Tertiary Lymphoid Structures in Microorganism-Related Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview of TLSs
2.1. Structure and Composition of TLSs
2.2. Identification and Detection of TLSs
2.3. Classification of TLSs
2.3.1. Presence, Quantity, and Density
2.3.2. Location
2.3.3. Differentiation
2.3.4. Composition
3. Microorganisms and Cancer
3.1. Carcinogenic Microorganisms
3.2. Other Microorganisms
4. TLSs in Microorganism-Associated Cancer
4.1. Virus-Associated
4.2. Bacteria-Associated
5. Generation Mechanism and Induced Strategy of TLSs
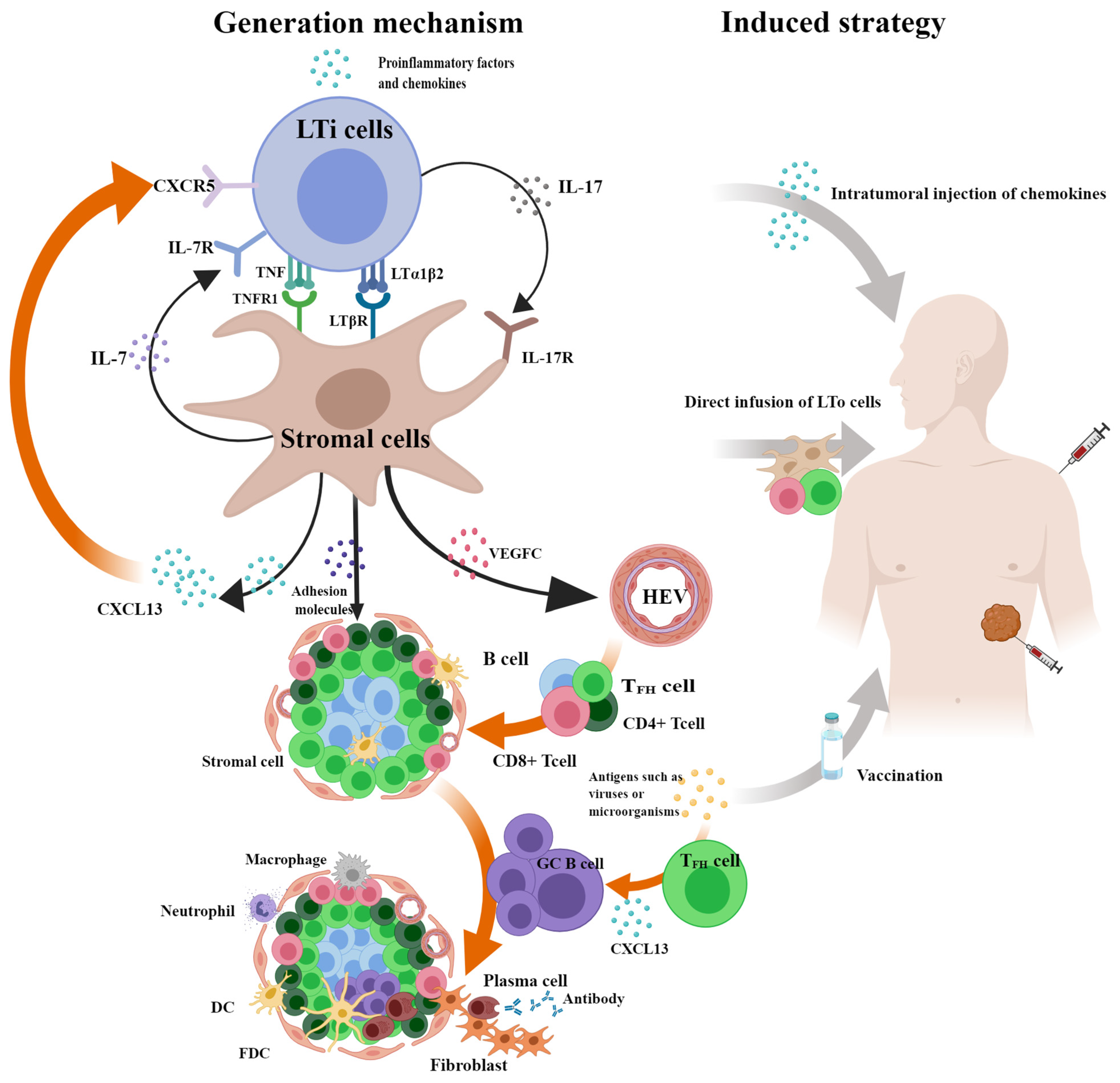
5.1. Generation Mechanism of TLSs
5.2. Induced Strategy of TLSs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Drayton, D.L.; Liao, S.; Mounzer, R.H.; Ruddle, N.H. Lymphoid organ development: From ontogeny to neogenesis. Nat. Immunol. 2006, 7, 344–353. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, X.; Tang, X.; Zou, W.; He, Y. Intratumoral tertiary lymphoid structures promote patient survival and immunotherapy response in head neck squamous cell carcinoma. Cancer Immunol. Immunother. 2022, 72, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, Z.; Guan, Z.; Zheng, S.; Lou, J.; Liu, W.; Cai, Q.; Si, Y. Follicle-like tertiary lymphoid structures: A potential biomarker for prognosis and immunotherapy response in patients with laryngeal squamous cell carcinoma. Front. Immunol. 2023, 14, 1096220. [Google Scholar] [CrossRef]
- Nakamura, S.; Ohuchida, K.; Hayashi, M.; Katayama, N.; Tsutsumi, C.; Yamada, Y.; Hisano, K.; Okuda, S.; Ohtsubo, Y.; Iwamoto, C.; et al. Tertiary lymphoid structures correlate with enhancement of antitumor immunity in esophageal squamous cell carcinoma. Br. J. Cancer 2023, 129, 1314–1326. [Google Scholar] [CrossRef]
- Zou, X.; Lin, X.; Cheng, H.; Chen, Y.; Wang, R.; Ma, M.; Liu, Y.; Dai, Z.; Tasiheng, Y.; Yan, Y.; et al. Characterization of intratumoral tertiary lymphoid structures in pancreatic ductal adenocarcinoma: Cellular properties and prognostic significance. J. ImmunoTherapy Cancer 2023, 11, e006698. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef]
- DiMaio, D.; Emu, B.; Goodman, A.L.; Mothes, W.; Justice, A. Cancer Microbiology. J. Natl. Cancer Inst. 2022, 114, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, A.T.; Cillo, A.R.; Tabib, T.; Liu, A.; Onkar, S.; Kunning, S.R.; Lampenfeld, C.; Atiya, H.I.; Abecassis, I.; Kurten, C.H.L.; et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 3349. [Google Scholar] [CrossRef] [PubMed]
- Neyt, K.; Perros, F.; GeurtsvanKessel, C.H.; Hammad, H.; Lambrecht, B.N. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012, 33, 297–305. [Google Scholar] [CrossRef]
- Thaunat, O.; Patey, N.; Caligiuri, G.; Gautreau, C.; Mamani-Matsuda, M.; Mekki, Y.; Dieu-Nosjean, M.C.; Eberl, G.; Ecochard, R.; Michel, J.B.; et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J. Immunol. 2010, 185, 717–728. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.M.; Italiano, A.; Sautes-Fridman, C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 2022, 19, 441–457. [Google Scholar] [CrossRef]
