Is Carmustine Wafer Implantation in Progressive High-Grade Gliomas a Relevant Therapeutic Option? Complication Rate, Predictors of Complications and Onco-Functional Outcomes in a Series of 53 Cases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Survival
2.4. Statistical Analysis
2.5. Standard Protocol Approvals and Registrations
3. Results
3.1. Characteristics of the Patients
3.1.1. Glioma Characteristics and Management at Diagnosis
3.1.2. Clinical Status and Management at Progression
3.2. Postoperative Course and Surgical Complications
3.2.1. Early Postoperative Complications and Rehospitalization Rate
3.2.2. Predictors of Early and Infectious Postoperative Complications
3.2.3. Delayed Complications and Functional Prognosis
3.3. Oncological Prognosis
3.3.1. Postoperative Management and Survival Analysis in Patients Managed for a Grade 4 Glioma
3.3.2. Predictors of PFS and OS in Patients with a Grade 4 Glioma
4. Discussion
4.1. Identification of Patients at Risk of Early Surgical Complications Following Reresection of HGG Associated with CWI
4.2. Functional Outcomes after the Reresection of HGG Associated with CWI
4.3. Oncological Outcomes Following Reresection of HGG Associated with CWI
4.4. Impact of CWI on the Onco-Functional Balance of Patients with Progressive HGG
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An Extent of Resection Threshold for Newly Diagnosed Glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual Tumor Volume versus Extent of Resection: Predictors of Survival after Surgery for Glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA Fluorescence-Guided Surgery versus White-Light Conventional Microsurgery for the Resection of Newly Diagnosed Glioblastomas (RESECT Study): A French Multicenter Randomized Phase III Study. J. Neurosurg. 2023, 140, 987–1000. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Nishikawa, R.; Yamasaki, F.; Arakawa, Y.; Muragaki, Y.; Narita, Y.; Tanaka, S.; Yamaguchi, S.; Mukasa, A.; Kanamori, M. Safety and Efficacy of Tumour-Treating Fields (TTFields) Therapy for Newly Diagnosed Glioblastoma in Japanese Patients Using the Novo-TTF System: A Prospective Post-Approval Study. Jpn. J. Clin. Oncol. 2023, 53, 371–377. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current Status and Recent Advances in Reirradiation of Glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of Patterns of Care, Prognostic Factors, and Use of Radiotherapy-Temozolomide Therapy with Survival in Patients with Newly Diagnosed Glioblastoma: A French National Population-Based Study. J. Neurooncol. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Au, K.; Davis, F.G.; Easaw, J.C.; Mehta, V.; Broad, R.; Chow, M.M.C.; Hockley, A.; Kaderali, Z.; Magro, E.; et al. Clinical Uncertainty and Equipoise in the Management of Recurrent Glioblastoma. Am. J. Clin. Oncol. 2021, 44, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Suchorska, B.; Weller, M.; Tabatabai, G.; Senft, C.; Hau, P.; Sabel, M.C.; Herrlinger, U.; Ketter, R.; Schlegel, U.; Marosi, C.; et al. Complete Resection of Contrast-Enhancing Tumor Volume Is Associated with Improved Survival in Recurrent Glioblastoma-Results from the DIRECTOR Trial. Neuro-Oncology 2016, 18, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.F.; et al. Single-Agent Bevacizumab or Lomustine versus a Combination of Bevacizumab plus Lomustine in Patients with Recurrent Glioblastoma (BELOB Trial): A Randomised Controlled Phase 2 Trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Cloughesy, T.; Samant, M.; Prados, M.