; Shi, R.; Zhang, F.; Liao, T.; Lv, G.; Zhu, Z.; Jiao, L.; et al. Integrated proteogenomic characterization of medullary thyroid carcinoma. Crll Discov. 2022, 8, 120. [Google Scholar] [CrossRef]
- Juez, L.D.; Mercader, E.; Amunategui, I.; Febrero, B.; Rodriguez, J.M.; Gomez-Ramirez, J.; MECANO Collaboration Group. Extension of Prophylactic Surgery in Medullary Thyroid Carcinoma. Differences Between Sporadic and Hereditary Tumours According to Calcitonin Levelsand Lymph NodeInvolvement. World J Surg. 2022, 46, 820–828. [Google Scholar] [CrossRef]
- Shaghaghi, A.; Salari, A.; Jalaeefar, A.; Shirkhoda, M. Management of lymph nodes in medullary thyroid carcinoma: A review. Ann. Med. Surg. 2022, 81, 104538. [Google Scholar] [CrossRef]
- Roy, M.; Chen, H.; Sippel, R.S. Current Understanding and Management of Medullary Thyroid Cancer. Oncologist 2013, 18, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.S.; de Castroneves, L.A.; Sado, H.N.; Sapienza, M.T.; de Oliveira Hoff, A.A.F.; Buchpiguel, C.A. Bone and Calcified Soft Tissue Metastases of Medullary Thyroid Carcinoma Better Characterized on 18F-Fluoride PET/CT than on 68Ga-Dotatate PET/CT. Nucl. Med. Mol. Imaging 2018, 52, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Rasul, S.; Hartenbach, S.; Rebhan, K.; Gollner, A.; Karanikas, G.; Mayerhoefer, M.; Mazal, P.; Hacker, M.; Hartenbach, M. [18F]DOPA PET/ceCT in diagnosis and staging of primary medullary thyroid carcinoma prior to surgery. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.S.; Jesus, T.T.; Cardoso, L.; Soares, P.; Vinagre, J. Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments. J. Pers. Med. 2023, 13, 1132. [Google Scholar] [CrossRef]
- Trimboli, P.; Ferrarazzo, G.; Cappelli, C.; Piccardo, A.; Castellana, M.; Barizzi, J. Thyroid nodules with Indeterminate FNAC According to the Italian Classification System: Prevalance, Rate of Operation, and Impact on Risk of Malignancy. An Updated Systematic Review and Meta-analysis. Endo Pathol. 2022, 33, 457–471. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Shen, C.-K.; Du, T.-X.; Li, Z.-M.; Shi, X.; Wang, Y.; Wei, W.-J. Survival Outcome and Optimal Candidates of Primary Tumor Resection for Patients with Metastatic Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2024, dgae214. [Google Scholar] [CrossRef]
- Brammen, L.; Niederle, M.B.; Riss, P.; Scheuba, C.; Selberherr, A.; Karanikas, G.; Bodner, G.; Koperek, O.; Niederle, B. Medullary Thyroid Carcinoma: Do Ultrasonography and F-DOPA-PER—CT Influence the Initial Surgical Strategy? Ann. Surg. Oncol. 2018, 25, 3919–3927. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, H.; Lv, M.; Li, N. Preoperative Serum Calcitonin Level and Ultrasonographic Characteristics Predict the Risk of Metastatic Medullary Thyroid Carcinoma: Functional Carcinoma Analysis of Calcitonin Related Genes. Dis. Mrkers. 2020, 2022, 9980185. [Google Scholar] [CrossRef]
- Gambardella, C.; Offi, C.; Clarizia, G.; Romano, R.M.; Cozzolino, I.; Montella, M.; Di Crescenzo, R.M.; Mascolo, M.; Cangiano, A.; Di Martino, S.; et al. Medullary thyroid carcinoma with double negative calcitonin and CEA: A case report and update of literature review. BMC Endocr. Discord 2019, 19, 103. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Chen, G.; Xue, S. Early postoperative prediction of the risk of distant metastases in medullary thyroid cancer. Front. Endocrinol. 2023, 14, 1209978. [Google Scholar] [CrossRef]
- Wienliang, Y.; Zhang, Y. Oncologic outcomes of calcitonin-negative medullary thyroid carcinoma. Front. Endocrinol. 2022, 13, 1025629. [Google Scholar] [CrossRef]
- Trimboli, P.; Camponovo, C.; Ruinelli, L. The dilemma of routine testing for calcitonin thyroid nodule’s patients to detect or exclude medullary carcinoma: One single negative test should be valuable as rule-out strategy to avoid further calcitonin measurements over time. Endocrine 2022, 77, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Asa, S.L.; Ezzat, S.; Sawka, A.M.; Goldstein, D. Diagnosis and pathologic characteristics of medullary thyroid carcinoma-review of current guidelines. Curr. Oncol. 2019, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.S.; Kang, M.J.; Youn, H.J.; Kim, K.M. Diagnostic pitfall of thyroid fine-needle aspiration induced fibrosis: Follicular adenoma mimicking medullary thyroid carcinoma in frozen section. Diagn. Pathol. 2021, 16, 25. [Google Scholar] [CrossRef]
- Ciarletto, A.M.; Narick, C.; Malchoff, C.D.; Massoll, N.A.; Labourier, E.; Haugh, K.; Mireskandari, A.; Finkelstein, S.D.; Kumar, G. Analytical and Clinical Validation of Pairwise MicroRNA Expression Analysis to Identify Medullary Thyroid Cancer in Thyroid Fine-Needle Aspiration Samples. Cancer Cytopathol. 2021, 129, 239–249. [Google Scholar] [CrossRef]