- Wu, H.; Xia, L.; Jia, D.; Zou, H.; Jin, G.; Qian, W.; Xu, H.; Li, T. PD-L1(+) regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol. Immunol. 2020, 119, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Collison, J. Immunofibroblasts are the cornerstone of TLS formation in pSS. Nat. Rev. Rheumatol. 2019, 15, 513. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.; Girard, J.P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 2021, 24, 719–753. [Google Scholar] [CrossRef]
- Kiessler, M.; Plesca, I.; Sommer, U.; Wehner, R.; Wilczkowski, F.; Muller, L.; Tunger, A.; Lai, X.; Rentsch, A.; Peuker, K.; et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J. Immunother. Cancer 2021, 9, e001813. [Google Scholar] [CrossRef]
- Wen, S.; Chen, Y.; Hu, C.; Du, X.; Xia, J.; Wang, X.; Zhu, W.; Wang, Q.; Zhu, M.; Chen, Y.; et al. Combination of Tertiary Lymphoid Structure and Neutrophil-to-Lymphocyte Ratio Predicts Survival in Patients with Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 788640. [Google Scholar] [CrossRef]
- Chen, L.; Oke, T.; Siegel, N.; Cojocaru, G.; Tam, A.J.; Blosser, R.L.; Swailes, J.; Ligon, J.A.; Lebid, A.; Morris, C.; et al. The Immunosuppressive Niche of Soft-Tissue Sarcomas is Sustained by Tumor-Associated Macrophages and Characterized by Intratumoral Tertiary Lymphoid Structures. Clin. Cancer Res. 2020, 26, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, A.; Derangere, V.; Boidot, R.; Apetoh, L.; Vincent, J.; Orry, D.; Fraisse, J.; Causeret, S.; Martin, F.; Arnould, L.; et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology 2016, 5, e1054598. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Kissick, H.T.; Patel, M.R.; Cardenas, M.A.; Prokhnevska, N.; Obeng, R.C.; Nasti, T.H.; Griffith, C.C.; Im, S.J.; Wang, X.; et al. Functional HPV-specific PD-1(+) stem-like CD8 T cells in head and neck cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef]
- Wennhold, K.; Thelen, M.; Lehmann, J.; Schran, S.; Preugszat, E.; Garcia-Marquez, M.; Lechner, A.; Shimabukuro-Vornhagen, A.; Ercanoglu, M.S.; Klein, F.; et al. CD86(+) Antigen-Presenting B Cells Are Increased in Cancer, Localize in Tertiary Lymphoid Structures, and Induce Specific T-cell Responses. Cancer Immunol. Res. 2021, 9, 1098–1108. [Google Scholar] [CrossRef]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Uribe-Uribe, N.O.; Espinosa-Gonzalez, R.; Kast, W.M.; Khader, S.A.; Rangel-Moreno, J. A Unique Cellular and Molecular Microenvironment Is Present in Tertiary Lymphoid Organs of Patients with Spontaneous Prostate Cancer Regression. Front. Immunol. 2017, 8, 563. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Li, B.; Han, Z.; Shen, J.; Xia, Y.; Li, R. Development and Validation of a Machine Learning Model for Detection and Classification of Tertiary Lymphoid Structures in Gastrointestinal Cancers. JAMA Network Open 2023, 6, e2252553. [Google Scholar] [CrossRef] [PubMed]