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.A.; Paleologos, N.; et al. Corticosteroid Use in Patients with Glioblastoma at First or Second Relapse Treated with Bevacizumab in the BRAIN Study. Oncologist 2010, 15, 1329–1334. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Champeaux Depond, C.; Bauchet, L.; Elhairech, D.; Tuppin, P.; Jecko, V.; Weller, J.; Metellus, P. Survival After Newly-Diagnosed High-Grade Glioma Surgery: What Can We Learn From the French National Healthcare Database? Brain Tumor Res. Treat. 2024, 12, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Caire, F.; Guyotat, J.; Menei, P.; Metellus, P.; Pallud, J. Neuro-Oncology Club of the French Neurosurgical Society Carmustine Wafer Implantation for High-Grade Gliomas: Evidence-Based Safety Efficacy and Practical Recommendations from the Neuro-Oncology Club of the French Society of Neurosurgery. Neurochirurgie 2017, 63, 433–443. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G. Placebo-Controlled Trial of Safety and Efficacy of Intraoperative Controlled Delivery by Biodegradable Polymers of Chemotherapy for Recurrent Gliomas. The Polymer-Brain Tumor Treatment Group. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival Outcomes and Safety of Carmustine Wafers in the Treatment of High-Grade Gliomas: A Meta-Analysis. J. Neurooncol. 2015, 122, 367–382. [Google Scholar] [CrossRef]
- Ono, T.; Suzuki, H.; Nanjo, H.; Shimizu, H. Clinical Course after Carmustine Wafer Implantation for Newly Diagnosed Adult-Type Diffuse Gliomas; A Controlled Propensity Matched Analysis of a Single Center Cohort. J. Neurooncol. 2024, 168, 393–404. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Noel, G.; Corns, R.; Lechapt-Zalcman, E.; Duntze, J.; Pavlov, V.; Guyotat, J.; Hieu, P.D.; Le Reste, P.-J.; et al. Long-Term Results of Carmustine Wafer Implantation for Newly Diagnosed Glioblastomas: A Controlled Propensity-Matched Analysis of a French Multicenter Cohort. Neuro-Oncology 2015, 17, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Ammar, H.; Moiraghi, A.; Peeters, S.; Baroud, M.; Zah-Bi, G.; Benzakoun, J.; Parraga, E.; Oppenheim, C.; Benevello, C.; et al. Discriminating Surgical Bed Cysts from Bacterial Brain Abscesses after Carmustine Wafer Implantation in Newly Diagnosed IDH-Wildtype Glioblastomas. Neurosurg. Rev. 2022, 45, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Peeters, S.; Zanello, M.; Bou Nassif, R.; Abi Lahoud, G.; Dezamis, E.; Parraga, E.; Lechapt-Zalcmann, E.; Dhermain, F.; Dumont, S.; et al. Extent of Resection and Carmustine Wafer Implantation Safely Improve Survival in Patients with a Newly Diagnosed Glioblastoma: A Single Center Experience of the Current Practice. J. Neurooncol. 2017, 135, 83–92. [Google Scholar] [CrossRef]
- Champeaux, C.; Weller, J. Implantation of Carmustine Wafers (Gliadel®) for High-Grade Glioma Treatment. A 9-Year Nationwide Retrospective Study. J. Neurooncol. 2020, 147, 159–169. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.C.; Lautenbach, E.; Brennan, P.J.; Lustig, R.A.; Fishman, N.O. Risk Factors for Postcraniotomy Surgical Site Infection after 1,3-Bis (2-Chloroethyl)-1-Nitrosourea (Gliadel) Wafer Placement. Clin. Infect. Dis. 2003, 36, 759–765. [Google Scholar] [CrossRef]