- Ferrarazzo, G.; Camponovo, C.; Deandrea, M.; Piccardo, A.; Scappaticcio, L.; Trimboli, P. Suboptimal accuracy of ultrasound and ultrasound-based risk stratification systems in detecting medullary thyroid carcinoma should not be overlooked. Findings from a systematic review with meta-analysis. Clin. Endocrinol. 2022, 97, 532–540. [Google Scholar] [CrossRef]
- Torresan, F.; Armellin, C.; Iacobone, M. Management of medullary carcinoma. Ann. Thyroid 2020, 5, 16. [Google Scholar] [CrossRef]
- Makri, A.; Akshintala, S.; Derse-Anthony, C.; Del Ribero, J.; Widemann, B.; Stratakis, C.A.; Glod, J.; Lodish, M. Pheochromocytoma in children and adlolescents with multiple endocrine Neoplasia Type 2B. J. Clin. Endocrinol. Metab. 2019, 104, 7–12. [Google Scholar] [CrossRef]
- Opsahl, E.M.; Brauckhoff, M.; Schlichting, E.; Helset, K.; Svartberg, J.; Brauckhoff, K.; Maehle, L.; Engebretsen, L.F.; Sigstad, E.; Groholt, K.K.; et al. A Nationwide Study of Multiple Endocrine Neoplasia Type 2A in Norway: Predictive and Prognostic Factors for the Clinical Course of Medullary Thyroid Carcinoma. Thyroid 2016, 26, 1225–1238. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kwon, H.J.; Kim, B.K.; Kwon, S.K.; Park, Y.H.; Kim, J.H.; Jung, S.B.; Lee, C.H.; Lee, S.K.; Uchino, S. A Case of Medullary Thyroid Carcinoma with de novo V804M RET Germline Mutation. J. Korean Med. Sci. 2013, 28, 156–159. [Google Scholar] [CrossRef]
- Lombardo, F.; Baudin, E.; Chiefari, E.; Arturi, F.; Bardet, S.; Caillou, B.; Conte, C.; Dallaiccola, B.; Giuffrida, D.; Bidart, J.-M.; et al. Familial Medullary Thyroid Carcinoma: Clinical Variability and Low Aggressiveness Associated with RET Mutation at Codon 804. J. Clin. Endocrinol. Metab. 2002, 87, 1674–1680. [Google Scholar] [CrossRef][Green Version]
- Dabir, T.; Hunter, S.J.; Russell, C.F.J.; McCall, D.; Morrison, P.J. The RET Mutation E768D Confers a Late-onset Familial Medullary Thyroid Carcinoma—Only Phenotype with Incomplete Penetrance: Implications for Screening and Management of Carrier Status. Fam. Cancer 2006, 5, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.S.; Hu, M.I.; Chiang, Y.-J.; Graham, P.H.; Fisher, S.B.; Sosa, J.A.; Perrier, N.; Brown, S.; Holla, V.R.; Dadu, R.; et al. Next-Generation Sequencing in Sporadic Medullary Thyroid Cancer Patients: Mutation Profile and Disease Aggressiveness. J. Endocr. Soc. 2024, 8, bvae048. [Google Scholar] [CrossRef] [PubMed]
- Al-Kurd, A.; Gross, D.J.; Zangen, A.; Atlan, K.; Mazeh, H.; Grozinsky-Glasberg, S. Bilateral Medullary thyroid carcinoma in a 3-year-old female patient with Multiple Endocrine Neoplasia 2A Syndrome Undergoing prophylactic Thyroidectomy:Should current guidelines be revised? Eur. Thyroid J. 2018, 79, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Licata, L.; Di Fidio, C.A.; Vacante, M.; Basile, F.; Biondi, A.; Richiusa, P.; Gurrera, A.; Ciuni, R. A Rare Case of Negative Serum Calcitonin in Metastatic Medullary Thyroid Carcinoma: Diagnosis, Treatment, and Follow-up Strategy. Am J Case Rep. 2022, 23, e935207-1–e935207-9. [Google Scholar] [CrossRef]
- Murphy, D.C.; Johnson, S.J.; Aspinall, S. Calcitonin-negative medullary thyroid carcinoma: The ‘triple-negative’ phenotype. Ann. R. Coll. Surg. Engl. 2020, 102, e63–e66. [Google Scholar] [CrossRef]
- Niederle, B. Screening for medullary carcinoma of the thyroid. BJS 2014, 101, 1625–1626. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Sun, J.; Yin, L.; Guo, R.; Yan, Z. Clinical value of color Doppler Ultrasound combined with serum tumor markers for the diagnosis of medullary thyroid carcinoma. Oncol. Lett. 2021, 22, 561. [Google Scholar] [CrossRef]
- Kaliszewski, K.; Ludwig, M.; Ludwig, B.; Mikula, A.; Greniuk, M.; Rudnicki, J. Update on the Diagnosis and Management of Medullary Thyroid Cancer: What Has Changed in Recent Years? Cancers 2022, 14, 3643. [Google Scholar] [CrossRef]
- Guo, Z.-T.; Tian, K.; Xie, X.-Y.; Zhang, Y.-H.; Fang, D.-B. Machine Learning for Predicting Distant Metastasis of Medullary Thyroid Carcinoma Using SEER Database. Int. J. Endocrinol. 2023, 2023, 9965578. [Google Scholar] [CrossRef]