- Barmpoutis, P.; Di Capite, M.; Kayhanian, H.; Waddingham, W.; Alexander, D.C.; Jansen, M.; Kwong, F.N.K. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PLoS ONE 2021, 16, e0256907. [Google Scholar] [CrossRef]
- Klein, C.; Devi-Marulkar, P.; Dieu-Nosjean, M.C.; Germain, C. Development of Tools for the Selective Visualization and Quantification of TLS-Immune Cells on Tissue Sections. Methods Mol. Biol. 2018, 1845, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Meylan, M.; Petitprez, F.; Becht, E.; Bougouin, A.; Pupier, G.; Calvez, A.; Giglioli, I.; Verkarre, V.; Lacroix, G.; Verneau, J.; et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 2022, 55, 527–541.e5. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, L.; Rong, H.; Ou, Z.; Cai, T.; Liu, N.; Li, B.; Zhang, L.; Wu, F.; Lan, T.; et al. Single-cell profiling of tumor-infiltrating TCF1/TCF7(+) T cells reveals a T lymphocyte subset associated with tertiary lymphoid structures/organs and a superior prognosis in oral cancer. Oral. Oncol. 2021, 119, 105348. [Google Scholar] [CrossRef]
- Dai, S.; Zeng, H.; Liu, Z.; Jin, K.; Jiang, W.; Wang, Z.; Lin, Z.; Xiong, Y.; Wang, J.; Chang, Y.; et al. Intratumoral CXCL13(+)CD8(+)T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J. Immunother. Cancer 2021, 9, e001823. [Google Scholar] [CrossRef]
- Nakamura, M.; Magara, T.; Kano, S.; Matsubara, A.; Kato, H.; Morita, A. Tertiary Lymphoid Structures and Chemokine Landscape in Virus-Positive and Virus-Negative Merkel Cell Carcinoma. Front. Oncol. 2022, 12, 811586. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Rizk, V.T.; Ma, Z.; Cheng, C.H.; Berglund, A.E.; Coppola, D.; Khalil, F.; Mule, J.J.; Soliman, H.H. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: Correlation with clinical outcomes. Breast Cancer Res. 2017, 19, 71. [Google Scholar] [CrossRef]
- Andersson, A.; Larsson, L.; Stenbeck, L.; Salmen, F.; Ehinger, A.; Wu, S.Z.; Al-Eryani, G.; Roden, D.; Swarbrick, A.; Borg, A.; et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat. Commun. 2021, 12, 6012. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Rajamanickam, V.; Bui, C.; Bernard, B.; Pucilowska, J.; Ballesteros-Merino, C.; Schmidt, M.; McCarty, K.; Philips, M.; Piening, B.; et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 2021, 10, 1900635. [Google Scholar] [CrossRef]