- Samis Zella, M.A.; Wallocha, M.; Slotty, P.J.; Isik, G.; Hänggi, D.; Schroeteler, J.; Ewelt, C.; Steiger, H.-J.; Sabel, M. Evaluation of Post-Operative Complications Associated with Repeat Resection and BCNU Wafer Implantation in Recurrent Glioblastoma. Acta Neurochir. 2014, 156, 313–323. [Google Scholar] [CrossRef]
- Matsuda, R.; Maeoka, R.; Tokuda, N.; Nakazawa, T.; Morimoto, T.; Kotsugi, M.; Takeshima, Y.; Tamura, K.; Yamada, S.; Nishimura, F.; et al. Intraoperative Ventricular Opening Has No Effect on Complication Development Following BCNU Wafer Implantation for Malignant Glioma. World Neurosurg. 2023, 171, e707–e713. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Metellus, P.; Parot-Schinkel, E.; Loiseau, H.; Capelle, L.; Jacquet, G.; Guyotat, J. Neuro-oncology Club of the French Society of Neurosurgery Biodegradable Carmustine Wafers (Gliadel) Alone or in Combination with Chemoradiotherapy: The French Experience. Ann. Surg. Oncol. 2010, 17, 1740–1746. [Google Scholar] [CrossRef]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The Role of Gliadel Wafers in the Treatment of High-Grade Gliomas. Expert. Rev. Anticancer. Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef]
- Gutenberg, A.; Bock, H.C.; Brück, W.; Doerner, L.; Mehdorn, H.M.; Roggendorf, W.; Westphal, M.; Felsberg, J.; Reifenberger, G.; Giese, A. MGMT Promoter Methylation Status and Prognosis of Patients with Primary or Recurrent Glioblastoma Treated with Carmustine Wafers. Br. J. Neurosurg. 2013, 27, 772–778. [Google Scholar] [CrossRef]
- Haim, O.; Agur, A.; Efrat, O.-T.; Valdes, P.; Ram, Z.; Grossman, R. The Clinical Significance of Radiological Changes Associated with Gliadel Implantation in Patients with Recurrent High Grade Glioma. Sci. Rep. 2023, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Dörner, L.; Ulmer, S.; Rohr, A.; Mehdorn, H.M.; Nabavi, A. Space-Occupying Cyst Development in the Resection Cavity of Malignant Gliomas Following Gliadel® Implantation--Incidence, Therapeutic Strategies, and Outcome. J. Clin. Neurosci. 2011, 18, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Rossetto, M.; Ciccarino, P.; Del Moro, G.; Rotilio, A.; Manara, R.; Paola Gardiman, M.; Denaro, L.; d’Avella, D.; Scienza, R. The First 3 Months after BCNU Wafers Implantation in High-Grade Glioma Patients: Clinical and Radiological Considerations on a Clinical Series. Acta Neurochir. 2010, 152, 1923–1931. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Karschnia, P.; Dono, A.; Young, J.S.; Juenger, S.T.; Teske, N.; Häni, L.; Sciortino, T.; Mau, C.Y.; Bruno, F.; Nunez, L.; et al. Prognostic Evaluation of Re-Resection for Recurrent Glioblastoma Using the Novel RANO Classification for Extent of Resection: A Report of the RANO Resect Group. Neuro-Oncology 2023, 25, 1672–1685. [Google Scholar] [CrossRef]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Maira, G.; Mangiola, A. Safety and Efficacy of Gliadel Wafers for Newly Diagnosed and Recurrent Glioblastoma. Acta Neurochir. 2012, 154, 1371–1378. [Google Scholar] [CrossRef]
- Shah, R.S.; Homapour, B.; Casselden, E.; Barr, J.G.; Grundy, P.L.; Brydon, H.L. Delayed Post-Operative Haemorrhage after Carmustine Wafer Implantation: A Case Series from Two UK Centres. Br. J. Neurosurg. 2014, 28, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Denaro, L.; Rossetto, M.; Ciccarino, P.; Manara, R.; Lombardi, G.; Del Moro, G.; Rotilio, A.; d’Avella, D.; Scienza, R. Postoperative Seizure in High Grade Glioma Patients Treated with BCNU Wafers. A Mono-Institutional Experience. J. Neurooncol. 2011, 105, 275–280. [Google Scholar] [CrossRef]