- Wilczynska, M.; Suchnmiel, M.; Solowski, G.; Hubalewska-Dydejczyk, A.; Tofimiuk Muldener, M. Disseminated medullary thyroid cancer—An alternative therapeutic approach. Endokrynol. Pok. 2022, 73, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest. Endosc. 2020, 92, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.L.; Li, Q.L.; Tong, J.; Shi, L.-N.; Liv, W.X.; Xu, Y.; Cheng, J.; Du, T.T.; Li, J.; Cui, X.W. Artificial Intelligence in thyroid ultrasound. Front. Oncol. 2023, 13, 1060702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.R.; Du, B.; Liu, H.Q.; Chen, C. Artificial Intelligence for Personalized Medicine in Thyroid Cancer: Current Status and Future Perspectives. Front. Oncol. 2020, 2, 604051. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Meng, J.; Zhang, Y. miR-592 acts as an oncogene and promotes medullary thyroid cancer tumorigenesis by targeting cyclin-dependent kinase 8. Mol. Med. Rep. 2020, 22, 3316–3326. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Chen, Y.; eFeng, L.; Zheng, C.; Liu, L.; Hu, L.; Pan, B. HADCNet: Automatic segmentation of COVID-19 infection based on a hybrid attention dense connected network with dilated convolution. Comput. Biol. Med. 2022, 149, 105981. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Ye, Z.; Xu, P.; Xia, X.; Guo, M. Prediction of lung metastases in thyroid cancer using machine learning based on SEER database. Cancer Med. 2022, 11, 2503–2515. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Q.; Wang, J.; Tan, Z.; Li, Q.; Ge, M. Molecular basis and targeted therapy in thyroid cancer: Progress and opportunities. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188928. [Google Scholar] [CrossRef]
- Shi, B.; Chen, J.; Chen, Y.; Lin, W.; Yang, J.; Chen, Y.; Wu, C.; Huang, Z. Prediction of recurrent spontaneous abortion using evolutionary machine learning with joint self-adaptive sime mould algorithm. Comput. Biol. Med. 2022, 148, 105885. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Lin, Q.; Qi, Q.; Hou, S.; Chen, Z.; Jiang, N.; Zhang, L.; Lin, C. Application of Pet-CT Fusion Deep Learning Imaging in Precise Radiotherapy of Thyroid Cancer. J. Healthc. Eng. 2021, 2021, 2456429. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Muzakky, H.; Lee, Y.C.; Lin, Y.J.; Chao, T.K. Annotation-Free Deep Learning-Based Prediction of Thyroid Molecular Cancer Biomarker BRAF (V600E) from Cytological Slides. Int. J. Mol. Sci. 2023, 24, 2521. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, M.; Niloka, H.; Suzuki, A.; Abe, M.; Arai, Y.; Nagahara, H.; Miyauchi, A.; Akamizu, T. Application of deep learning as an ancillary diagnostic tool for thyroid FNA cytology. Cancer Cytopathol. 2023, 131, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Xi, N.M.; Wang, L.; Yang, W. Improving the diagnosis of thyroid cancer by machine learning and clinical data. Sci. Rep. 2022, 12, 11143. [Google Scholar] [CrossRef]
- Gao, L.; Xi, X.; Jiang, Y.; Yang, X.; Wang, Y.; Zhu, S.; Lai, X.; Zhang, X.; Zhao, R.; Zhang, B. Comparison among TIRADS (ACR TI-RADS and KWAK-TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine 2019, 64, 90–96. [Google Scholar] [CrossRef]
- Radebe, L.; van der Kaay, D.C.M.; Wasserman, J.D.; Goldenberg, A. Predicting Malignancy in Pediatric Thyroid Nodules: Early Experience with Machine Learning for Clinical Decision Support. J. Clin. Endocrinol. Metab. 2021, 106, e5236–e5246. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, C.; Ye, J.; Chang, L.F.; Xu, Q.; Shi, B.; Liu, L.; Yin, Y. A comparison between deep learning convolutional neural networks and radiologists in the differentiation of benign and malignant thyroid nodules on CT images. Endokrynol. Pol. 2021, 72, 217–225. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, B. Radiomics Features of Different Sizes of Medullary Thyroid Carcinoma (MTC) and Papillary Thyroid Carcinoma (PTC) Tumors: A Comparative Study. Clin. Med. Insights Oncol. 2022, 16, 11795549221097675. [Google Scholar] [CrossRef]
- Wong, C.M.; Kezlarian, B.E.; Lin, O. Current status of machine learning in thyroid cytopathology. J. Pathol. Inform. 2023, 14, 100309. [Google Scholar] [CrossRef]
- Maurea, S.; Stanzione, A.; Klain, M. Thyroid Cancer Radiomics: Navigating Challenges in a Developing Landscape. Cancers 2023, 15, 5884. [Google Scholar] [CrossRef]
- Cordes, M.; Gotz, T.I.; Coerper, S.; Kuwert, T.; Schmidkonz, C. Ultrasound characteristics of follicular and parafollicular thyroid neoplasms: Diagnostic performance of artificial neural network. Thyroid Res. 2023, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M. Digital Pathology and Artificial Intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, A.; Cuocolo, R.; Ugga, L.; Verde, F.; Romeo, V.; Brunetti, A.; Maurea, D. Oncologic Imaging and Radiomics: A Walkthrough Review of Methodological Challenges. Cancers 2022, 14, 4871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ye, C.; Hu, Y.; Li, C.; Li, X. Cascade and Fusion of Multitask Convolutional Neural Networks for Detection of Thyroid Nodules in Contrast-Enhanced CT. Comput. Intell. Neurosci. 2019, 2019, 7401235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Cheng, S.; Qian, K.; Yue, K.; Liu, H. Automatic Recognition and Classification System of Thyroid Nodules in CT Images Based on CNN. Comput. Intell. Neurosci. 2021, 2021, 5540186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiao, L.; Li., H.; Wang, Z.; Sun, H.; Feng, G.; Yin, D. Machine learning based on SEER database to predict distant metastasis of thyroid cancer. Endocrine. 2024, 84, 1040–1050. [Google Scholar] [CrossRef]