- Qin, M.; Hamanishi, J.; Ukita, M.; Yamanoi, K.; Takamatsu, S.; Abiko, K.; Murakami, R.; Miyamoto, T.; Suzuki, H.; Ueda, A.; et al. Tertiary lymphoid structures are associated with favorable survival outcomes in patients with endometrial cancer. Cancer Immunol. Immunother. 2022, 71, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Vanhersecke, L.; Brunet, M.; Guegan, J.P.; Rey, C.; Bougouin, A.; Cousin, S.; Moulec, S.L.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Lin, Q.; Tao, P.; Wang, J.; Ma, L.; Jiang, Q.; Li, J.; Zhang, G.; Liu, J.; Zhang, Y.; Hou, Y.; et al. Tumor-associated tertiary lymphoid structure predicts postoperative outcomes in patients with primary gastrointestinal stromal tumors. Oncoimmunology 2020, 9, 1747339. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, W.Q.; Han, X.; Gao, H.L.; Xu, S.S.; Li, S.; Li, T.J.; Xu, H.X.; Li, H.; Ye, L.Y.; et al. Infiltrating pattern and prognostic value of tertiary lymphoid structures in resected non-functional pancreatic neuroendocrine tumors. J. Immunother. Cancer 2020, 8, e001188. [Google Scholar] [CrossRef]
- Benzerdjeb, N.; Dartigues, P.; Kepenekian, V.; Valmary-Degano, S.; Mery, E.; Averous, G.; Chevallier, A.; Laverriere, M.H.; Villa, I.; Harou, O.; et al. Tertiary lymphoid structures in epithelioid malignant peritoneal mesothelioma are associated with neoadjuvant chemotherapy, but not with prognosis. Virchows Arch. 2021, 479, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.T.; Young, S.J.; Meneveau, M.O.; Wages, N.A.; Engelhard, V.H.; Slingluff, C.L., Jr.; Mauldin, I.S. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J. Immunother. Cancer 2021, 9, e002273. [Google Scholar] [CrossRef] [PubMed]
- Silina, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Tanaka, N.; Takamatsu, K.; Hakozaki, K.; Takahashi, R.; Anno, T.; Kufukihara, R.; Shojo, K.; Mikami, S.; Shinojima, T.; et al. Unique characteristics of tertiary lymphoid structures in kidney clear cell carcinoma: Prognostic outcome and comparison with bladder cancer. J. Immunother. Cancer 2022, 10, e003883. [Google Scholar] [CrossRef]
- Posch, F.; Silina, K.; Leibl, S.; Mundlein, A.; Moch, H.; Siebenhuner, A.; Samaras, P.; Riedl, J.; Stotz, M.; Szkandera, J.; et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 2018, 7, e1378844. [Google Scholar] [CrossRef]
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; Luciani, A.; Amaddeo, G.; Derman, J.; Charpy, C.; et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 2019, 70, 58–65. [Google Scholar] [CrossRef]
- Mauldin, I.S.; Jo, J.; Wages, N.A.; Yogendran, L.V.; Mahmutovic, A.; Young, S.J.; Lopes, M.B.; Slingluff, C.L., Jr.; Erickson, L.D.; Fadul, C.E. Proliferating CD8(+) T Cell Infiltrates Are Associated with Improved Survival in Glioblastoma. Cells 2021, 10, 3378. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, L.; Paracchini, L.; Pezzuto, F.; Olteanu, G.E.; Moracci, L.; Vedovelli, L.; De Simone, I.; Bosetti, C.; Lupi, M.; Amodeo, R.; et al. Epithelioid Pleural Mesothelioma Is Characterized by Tertiary Lymphoid Structures in Long Survivors: Results from the MATCH Study. Int. J. Mol. Sci. 2022, 23, 5786. [Google Scholar] [CrossRef] [PubMed]