- Roux, A.; Elia, A.; Aboubakr, O.; Moiraghi, A.; Simboli, G.A.; Tauziede-Espariat, A.; Dezamis, E.; Parraga, E.; Benevello, C.; Fathallah, H.; et al. Efficacy and Safety of Carmustine Wafer Implantation After Ventricular Opening in Glioblastomas, Isocitrate Dehydrogenase-Wildtype, in Adults. Neurosurgery 2024, 94, 1227–1236. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rossetto, M.; Ciccarino, P.; Denaro, L.; Rotilio, A.; d’Avella, D.; Scienza, R. Carmustine Wafer Implantation When Surgical Cavity Is Communicating with Cerebral Ventricles: Technical Considerations on a Clinical Series. World Neurosurg. 2011, 76, 156–159; discussion 67–68. [Google Scholar] [CrossRef]
- Roux, A.; Aboubakr, O.; Elia, A.; Moiraghi, A.; Benevello, C.; Fathallah, H.; Parraga, E.; Oppenheim, C.; Chretien, F.; Dezamis, E.; et al. Carmustine Wafer Implantation for Supratentorial Glioblastomas, IDH-Wildtype in “Extreme” Neurosurgical Conditions. Neurosurg. Rev. 2023, 46, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Dai, R.-Y.; Chen, Z.; Zhang, Y.-H.; He, X.-Z.; Zhou, J. Efficacy and Safety of Carmustine Wafers in the Treatment of Glioblastoma Multiforme: A Systematic Review. Turk. Neurosurg. 2014, 24, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A Phase 3 Trial of Local Chemotherapy with Biodegradable Carmustine (BCNU) Wafers (Gliadel Wafers) in Patients with Primary Malignant Glioma. Neuro-Oncology 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Prajapati, H.P.; Ansari, A. Updates in the Management of Recurrent Glioblastoma Multiforme. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2023, 84, 174–187. [Google Scholar] [CrossRef]
- Champeaux-Depond, C.; Jecko, V.; Weller, J.; Constantinou, P.; Tuppin, P.; Metellus, P. Newly Diagnosed High-Grade Glioma Surgery with Carmustine Wafers Implantation. A Long-Term Nationwide Retrospective Study. World Neurosurg. 2023, 173, e778–e786. [Google Scholar] [CrossRef]
- Catalán-Uribarrena, G.; Bilbao-Barandica, G.; Pomposo-Gaztelu, I.; Undabeitia-Huertas, J.; Ruiz de Gopegui-Ruiz, E.; Galbarriatu-Gutiérrez, L.; Canales-Llantada, M.; Aurrecoechea-Obieta, J.; Igartua-Azkune, A.; Carbayo-Lozano, G. Prognostic Factors and Survival in a Prospective Cohort of Patients with High-Grade Glioma Treated with Carmustine Wafers or Temozolomide on an Intention-to-Treat Basis. Acta Neurochir. 2012, 154, 211–222; discussion 222. [Google Scholar] [CrossRef] [PubMed]
- Champeaux-Depond, C.; Jecko, V.; Weller, J.; Constantinou, P.; Tuppin, P.; Metellus, P. Recurrent High Grade Glioma Surgery with Carmustine Wafers Implantation: A Long-Term Nationwide Retrospective Study. J. Neurooncol. 2023, 162, 343–352. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III Randomized Trial of CED of IL13-PE38QQR vs Gliadel Wafers for Recurrent Glioblastoma. Neuro-Oncology 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Subach, B.R.; Witham, T.F.; Kondziolka, D.; Lunsford, L.D.; Bozik, M.; Schiff, D. Morbidity and Survival after 1,3-Bis(2-Chloroethyl)-1-Nitrosourea Wafer Implantation for Recurrent Glioblastoma: A Retrospective Case-Matched Cohort Series. Neurosurgery 1999, 45, 17–22; discussion 22–23. [Google Scholar] [CrossRef]
- Metellus, P.; Coulibaly, B.; Nanni, I.; Fina, F.; Eudes, N.; Giorgi, R.; Barrie, M.; Chinot, O.; Fuentes, S.; Dufour, H.; et al. Prognostic Impact of O6-Methylguanine-DNA Methyltransferase Silencing in Patients with Recurrent Glioblastoma Multiforme Who Undergo Surgery and Carmustine Wafer Implantation: A Prospective Patient Cohort. Cancer 2009, 115, 4783–4794. [Google Scholar] [CrossRef]