- Krishnan, G.; Singh, S.; Pathania, M.; Gosavi, S.; Abhishek, S.; Parchani, A.; Dhar, M. Artificial intelligence in clinical medicine: Catalyzing a sustainable global healthcare paradigm. Front. Artif. Intell. 2023, 6, 1227091. [Google Scholar] [CrossRef]
- Ma, X.; Han, X.; Zhang, L. An Improved k-Nearest Neighbor Algorithm for Recognition and Classification of Thyroid Nodules. J. Ultrasound Med. 2024, 43, 1025–1036. [Google Scholar] [CrossRef]
- Habchi, Y.; Himeur, Y.; Kheddar, H.; Boukabou, A.; Atalla, S.; Chouchane, A.; Ouamane, A.; Mansoor, W. AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems 2023, 11, 519. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Canes, A.; Steinberg, D.; Lyashevska, O.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Predictive analytics with gradient boosting in clinical medicine. Ann. Transl. Med. 2019, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- BuHamra, S.S.; Almutairi, A.N.; Buhamrah, A.K.; Almadani, S.H.; Alibrahim, Y.A. An NLP tool for data extraction from electronic health records: COVID-19 mortalities and comorbidities. Front. Public. Health 2022, 10, 1070870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco, E.F.; Rana, P.; Cruz, A.; Calderón, V.V.; Azevedo, V.; Ramos, R.T.J.; Ghosh, P. Performance Comparison of Deep Learning Autoencoders for Cancer Subtype Detection Using Multi-Omics Data. Cancers 2021, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kamel Boulos, M.N.; Peng, G.; Vopham, T. An overview of GeoAI applications in health and healthcare. Int. J. Health Geogr. 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-J.; Wang, H.-Y.; Lin, T.-W.; Lu, J.-J.; Hsieh, C.-H.; Liao, C.-T. Development of a Machine Learning Model for Survival Risk Stratification of Patients with Advanced Oral Cancer. JAMA Netw. Open 2020, 3, e2011768. [Google Scholar] [CrossRef] [PubMed]
- Rathi, R.; Vakharia, A.; Shadab, M. Lean six sigma in the healthcare sector: A systematic literature review. Mater. Today Proc. 2022, 50, 773–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dean, D.S.; Gharib, H. Epidemiology of thyroid nodules. Best. Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhao, X.; Guo, Q.; Li, Z.; Wei, M.; Niu, L.A. Combining radiomics with thyroid imaging reporting and data system to predict lateral cervical lymph node metastases in medullary thyroid cancer. BMC Med. Imaging 2024, 24, 64. [Google Scholar] [CrossRef]
- Gatta, R.; Depeursinge, A.; Ratib, O.; Michielin, O.; Leimgruber, A. Integrating radiomics into holomics for personalized oncology: From algorithms to bedside. Eur. Radiol. Exp. 2020, 4, 11. [Google Scholar] [CrossRef]
- Piccardo, A.; Puntoni, M.; Treglia, G.; Foppiani, L.; Bertagna, F.; Paparo, F.; Massollo, M.; Dib, B.; Paone, G.; Arlandini, A.; et al. Thyroid nodules with indeterminate cytology: Prospective comparison between 18F-FDG-PER/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur. J. Endocrinol. 2016, 174, 693–703. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, X.; Diao, W.; Mu, J.; Cheng, Y.; Jia, Z. Radiomics in Differentiated Thyroid Cancer and Nodules Explorations, Applications, and Limitations. Cancers 2021, 13, 2436. [Google Scholar] [CrossRef]
- Le, N.Q.K.; Kha, Q.H.; Nguyen, V.H.; Chen, Y.-C.; Cheng, S.-J.; Chen, C.-Y. Machine Learning-Based Radiomics Signatures for EGFR and KRAS Mutations Prediction in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9254. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Ludwig, B.; Mikula, A.; Biernat, S.; Rudnicki, J.; Kaliszewski, K. The Use of Artificial Intelligence in the Diagnosis and Classification of Thyroid Nodules: An Update. Cancers 2023, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, Y.H.; Seo, H.S.; Lee, K.Y.; Suh, S.-I.; Ryoo, I.; You, S.-H.; Kim, B.; Yang, K.-S. Dual-energy CT iodine quantification for characterizing focal thyroid lesions. Head Neck 2019, 41, 1024–1031. [Google Scholar] [CrossRef]
- Gupta, R.; Kurc, T.; Sharma, A.; Almeida, J.S.; Saltz, J. The Emergence of Pathomics. Curr. Pathol. Rep. 2019, 7, 73–84. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Zha, X.; Xu, Y.; Yin, Z.; Rixiati, Y.; Yu, F. A Proposed Modified Staging System for Medullary Thyroid Cancer: A SEEE Analysis with Multicenter Validation. Oncologist 2024, 29, e59–e67. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Wei, S.; LiVosi, V.A.; Montone, K.T.; Morrissette, J.J.D.; Baloch, Z.W. Detection of Molecular Alterations in Medullary Thyroid Carcinoma Using Next-Generation Sequencing: An Institutional Experience. Endocr. Pathol. 2016, 27, 359–362. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, D.; Liu, M.; Zhang, M.; Peng, Q. Identification and interaction analysis of key miRNAs in medullary thyroid carcinoma by bioinformatics analysis. Mol. Med. Rep. 2019, 20, 2316–2324. [Google Scholar] [CrossRef]