- Kurten, C.H.L.; Kulkarni, A.; Cillo, A.R.; Santos, P.M.; Roble, A.K.; Onkar, S.; Reeder, C.; Lang, S.; Chen, X.; Duvvuri, U.; et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat. Commun. 2021, 12, 7338. [Google Scholar] [CrossRef]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; van Montfoort, M.L.; Einerhand, S.; Jonkman, L.; Voskuilen, C.S.; Peters, D.; Sanders, J.; Lubeck, Y.; et al. The Tumor Immune Landscape and Architecture of Tertiary Lymphoid Structures in Urothelial Cancer. Front. Immunol. 2021, 12, 793964. [Google Scholar] [CrossRef]
- Ding, G.Y.; Ma, J.Q.; Yun, J.P.; Chen, X.; Ling, Y.; Zhang, S.; Shi, J.Y.; Chang, Y.Q.; Ji, Y.; Wang, X.Y.; et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J. Hepatol. 2022, 76, 608–618. [Google Scholar] [CrossRef]
- Jacquelot, N.; Tellier, J.; Sl, N.; Gt, B. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar] [CrossRef]
- Meylan, M.; Petitprez, F.; Lacroix, L.; Di Tommaso, L.; Roncalli, M.; Bougouin, A.; Laurent, A.; Amaddeo, G.; Sommacale, D.; Regnault, H.; et al. Early Hepatic Lesions Display Immature Tertiary Lymphoid Structures and Show Elevated Expression of Immune Inhibitory and Immunosuppressive Molecules. Clin. Cancer Res. 2020, 26, 4381–4389. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Zhang, G.; Wang, Y.; He, M.; Xu, Q.; Xu, C.; Liu, H. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 2021, 9, e001136. [Google Scholar] [CrossRef]
- Li, J.P.; Wu, C.Y.; Chen, M.Y.; Liu, S.X.; Yan, S.M.; Kang, Y.F.; Sun, C.; Grandis, J.R.; Zeng, M.S.; Zhong, Q. PD-1(+)CXCR5(−)CD4(+) Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer 2021, 9, e002101. [Google Scholar] [CrossRef]
- Sanchez-Alonso, S.; Setti-Jerez, G.; Arroyo, M.; Hernandez, T.; Martos, M.I.; Sanchez-Torres, J.M.; Colomer, R.; Ramiro, A.R.; Alfranca, A. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother. Cancer 2020, 8, e001187. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, S.; Clarke, J.; Ramirez-Suastegui, C.; Panwar, B.; Madrigal, A.; Chee, S.J.; Karydis, I.; Woo, E.; Alzetani, A.; Elsheikh, S.; et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nat. Immunol. 2021, 22, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhu, S.; Lu, H.; Soutto, M.; Bhat, N.; Chen, Z.; Peng, D.; Lin, J.; Lu, J.; Li, P.; et al. Helicobacter pylori-induced RASAL2 Through Activation of Nuclear Factor-kappaB Promotes Gastric Tumorigenesis via beta-catenin Signaling Axis. Gastroenterology 2022, 162, 1716–1731.e17. [Google Scholar] [CrossRef]
- Chonwerawong, M.; Ferrand, J.; Chaudhry, H.M.; Higgins, C.; Tran, L.S.; Lim, S.S.; Walker, M.M.; Bhathal, P.S.; Dev, A.; Moore, G.T.; et al. Innate Immune Molecule NLRC5 Protects Mice from Helicobacter-induced Formation of Gastric Lymphoid Tissue. Gastroenterology 2020, 159, 169–182.e8. [Google Scholar] [CrossRef]