- Attenello, F.J.; Mukherjee, D.; Datoo, G.; McGirt, M.J.; Bohan, E.; Weingart, J.D.; Olivi, A.; Quinones-Hinojosa, A.; Brem, H. Use of Gliadel (BCNU) Wafer in the Surgical Treatment of Malignant Glioma: A 10-Year Institutional Experience. Ann. Surg. Oncol. 2008, 15, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Cesselli, D.; Isola, M.; Toniato, G.; Pauletto, G.; Sciacca, G.; Fabbro, S.; Pegolo, E.; Rizzato, S.; Beltrami, A.P.; et al. Combining Clinical and Molecular Data to Predict the Benefits of Carmustine Wafers in Newly Diagnosed High-Grade Gliomas. Curr. Treat. Options Neurol. 2018, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Lechapt-Zalcman, E.; Levallet, G.; Dugué, A.E.; Vital, A.; Diebold, M.-D.; Menei, P.; Colin, P.; Peruzzy, P.; Emery, E.; Bernaudin, M.; et al. O(6) -Methylguanine-DNA Methyltransferase (MGMT) Promoter Methylation and Low MGMT-Encoded Protein Expression as Prognostic Markers in Glioblastoma Patients Treated with Biodegradable Carmustine Wafer Implants after Initial Surgery Followed by Radiotherapy with Concomitant and Adjuvant Temozolomide. Cancer 2012, 118, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Schott, R.; Froelich, S.; Gaub, M.-P.; Boyer, P.; Fischer-Lokou, D.; Dufour, P.; Kehrli, P.; Maitrot, D. Retrospective Comparison of Chemoradiotherapy Followed by Adjuvant Chemotherapy, with or without Prior Gliadel Implantation (Carmustine) after Initial Surgery in Patients with Newly Diagnosed High-Grade Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 749–755. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Kone, L.; Bettegowda, C.; Weingart, J.D.; Olivi, A.; Lim, M.; Quinones-Hinojosa, A.; Gallia, G.L.; Brem, H. Risk of Surgical Site Infection in 401 Consecutive Patients with Glioblastoma with and without Carmustine Wafer Implantation. Neurol. Res. 2015, 37, 717–726. [Google Scholar] [CrossRef]
- Sabel, M.; Giese, A. Safety Profile of Carmustine Wafers in Malignant Glioma: A Review of Controlled Trials and a Decade of Clinical Experience. Curr. Med. Res. Opin. 2008, 24, 3239–3257. [Google Scholar] [CrossRef]
- Salle, H.; Deluche, E.; Couvé-Deacon, E.; Beaujeux, A.-C.; Pallud, J.; Roux, A.; Dagain, A.; de Barros, A.; Voirin, J.; Seizeur, R.; et al. Surgical Site Infections after Glioblastoma Surgery: Results of a Multicentric Retrospective Study. Infection 2021, 49, 267–275. [Google Scholar] [CrossRef]
- De Bonis, P.; Albanese, A.; Lofrese, G.; de Waure, C.; Mangiola, A.; Pettorini, B.L.; Pompucci, A.; Balducci, M.; Fiorentino, A.; Lauriola, L.; et al. Postoperative Infection May Influence Survival in Patients with Glioblastoma: Simply a Myth? Neurosurgery 2011, 69, 864–868; discussion 868–869. [Google Scholar] [CrossRef]
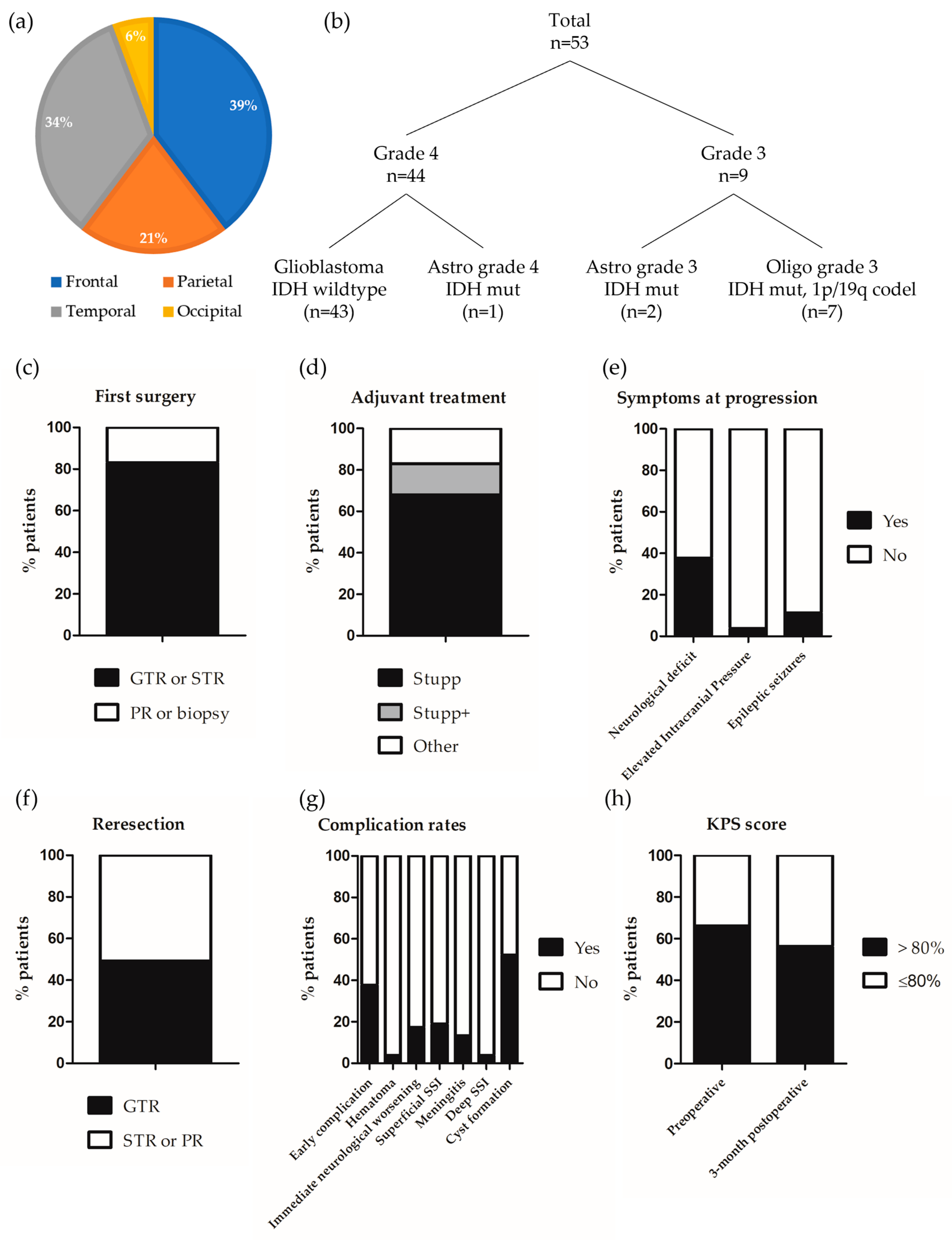
| Whole Series (n = 53) | Grade 4 Gliomas Only (n = 44) | |
|---|---|---|
| DEMOGRAPHIC DATA | ||
| Sex | ||
| Female | 19 (35.8) | 19 (43.2) |
| Male | 34 (64.2) | 25 (56.8) |
| Age (years) mean ± SD | 55 ± 10.9 | 56 ± 11.4 |
| Age categories | ||
| ≤60 years | 35 (66.0) | 26 (59.1) |
| >60 years | 18 (34.0) | 18 (40.9) |
| MEDICAL HISTORY | ||
| Immunosuppression | ||
| Yes | 10 (18.9) | 9 (20.5) |
| No | 43 (81.1) | 35 (79.5) |
| Chronic infectious site | ||
| Yes | 4 (7.5) | 4 (9.1) |
| No | 49 (92.5) | 40 (90.9) |
| LOCATION AND HISTO-MOLECULAR CHARACTERISTICS OF THE GLIOMA | ||
| Location | ||