- Censi, S.; Bertazza, L.; Piva, I.; Manso, J.; Benna, C.; Iacobone, M.; Mondin, A.; Plebani, M.; Faggian, D.; Galuppini, F.; et al. Serum miR-375 for Diagnostic and Prognostic Purposes in Medullary Thyroid Carcinoma. Front. Endocrinol. 2021, 12, 647369. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chang, C.C.; Huang, H.H.; Lin, C.Y.; Yeh, K.T.; Chang, J.G. Detection of Molecular Alterations in Taiwanese Patients with Medullary Thyroid Cancer Using Whol-Exome Sequencing. Endocr. Pathol. 2018, 29, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Shabani, N.; Razaviyan, J.; Paryan, M.; Tavangar, S.M.; Azizi, F.; Mohammadi-Yeganeh, S.; Hedayati, M. Evaluation of miRNAs expression in medullary thyroid carcinoma tissue samples: miR-34a and miR-144 as promising overexpressed markers in MTC. Hum. Pathol. 2018, 79, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Jackson, N.; Gundara, J.S.; Zhao, J.T.; Gill, A.J.; Delbridge, L.; Robinson, B.G.; Sidhu, S.B. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin. Cancer Res. 2011, 17, 4772–4781. [Google Scholar] [CrossRef]
- Jajin, M.G.; Abooshahab, R.; Hooshmand, K.; Moradi, A.; Siadat, S.D.; Mirzazadeh, R.; Chegini, K.G.; Hedayati, M. Gas chromatography-mass spectrometry-based untargeted metabolomics reveals metabolic perturbations in medullary thyroid carcinoma. Sci. Rep. 2022, 12, 8397. [Google Scholar] [CrossRef] [PubMed]
- Krneta, M.P.; Saranovic, D.S.; Teodotrovic, L.M.; Krajcinovic, N.; Avramovic, N.; Bojovic, Z.; Bukumiric, Z.; Markobic, I.; Rajsic, S.; Djorovic, B.B.; et al. Prediction of Cervical Lymph Node Metastasis in Clinically Node-Negative T1 and T2 Papillary Thyroid Carcinoma Using Supervised Machine Learning Approach. J. Clin. Med. 2023, 12, 3641. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, X.; Zhao, Z.Y.; Yan, J.B.; Yao, D.Y. A rare coexistence of parathyroid adenoma and medullary and papillary thyroid carcinoma. Int. J. Clin. Exp. Pathol. 2020, 13, 2387–2391. [Google Scholar]
- Innella, G.; Rossi, C.; Romagnoli, M.; Repaci, A.; Bianchi, D.; Cantarini, M.E.; Mrtorona, D.; Godino, L.; Pession, A.; Percesepe, A.; et al. Result and Clinical Interpretation of Germline RET Analysis in a Series of Patients with Medullary Thyroid Carcinoma: The Challenge of the Variants of Uncertain Significance. Cancers 2020, 12, 3268. [Google Scholar] [CrossRef]
- Ghazani, A.A.; Breen, K.M.; Dwan, M.; Barletta, J.A.; Vatnick, D.R.; Stokes, S.M.; Block, C.; Doherty, G.M.; Cohn, A.Y.; Marqusee, E.; et al. Unexpected Pathogenic RET p.V804M Variant Leads to the Clinical Diagnosis and Management of Medullary Thyroid Carcinoma. Am. J. Case Rep. 2020, 21, e927415. [Google Scholar] [CrossRef]
- Skalnaniak, A.; Trofimiuk-Mudldner, M.; Przybylik-Mazerek, E.; Hubalewska-Dydejczyk, A. Modifier Role of Common RET Variants in Sporadic Medullary Thyroid Carcinoma. Int. J. Mol. Sci. 2021, 22, 11794. [Google Scholar] [CrossRef]
- Randolph, G.W.; Sosa, J.A.; Hao, Y.; Angell, T.E.; Shonka, D.C., Jr.; LiVolsi, V.A.; Ladenson, P.W.; Blevins, T.C.; Duh, Q.-Y.; Ghossein, R.; et al. Preoperative Identification of Medullary Thyroid Carcinoma (MTC): Clinical Validation of the Afirma MTC RNA-Sequencing Classifier. Thyroid 2022, 32, 1069–1076. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, C.; Li, Y.; Huang, T.; Wang, J.; Lin, C. Artificial intelligence assisted diagnosis of early tc markers and its application. Discov. Oncol. 2024, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial intelligence assists precision medicine in cancer treatment. Front. Oncol. 2023, 12, 998222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aksoy, Y.A.; Xu, B.; Viswanathan, K.; Ahadi, M.S.; Al Ghuzlan, A.; Alzumaili, B.; eBani, M.-A.; Barletta, J.A.; Chau, N.; Chou, A.; et al. Novel prognostic nomogram for predicting recurrence-free survival in medullary thyroid carcinoma. Histopathology 2024, 84, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Munk, K.; Ilina, D.; Ziemba, L.; Brader, G.; Molin, E.M. Holomics—A user-friendly R shiny application for multi-omics data integration and analysis. BMC Bioinform. 2024, 25, 93. [Google Scholar] [CrossRef]
- López, D.M.; Rico-Olarte, C.; Blobel, B.; Hullin, C. Challenges and solutions for transforming health ecosystems in low- and middle-income countries through artificial intelligence. Front. Med. 2022, 9, 958097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vadhiraj, V.V.; Simpkin, A.; O’Connell, J.; Ospina, N.S.; Maraka, S.; O’Keeffe, D.T. Ultrasound Image Classification of Thyroid Nodules Using Machine Learning Techniques. Medicina. 2021, 57, 527. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Jin, L.; Guo, Q.; Wei, M.; Li, Z.; Niu, L.; Liu, Z.; An, C. The machine learning-based model for lateral lymph node metastasis of thyroid medullary carcinoma improved the prediction ability of occult metastasis. Cancer Med. 2024, 13, e7155. [Google Scholar] [CrossRef]