- Oster, P.; Vaillant, L.; Riva, E.; McMillan, B.; Begka, C.; Truntzer, C.; Richard, C.; Leblond, M.M.; Messaoudene, M.; Machremi, E.; et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut 2022, 71, 457–466. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- DeCaprio, J.A. Molecular Pathogenesis of Merkel Cell Carcinoma. Annu. Rev. Pathol. 2021, 16, 69–91. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef]
- Ringelhan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160274. [Google Scholar] [CrossRef]
- Engels, E.A.; Biggar, R.J.; Hall, H.I.; Cross, H.; Crutchfield, A.; Finch, J.L.; Grigg, R.; Hylton, T.; Pawlish, K.S.; McNeel, T.S.; et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer 2008, 123, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Kürten, C.H.L.; Tabib, T.; Qi, Z.; Onkar, S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.J.; et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020, 52, 183–199.e9. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef]
- Hong, J.; Guo, F.; Lu, S.Y.; Shen, C.; Ma, D.; Zhang, X.; Xie, Y.; Yan, T.; Yu, T.; Sun, T.; et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut 2021, 70, 2123–2137. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-kappaB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Alam, A.; Levanduski, E.; Denz, P.; Villavicencio, H.S.; Bhatta, M.; Alhorebi, L.; Zhang, Y.; Gomez, E.C.; Morreale, B.; Senchanthisai, S.; et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 2022, 40, 153–167.e11. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; Gonzalez, A.; et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022, 185, 3789–3806.e17. [Google Scholar] [CrossRef]
- Liu, N.-N.; Jiao, N.; Tan, J.-C.; Wang, Z.; Wu, D.; Wang, A.-J.; Chen, J.; Tao, L.; Zhou, C.; Fang, W.; et al. Multi-kingdom microbiota analyses identify bacterial–fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 2022, 7, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.N.; Rebernick, R.J.; Goyert, J.; Singhal, R.; Kuljanin, M.; Kerk, S.A.; Huang, W.; Das, N.K.; Andren, A.; Solanki, S.; et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022, 40, 185–200.e6. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Liu, W.-X.; Zhao, L.-Y.; Huang, D.; Liu, X.-D.; Chan, H.; Zhang, Y.; Zeng, J.-D.; Coker, O.O.; et al. Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting β-Galactosidase. Gastroenterology 2021, 160, 1179–1193.e14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tinoco, R.; Elmen, L.; Segota, I.; Xian, Y.; Fujita, Y.; Sahu, A.; Zarecki, R.; Marie, K.; Feng, Y.; et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5(-/-) mice. Nat. Commun. 2019, 10, 1492. [Google Scholar] [CrossRef]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The Microbial Metabolite Butyrate Induces Expression of Th1-Associated Factors in CD4(+) T Cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Cheng, N.; Li, P.; Cheng, H.; Zhao, X.; Dong, M.; Zhang, Y.; Zhao, P.; Chen, J.; Shao, C. Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and -Negative Gastric Carcinoma. Front. Immunol. 2021, 12, 692859. [Google Scholar] [CrossRef]