| Frontal | 21 (39.6) | 14 (31.8) |
| Parietal | 11 (20.8) | 10 (22.7) |
| Temporal | 18 (34.0) | 17 (38.7) |
| Occipital | 3 (5.7) | 3 (6.8) |
| Integrated diagnosis | ||
| Glioblastoma IDH wild type | 43 (81.1) | 43 (97.7) |
| Astrocytoma grade 4 IDH mutant | 1 (1.9) | 1 (2.3) |
| Astrocytoma grade 3 IDH mutant | 2 (3.8) | 0 (0) |
| Oligodendroglioma grade 3 IDH mutant, 1p/19q co-deleted | 7 (13.2) | 0 (0) |
| EGFR status | ||
| Amplified | 20 (41.6) | 20 (46.5) |
| Non amplified | 28 (58.3) | 23 (53.5) |
| Missing | 5 | 0 |
| TERT status | ||
| Mutation (C228T or C250T) | 36 (90.0) | 32 (88.9) |
| Wild-type | 4 (10.0) | 4 (11.1) |
| Missing | 13 | 8 |
| MGMT status | ||
| Methylated | 33 (75.0) | 29 (72.5) |
| Unmethylated | 11 (25.0) | 11 (27.5) |
| Missing | 9 | 4 |
| INITIAL MANAGEMENT | ||
| Extension of the first surgery | ||
| GTR or STR | 44 (83.0) | 40 (90.9) |
| Partial resection or biopsy | 9 (17.0) | 4 (9.1) |
| Adjuvant treatment | ||
| Stupp | 36 (67.9) | 35 (79.5) |
| Stupp + | 8 (15.1) | 8 (18.2) |
| Other | 9 (17.0) | 1 (2.3) |
| CLINICAL DATA AT PROGRESSION | ||
| Neurological deficit | ||
| Yes 1 | 20 (37.7) | 18 (40.9) |
| No | 33 (62.3) | 26 (59.1) |
| Elevated Intracranial Pressure | ||
| Yes | 2 (3.8) | 2 (4.5) |
| No | 51 (96.2) | 42 (95.5) |
| Epileptic seizures | ||
| Yes | 6 (11.3) | 5 (11.4) |
| No | 47 (88.7) | 39 (88.6) |
| Preoperative KPS score | ||
| >80% | 35 (66.0) | 29 (65.9) |
| ≤80% | 18 (34.0) | 15 (34.1) |
| Pre-operative antibiotics intake | ||
| Yes 2 | 7 (13.2) | 7 (15.9) |
| No | 46 (86.8) | 37 (84.1) |
| Pre-operative corticosteroids intake | ||
| Yes | 24 (45.3) | 22 (50.0) |
| No | 29 (54.7) | 22 (50.0) |
| SURGICAL MANAGEMENT AT PROGRESSION | ||
| Time between the first resection and the reresection (months) mean ± SD | 38.7 ± 49.6 | 24.7 ± 20.9 |
| Extent of the reresection | ||
| GTR | 26 (49.1) | 21 (47.7) |
| STR or partial resection | 27 (50.9) | 23 (52.3) |
| Ventricular opening | ||
| Yes | 23 (48.9) | 22 (55.0) |
| No | 24 (51.1) | 18 (45.0) |
| Missing | 6 | 4 |
| Number of implanted Carmustine wafers | ||
| <8 | 17 (32.7) | 32 (72.7) |
| 8 | 34 (65.4) | 12 (27.3) |
| >8 | 1 (1.9) | 0 (0) |
| Missing | 1 | 0 |
| Whole Series (n = 53) | Grade 4 Gliomas Only (n = 44) | |
|---|---|---|
| Length of hospital stay (day) mean ± SD | 8.6 ± 3.0 | 8.6 ± 3.2 |
| Dose of corticosteroids during the 3 postoperative weeks (eq mg hydrocortisone) mean ± SD | 141.5 ± 131.5 | 151.5 ± 133.5 |
| Post-surgical hematoma | ||
| Yes | 2 (3.8) | 1 (2.3) |
| No | 51 (96.2) | 43 (97.7) |
| Immediate neurological worsening | ||
| Yes 1 | 9 (17.3) | 9 (20.9) |
| No | 43 (82.7) | 34 (79.1) |
| Missing | 1 | 1 |
| Superficial SSI | ||
| Yes | 10 (18.9) | 7 (15.9) |
| No | 43 (81.1) | 37 (84.1) |
| Meningitis | ||
| Yes | 7 (13.2) | 4 (9.1) |
| No | 46 (86.8) | 40 (90.9) |
| Deep SSI | ||
| Yes | 2 (3.8) | 1 (2.3) |
| No | 51 (96.2) | 43 (97.7) |
| Early rehospitalization | ||
| Yes | 19 (35.8) | 14 (31.8) |
| No | 34 (64.2) | 30 (68.2) |
| Cyst formation | ||
| Yes | 25 (52.1) | 19 (48.7) |
| No | 23 (47.9) | 20 (51.3) |
| Missing | 5 | 5 |
| 3-month postoperative KPS | ||
| >80% | 27 (56.2) | 23 (56.1) |
| ≤80% | 21 (43.8) | 18 (43.9) |
| Missing | 5 | 3 |
| 3-month postoperative general or neurological worsening | ||
| Yes 2 | 23 (47.9) | 21 (56.1) |
| No | 25 (52.1) | 18 (43.9) |
| Missing | 5 | 5 |