- Cote, G.J.; Evers, C.; Hu, M.I.; Grubbs, E.G.; Williams, M.D.; Hau, T.; Duose, D.Y.; Houston, M.R.; Bui, J.H.; Mehrotra, M.; et al. Prognostic Significance of Circulating RET M918T Mutated Tumor DNA in Patients with Advanced Medullary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 3591–3599. [Google Scholar] [CrossRef]
- Gao, X.; Ran, X.; Ding, W. The progress of radiomics in thyroid nodules. Front. Oncol. 2023, 13, 1109319. [Google Scholar] [CrossRef]
- Shen, C.; Shi, X.; Wen, D.; Zhang, Y.; Du, Y.; Zhang, Y.; Ma, B.; Tang, H.; Yin, M.; Huang, N.; et al. Comprehensive DNA Methylation Profiling of Medullary Thyroid Carcinoma: Molecular Classification, Potential Therapeutic Target, and Classifier System. Clin. Cancer Res. 2024, 30, 127–138. [Google Scholar] [CrossRef]
- Minna, E.; Romeo, P.; Dugo, M.; De Cecco, L.; Aiello, A.; Pistore, F.; Carenzo, A.; Greco, A.; Borrello, M.G. Medullary Thyroid Carcinoma Mutational Spectrum Update and Signalling-Type Inference by Transcriptional Profiles: Literature Meta-Analysis and Study of Tumor Samples. Cancers 2022, 14, 1951. [Google Scholar] [CrossRef] [PubMed]
- Guma, J.; Pena, K.B.; Riu, F.; Lucia-Gozalvez, C.; Vidaller, A.; Maldonado, V.; Parada, D. Blood Liquid Biopsy in an Advanced Medullary Thyroid Carcinoma: A Case Study with Rearranged during Transfection Heterogeneity. Pathobiology 2023, 90, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Erickson, T.A.; Zhang, L.; Ellison, K.; Rivard, C.J.; Sams, S.; Hirsch, F.R.; Haugen, B.R.; French, J.D. Comprehensive Immune Profiling of Medullary Thyroid Cancer. Thyroid 2020, 30, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, H.; Li, X. Serum Biochemical Markers of Medullary Thyroid Carcinoma: An Update. Cancer Manag. Res. 2024, 16, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, L.; Gu, P.; Zhang, G.; Ruan, X.; Zhao, J.; Zheng, X.; Wei, S.; Gao, M. Changes of biochemical factors and the effect on recurrence of medullary thyroid carcinoma after surgery. Heliyon 2024, 10, e29857. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Wei, T.; Li, Z.; Zhu, J.; Chen, Y.W. Well-defined survival outcome disparity across age cutoffs at 45 and 60 for medullary thyroid carcinoma: A long-term restrospective cohort study of 3601 patients. Front. Endocrinol. 2024, 15, 1393904. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Canner, J.K.; Zeiger, M.A.; Mathur, A. Association between age and disease specific mortality in medullary thyroid cancer. Am. J. Surg. 2021, 221, 478–484. [Google Scholar] [CrossRef]
- Younis, H.A.; Eisa, T.A.E.; Nasser, M.; Sahib, T.M.; Noor, A.A.; Alyasiri, O.M.; Salisu, S.; Hayder, I.M.; Younis, H.A. A Systematic Review and Meta-Analysis of Artificial Intelligence Tools in Medicine and Healthcare: Applications, Considerations, Limitations, Motivation and Challenges. Diagnostics 2024, 14, 109. [Google Scholar] [CrossRef]
- Fei, X.; Wang, J.; Ying, S.; Hu, Z.; Shi, J. Projective parameter transfer based sparse multiple empirical kernel learning machine for diagnosis of brain disease. Neurocomputing 2020, 413, 271–283. [Google Scholar] [CrossRef]
- Yuan, X.; Wan, S.; Wang, W.; Chen, Y.; Lin, Y. A Mobile Application for Anticoagulation Management in Patients after Heart Valve Replacement: A Usability Study. Patient Prefer. Adherence 2024, 18, 2055–2066. [Google Scholar] [CrossRef]
| Algorithm | Application | Function | Example |
|---|---|---|---|
| CNN [125,126] | Image analysis (ultrasound, CT, MRI) | Automatically identify features indicative of MTC | Distinguish between benign and malignant thyroid nodules with high accuracy |
| SVM [127] | Classification of thyroid lesions | Find the optimal hyperplane that separates different classes of data points | Classify MTC patients based on genetic mutations and protein expression profiles |
| RF [128] | Classification and regression | Build multiple decision trees and merge them to improve predictive accuracy | Predict patient outcomes and treatment responses based on clinical, genetic, and proteomic data |
| KNN [129] | Classification and regression | Classify a data point based on the classification of its neighbors | Classify thyroid nodules based on ultrasound features to determine the likelihood of malignancy |
| ANN [130] | Complex pattern recognition | Process data in layers to learn intricate patterns | Integrate multi-omics data to predict disease progression and patient survival |