- Derks, S.; de Klerk, L.K.; Xu, X.; Fleitas, T.; Liu, K.X.; Liu, Y.; Dietlein, F.; Margolis, C.; Chiaravalli, A.M.; Da Silva, A.C.; et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann. Oncol. 2020, 31, 1011–1020. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yang, F.; Zhang, X.; Ren, X.; Wei, F. Relationship and prognostic significance of IL-33, PD-1/PD-L1, and tertiary lymphoid structures in cervical cancer. J. Leukoc. Biol. 2022, 112, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, A.; Quan, C.; Pan, Y.; Zhang, H.; Li, Y.; Gao, C.; Lu, H.; Wang, X.; Cao, P.; et al. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat. Commun. 2022, 13, 4594. [Google Scholar] [CrossRef]
- Lin, J.; Dai, Y.; Sang, C.; Song, G.; Xiang, B.; Zhang, M.; Dong, L.; Xia, X.; Ma, J.; Shen, X.; et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities. J. Immunother. Cancer 2022, 10, e004892. [Google Scholar] [CrossRef]
- Overacre-Delgoffe, A.E.; Bumgarner, H.J.; Cillo, A.R.; Burr, A.H.P.; Tometich, J.T.; Bhattacharjee, A.; Bruno, T.C.; Vignali, D.A.A.; Hand, T.W. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 2021, 54, 2812–2824.e4. [Google Scholar] [CrossRef] [PubMed]
- Goubet, A.G.; Lordello, L.; Alves Costa Silva, C.; Peguillet, I.; Gazzano, M.; Mbogning-Fonkou, M.D.; Thelemaque, C.; Lebacle, C.; Thibault, C.; Audenet, F.; et al. Escherichia coli-Specific CXCL13-Producing TFH Are Associated with Clinical Efficacy of Neoadjuvant PD-1 Blockade against Muscle-Invasive Bladder Cancer. Cancer Discov. 2022, 12, 2280–2307. [Google Scholar] [CrossRef]
- Wong-Rolle, A.; Dong, Q.; Zhu, Y.; Divakar, P.; Hor, J.L.; Kedei, N.; Wong, M.; Tillo, D.; Conner, E.A.; Rajan, A.; et al. Spatial meta-transcriptomics reveal associations of intratumor bacteria burden with lung cancer cells showing a distinct oncogenic signature. J. Immunother. Cancer 2022, 10, e004698. [Google Scholar] [CrossRef]
- Zhang, H.; AbdulJabbar, K.; Moore, D.A.; Akarca, A.; Enfield, K.S.S.; Jamal-Hanjani, M.; Raza, S.E.A.; Veeriah, S.; Salgado, R.; McGranahan, N.; et al. Spatial Positioning of Immune Hotspots Reflects the Interplay between B and T Cells in Lung Squamous Cell Carcinoma. Cancer Res. 2023, 83, 1410–1425. [Google Scholar] [CrossRef] [PubMed]
- Kinker, G.S.; Vitiello, G.A.F.; Diniz, A.B.; Cabral-Piccin, M.P.; Pereira, P.H.B.; Carvalho, M.L.R.; Ferreira, W.A.S.; Chaves, A.S.; Rondinelli, A.; Gusmão, A.F.; et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut 2023, 72, 1927–1941. [Google Scholar] [CrossRef]
- Wei, X.; Jin, Y.; Tian, Y.; Zhang, H.; Wu, J.; Lu, W.; Lu, X. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol. 2015, 37, 6581–6588. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef]