| Wound Infection | Meningitis | Deep Surgical Site Infection | Microbiological Agent 1 | Sex | Age (Years) | Immunosuppression | Chronic Infectious Site | Tumor Location 2 | Integrated Diagnosis 3 | Extension of the First Resection 4 | Adjuvant Treatment 5 | Preoperative Corticoids | Preoperative Antibiotics | Preoperative KPS (%) | Preoperative Neurological Deficit | Extent of the Reresection 4 | Lateral Ventricle Opening | Implanted Carmustine Wafers | Length of Hospital Stay (Days) | Postoperative Dose of Corticosteroids 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | - | - | MSSA | M | 38 | - | - | P | GBM | STR | S | - | - | 80 | + | GTR | + | 8 | 10 | 0 |
| 2 | + | + | - | STREP | M | 52 | - | - | F | O3 | STR | PCV | - | - | 90 | - | GTR | - | 6 | 14 | 0 |
| 3 | + | + | - | MSSA | M | 74 | - | - | T | GBM | STR | S | - | - | 90 | - | GTR | - | 6 | 8 | 220 |
| 4 | + | + | - | NA | M | 46 | + | - | F | GBM | STR | S | + | + | 90 | - | GTR | + | 7 | 6 | 240 |
| 5 | + | + | - | MSSA | M | 52 | - | - | T | O3 | PR | PCV-RT | - | - | 80 | + | STR | - | 8 | 7 | 0 |
| 6 | + | + | - | MSSA | F | 65 | - | - | F | GBM | STR | S | + | - | 80 | + | STR | + | 8 | 15 | 200 |
| 7 | + | + | - | EBC | M | 57 | - | - | P | GBM | STR | S | + | - | 90 | + | STR | NA | 6 | 22 | 0 |
| 8 | + | - | - | MSSA | M | 47 | - | - | O | GBM | STR | S+ | + | - | 90 | + | STR | - | 7 | 6 | 248 |
| 9 | + | - | + | MSSA + ECC | F | 53 | - | - | P | GBM | STR | S | + | - | 90 | - | STR | - | 8 | 7 | 0 |
| 10 | + | + | + | MSSA + CBA | M | 48 | - | - | P | A3 | STR | S | + | - | 80 | - | STR | NA | 8 | 7 | 200 |
| Factor | Early Postoperative Complications | Infectious Complications | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | ||||
| HR [95% CI] | p | HR [95% CI] | p | HR [95% CI] | p | |
| Sex (male) | 0.93 [0.29–3.01] | 0.91 | 1.09 [0.30–3.94] | 0.89 | 1.38 [0.31–6.12] | 0.67 |
| Age > 60 years | 0.38 [0.10–1.39] | 0.14 | 1.00 [0.95–1.07] | 0.82 | 0.42 [0.08–2.23] | 0.31 |
| Immunosuppression | 0.72 [0.16–3.20] | 0.67 | 0.42 [0.05–3.76] | 0.44 | ||
| Tumor location (parietal) | 2.40 [0.54–10.69] | 0.25 | 3.43 [0.61–19.40] | 0.16 | ||
| Integrated diagnosis of HGG | 0.75 [0.03–17.51] | 0.64 | 0.19 [0.01–3.39] | 0.25 | ||
| Preoperative corticosteroids | 1.00 [0.97–1.02] | 0.75 | 0.98 [0.94–1.02] | 0.31 | ||
| Preoperative antibiotics | 0.68 [0.12–3.19] | 0.67 | 0.69 [0.07–6.43] | 0.74 | ||
| Preoperative KPS (> 80%) | 0.19 [0.06–0.65] | 0.008 | 0.74 [0.05–10.00] | 0.81 | 0.97 [0.91–1.04] | 0.41 |
| Preoperative neurological deficit | 1.62 [0.48–5.40] | 0.43 | 5.35 [1.49–19.26] | 0.01 | 0.52 [0.10–2.76] | 0.44 |
| Extent of reresection (STR) | 2.17 [0.69–6.87] | 0.18 | 1.57 [0.39–6.37] | 0.53 | ||
| Lateral ventricle opening | 0.73 [0.22–2.45] | 0.61 | 0.57 [0.12–2.72] | 0.48 | ||
| Number of implanted Carmustine wafers | 0.98 [0.67–1.43] | 0.91 | 0.88 [0.54–1.42] | 0.60 | ||
| Length of hospital stay | 1.12 [0.92–1.36] | 0.25 | 1.21 [0.97–1.51] | 0.08 | ||
| Postoperative dose of corticosteroids | 1.01 [0.99–1.04] | 0.22 | 0.99 [0.96–1.02] | 0.50 | ||
| Factor | Neurological Worsening | |
|---|---|---|
| HR [95% CI] | p | |
| Sex (male) | 2.50 [0.96–6.49] | 0.06 |
| Age > 60 years | 2.50 [0.91–6.82] | 0.07 |
| Tumor location (parietal) | 0.65 [0.18–2.37] | 0.90 |
| Integrated diagnosis of HGG | 4.76 [0.59–35.80] | 0.15 |
| Preoperative corticosteroids | 3.84 [1.35–10.97] | 0.01 |
| Preoperative KPS (>80%) | 0.46 [0.13–1.59] | 0.22 |
| Preoperative neurological deficit | 0.95 [0.36–2.51] | 0.92 |