| GBM [131] | Classification and regression | Build models sequentially, each correcting errors of the previous ones | Predict recurrence risk in MTC patients by analyzing clinical and molecular data |
| RNN [130] | Time-series prediction and sequential data analysis | Maintain a memory of previous inputs to predict future outcomes | Analyze longitudinal patient data to predict future disease progression and treatment outcomes |
| Autoencoders [132] | Data dimensionality reduction and feature extraction | Compress data into a lower-dimensional representation and reconstruct it back | Identify key features in genetic and proteomic data that are most indicative of MTC |
| BN [130] | Probabilistic inference and decision-making | Represent variables and their conditional dependencies through directed acyclic graphs | Model relationships between genetic mutations, environmental factors, and MTC development |
| NLP [133] | Process and analyze unstructured clinical texts | Extract relevant information from EHRs, pathology reports, and the scientific literature | Extract patient data and clinical outcomes related to MTC, integrating with omics data for comprehensive analysis |
| Geolocation [134] | Epidemiology and public health planning | Mapping the geographical distribution of MTC cases to identify environmental and genetic risk factors; planning targeted screening programs and resource allocation | Identifying regions with higher incidence rates of MTC to implement targeted screening programs and allocate resources effectively; correlating regional dietary habits and environmental exposures with MTC incidence |
| Survival Analysis [135] | Prognostic predictions and patient stratification | Estimating time until events (disease progression, recurrence, death) and identifying prognostic factors | Developing risk stratification models based on clinical, genetic, and demographic variables to predict patient outcomes and tailor follow-up and monitoring strategies |
| Lean Six Sigma [136] | Process optimization and efficiency in clinical workflows | Streamlining clinical processes, reducing diagnostic errors, and improving treatment workflows by eliminating inefficiencies | Standardizing procedures for sample collection and data integration to reduce variability and improve the reliability of holomic analyses; ensuring consistent follow-through on diagnostic and treatment protocols |
| Name of miRNA | Expression | Consequences | References |
|---|---|---|---|
| miR-375 | Overexpressed | Diagnosis, lateral lymph nodes predicted, worse prognosis. Distinguishing hereditary from sporadic MTC. | [30,149,150] |
| miR-127 | Underexpressed | Aggressive sporadic disease. | [149,151] |
| miR-429 | Overexpressed | Not yet specified | [149] |
| miR-592 | Overexpressed | Poor prognosis. | [106] |
| miR-224 | Underexpressed | Poor prognosis | [30] |
| miR-199-5p | Underexpressed | Not yet specified | [149] |
| miR-199a-3p | Underexpressed | Not yet specified | [149] |
| miR-34a | Underexpressed | Biomarker of MTC | [152] |
| miR-9 | Underexpressed | Distinguishing hereditary versus sporadic | [153] |
| miR-21 | Overexpressed | Prediction of lymph node and distant metastasis | [30] |
| miR-144 | Overexpressed | Biomarker of MTC | [152] |
| miR-183 | Overexpressed | Prediction of lateral lymph node involvement, distant metastasis, and high mortality and distinguishing hereditary from sporadic MTC. | [30,153] |
| Application | Description | Examples/Impact |
|---|---|---|
| Enhanced diagnostic accuracy [101,162] | AI improves diagnostic accuracy for MTC by analyzing imaging data, integrating multiple data sources, including imaging, genetic, and clinical, and identifying subtle features indicative of MTC. Traditional methods like ultrasound and fine-needle aspiration can be inconclusive. | AI-powered image recognition systems distinguish between benign and malignant thyroid nodules more accurately than human radiologists, leading to an early and accurate diagnosis, which is essential for the effective treatment of MTC. |
| Personalized treatment plans [163] | AI personalizes treatment plans by analyzing genetic and molecular data to identify specific mutations and biomarkers associated with MTC. It predicts patient responses to targeted therapies, optimizing treatment efficacy and minimizing side effects. AI updates treatment recommendations as new data become available. | AI guides the selection of targeted therapies, such as tyrosine kinase inhibitors, ensuring that patients with MTC receive the most current and effective treatments based on their unique genetic profile. |