| Extent of reresection (STR) | 0.85 [0.31–2.32] | 0.75 |
| Lateral ventricle opening | 0.56 [0.21–1.44] | 0.22 |
| Number of implanted Carmustine wafers | 1.05 [0.82–1.36] | 0.69 |
| Early postoperative complication | 1.17 [0.43–3.16] | 0.76 |
| 3-month radiological progression | 3.69 [1.21–11.24] | 0.02 |
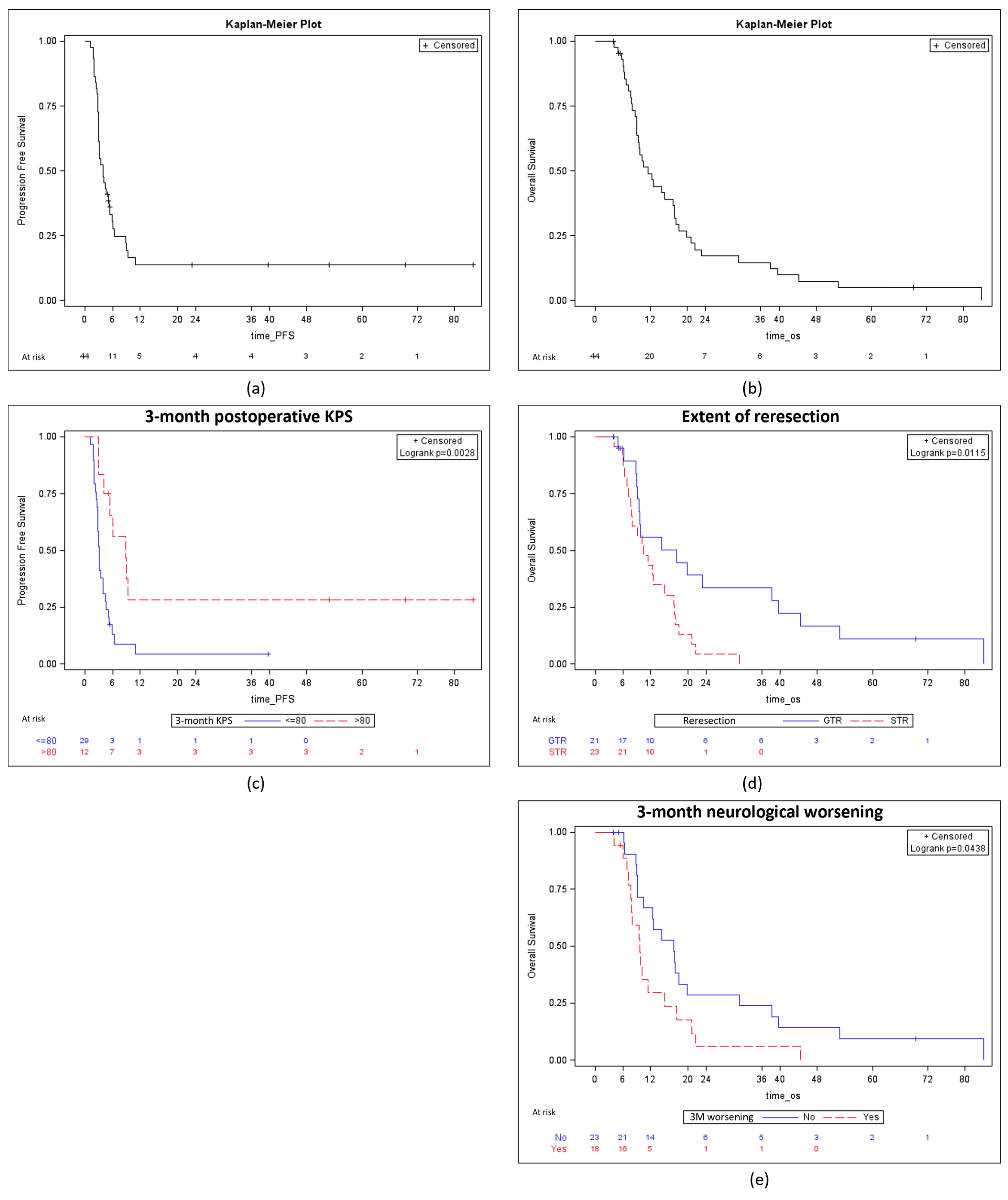
| Factor | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Multivariate Analysis 1 | ||||
| HR [95% CI] | p | HR [95% CI] | p | HR [95% CI] | p | |
| Sex (male) | 054 [0.27–1.05] | 0.07 | 0.92 [0.48–1.77] | 0.81 | 1.24 [0.62–2.46] | 0.54 |
| Age | 0.93 [0.46–1.86] | 0.83 | 1.02 [0.99–1.05] | 0.28 | 1.02 [0.99–1.05] | 0.28 |
| MGMT methylation | 0.60 [0.27–1.29] | 0.19 | 1.15 [0.54–2.41] | 0.71 | ||
| Extent of first resection: STR or GTR | 0.98 [0.34–2.77] | 0.96 | 1.04 [0.37–2.96] | 0.94 | ||
| Preoperative KPS > 80% | 1.02 [0.51–2.03] | 0.95 | 0.97 [0.93–1.00] | 0.04 | 0.97 [0.93–0.99] | 0.04 |
| Preoperative neurological deficit | 1.02 [0.53–1.99] | 0.95 | 1.97 [1.01–3.87] | 0.05 | ||
| Extent of reresection: GTR | 0.31 [0.15–0.62] | 0.001 | 0.40 [0.20–0.83] | 0.01 | 0.38 [0.18–0.80] | 0.01 |
| Number of implanted Carmustine wafer | 0.92 [0.71–1.20] | 0.53 | 0.95 [0.75–1.19] | 0.64 | ||
| 5-ALA use | 1.06 [0.54–2.06] | 0.86 | 1.21 [0.64–2.29] | 0.56 | ||
| Lateral ventricle opening | 0.43 [0.21–0.87] | 0.02 | 0.67 [0.35–1.29] | 0.23 | ||
| Early postoperative complication | 1.02 [0.53–1.97] | 0.94 | 1.45 [0.76–2.77] | 0.27 | ||
| 3-month neurological worsening | 2.33 [1.19–4.59] | 0.01 | 1.97 [1.01–3.87] | 0.04 | 1.79 [0.86–3.76] | 0.07 |
| 3-month postoperative KPS > 80% | 0.31 [0.14–0.69] | 0.003 | 0.37 [0.19–0.73] | 0.004 | 0.35 [0.17–0.72] | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkasdaris, G.; Berthiller, J.; Guyotat, J.; Jouanneau, E.; Gallet, C.; Meyronet, D.; Thomas, L.; Cartalat, S.; Seyve, A.; Honnorat, J.; et al. Is Carmustine Wafer Implantation in Progressive High-Grade Gliomas a Relevant Therapeutic Option? Complication Rate, Predictors of Complications and Onco-Functional Outcomes in a Series of 53 Cases. Cancers 2024, 16, 3465. https://doi.org/10.3390/cancers16203465
Gkasdaris G, Berthiller J, Guyotat J, Jouanneau E, Gallet C, Meyronet D, Thomas L, Cartalat S, Seyve A, Honnorat J, et al. Is Carmustine Wafer Implantation in Progressive High-Grade Gliomas a Relevant Therapeutic Option? Complication Rate, Predictors of Complications and Onco-Functional Outcomes in a Series of 53 Cases. Cancers. 2024; 16(20):3465. https://doi.org/10.3390/cancers16203465
Chicago/Turabian StyleGkasdaris, Grigorios, Julien Berthiller, Jacques Guyotat, Emmanuel Jouanneau, Clémentine Gallet, David Meyronet, Laure Thomas, Stéphanie Cartalat, Antoine Seyve, Jérôme Honnorat, and et al. 2024. "Is Carmustine Wafer Implantation in Progressive High-Grade Gliomas a Relevant Therapeutic Option? Complication Rate, Predictors of Complications and Onco-Functional Outcomes in a Series of 53 Cases" Cancers 16, no. 20: 3465. https://doi.org/10.3390/cancers16203465
APA StyleGkasdaris, G., Berthiller, J., Guyotat, J., Jouanneau, E., Gallet, C., Meyronet, D., Thomas, L., Cartalat, S., Seyve, A., Honnorat, J., Ducray, F., & Picart, T. (2024). Is Carmustine Wafer Implantation in Progressive High-Grade Gliomas a Relevant Therapeutic Option? Complication Rate, Predictors of Complications and Onco-Functional Outcomes in a Series of 53 Cases. Cancers, 16(20), 3465. https://doi.org/10.3390/cancers16203465