| Prognostic predictions [164] | AI develops predictive models to estimate disease progression and patient outcomes by integrating diverse data points like the stage of cancer, genetic mutations, and the patient’s characteristics. Machine learning algorithms analyze historical patient data to identify patterns and risk factors associated with recurrence or metastasis. | AI helps clinicians stratify patients into different risk categories and tailor follow-up and monitoring strategies, providing more accurate prognostic information and improving the long-term management of MTC. |
| Holomic integration [165] | Holomics integrates various omics data to provide a holistic view of MTC at the molecular level. AI analyzes and interprets complex datasets to identify gene expression patterns, detect protein biomarkers, and analyze metabolic profiles, offering a more complete understanding of the disease. | AI-enabled holomics uncovers novel insights into MTC pathogenesis and identifies new therapeutic targets, leading to better diagnostic and therapeutic strategies. |
| Comparative insights in HICs versus LMICs [166] | AI application varies between high-income countries (HICs) and low- and middle-income countries (LMICs). HICs benefit from advanced healthcare infrastructure and cutting-edge technologies, while LMICs face challenges like limited resources and insufficient training. AI can bridge these gaps by deploying diagnostic tools via mobile health platforms and optimizing resource use. | AI-driven diagnostic tools enable remote diagnosis and expert consultations in resource-limited settings, making high-quality cancer care more accessible and efficient. This reduces disparities between HICs and LMICs in MTC management. |
| Future prospects [167] | The future of AI in MTC investigation is promising, with continued advancements in AI algorithms and the growing availability of comprehensive holomic datasets. Collaborative efforts among researchers, clinicians, and AI experts will develop and validate tailored AI tools. The development of interpretable AI models will be crucial for clinical acceptance. | Expanding AI applications to other areas of thyroid cancer research, such as risk stratification and the discovery of novel therapeutic targets, holds great potential for improving patient outcomes. AI will play an increasingly integral role in the investigation and management of MTC, transforming the landscape of thyroid cancer care. |
| Target | Omics Option | References |
|---|---|---|
| Diagnosis | Fluidomics | [4] |
| Genomics | [165] | |
| Glycomics | [162] | |
| Metabolomics | [160] | |
| Pathomics | [12,145] | |
| Proteomics | [177] | |
| Radiomics | [104,111] | |
| Transcriptomics | [5] | |
| Staging | Fluidomics | [4,178] |
| Metabolomics | [155] | |
| Radiomics | [111,156,173] | |
| Pathomics | [146,147] | |
| Transcriptomics | [5] | |
| Risk stratification | Transcriptomics | [5,12,55] |
| Fluidomics | [4,99,173,176] | |
| Genomics | [94] | |
| Glycomics | [177] | |
| Immunomics | [47,174] | |
| Pathomics | [146,147,156] | |
| Radiomics | [173] | |
| Selection of treatment | Fluidomics | [176] |
| Genomics | [99] | |
| Immunomics | [47] | |
| Pathomics | [147] | |
| Radiomics | [141] | |
| Transcriptomics | [5,8,12] | |
| Follow-up | Delta radiomics | [143] |
| Fluidomics | [4] | |
| Genomics | [176] | |
| Metabolomics | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luvhengo, T.E.; Moeng, M.S.; Sishuba, N.T.; Makgoka, M.; Jonas, L.; Mamathuntsha, T.G.; Mbambo, T.; Kagodora, S.B.; Dlamini, Z. Holomics and Artificial Intelligence-Driven Precision Oncology for Medullary Thyroid Carcinoma: Addressing Challenges of a Rare and Aggressive Disease. Cancers 2024, 16, 3469. https://doi.org/10.3390/cancers16203469
Luvhengo TE, Moeng MS, Sishuba NT, Makgoka M, Jonas L, Mamathuntsha TG, Mbambo T, Kagodora SB, Dlamini Z. Holomics and Artificial Intelligence-Driven Precision Oncology for Medullary Thyroid Carcinoma: Addressing Challenges of a Rare and Aggressive Disease. Cancers. 2024; 16(20):3469. https://doi.org/10.3390/cancers16203469
Chicago/Turabian StyleLuvhengo, Thifhelimbilu Emmanuel, Maeyane Stephens Moeng, Nosisa Thabile Sishuba, Malose Makgoka, Lusanda Jonas, Tshilidzi Godfrey Mamathuntsha, Thandanani Mbambo, Shingirai Brenda Kagodora, and Zodwa Dlamini. 2024. "Holomics and Artificial Intelligence-Driven Precision Oncology for Medullary Thyroid Carcinoma: Addressing Challenges of a Rare and Aggressive Disease" Cancers 16, no. 20: 3469. https://doi.org/10.3390/cancers16203469
APA StyleLuvhengo, T. E., Moeng, M. S., Sishuba, N. T., Makgoka, M., Jonas, L., Mamathuntsha, T. G., Mbambo, T., Kagodora, S. B., & Dlamini, Z. (2024). Holomics and Artificial Intelligence-Driven Precision Oncology for Medullary Thyroid Carcinoma: Addressing Challenges of a Rare and Aggressive Disease. Cancers, 16(20), 3469. https://doi.org/10.3390/cancers16203469