- Garaud, S.; Dieu-Nosjean, M.C.; Willard-Gallo, K. T follicular helper and B cell crosstalk in tertiary lymphoid structures and cancer immunotherapy. Nat. Commun. 2022, 13, 2259. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Gao, Q.; Wang, Z.C.; Zhou, J.; Wang, X.Y.; Min, Z.H.; Shi, Y.H.; Shi, G.M.; Ding, Z.B.; Ke, A.W.; et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef]
- Finkin, S.; Yuan, D.; Stein, I.; Taniguchi, K.; Weber, A.; Unger, K.; Browning, J.L.; Goossens, N.; Nakagawa, S.; Gunasekaran, G.; et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 2015, 16, 1235–1244. [Google Scholar] [CrossRef]
- Hill, D.G.; Yu, L.; Gao, H.; Balic, J.J.; West, A.; Oshima, H.; McLeod, L.; Oshima, M.; Gallimore, A.; D’Costa, K.; et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int. J. Cancer 2018, 143, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Xu, A.S.; Ren, K.Y.M.; Visram, K.; Ren, R.; Berman, D.M.; Siemens, D.R. Tertiary Lymphoid Structures Associate with Tumour Stage in Urothelial Bladder Cancer. Bladder Cancer 2017, 3, 259–267. [Google Scholar] [CrossRef]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Vondenhoff, M.F.; Greuter, M.; Goverse, G.; Elewaut, D.; Dewint, P.; Ware, C.F.; Hoorweg, K.; Kraal, G.; Mebius, R.E. LTβR Signaling Induces Cytokine Expression and Up-Regulates Lymphangiogenic Factors in Lymph Node Anlagen. J. Immunol. 2009, 182, 5439–5445. [Google Scholar] [CrossRef]
- Peters, A.; Pitcher, L.A.; Sullivan, J.M.; Mitsdoerffer, M.; Acton, S.E.; Franz, B.; Wucherpfennig, K.; Turley, S.; Carroll, M.C.; Sobel, R.A.; et al. Th17 Cells Induce Ectopic Lymphoid Follicles in Central Nervous System Tissue Inflammation. Immunity 2011, 35, 986–996. [Google Scholar] [CrossRef]
- Lochner, M.; Ohnmacht, C.; Presley, L.; Bruhns, P.; Si-Tahar, M.; Sawa, S.; Eberl, G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J. Exp. Med. 2011, 208, 125–134. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Hao, Z.; Lin, M.; Xin, Z.; Chen, Y.; Ouyang, W.; Yang, Q.; Chen, X.; Zhou, H.; Zhang, W.; et al. Oncolytic adenovirus promotes vascular normalization and nonclassical tertiary lymphoid structure formation through STING-mediated DC activation. OncoImmunology 2022, 11, 2093054. [Google Scholar] [CrossRef] [PubMed]
- GeurtsvanKessel, C.H.; Willart, M.A.; Bergen, I.M.; van Rijt, L.S.; Muskens, F.; Elewaut, D.; Osterhaus, A.D.; Hendriks, R.; Rimmelzwaan, G.F.; Lambrecht, B.N. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 2009, 206, 2339–2349. [Google Scholar] [CrossRef]
- Chaurio, R.A.; Anadon, C.M.; Lee Costich, T.; Payne, K.K.; Biswas, S.; Harro, C.M.; Moran, C.; Ortiz, A.C.; Cortina, C.; Rigolizzo, K.E.; et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 2022, 55, 115–128.e9. [Google Scholar] [CrossRef]
- Delvecchio, F.R.; Fincham, R.E.A.; Spear, S.; Clear, A.; Roy-Luzarraga, M.; Balkwill, F.R.; Gribben, J.G.; Bombardieri, M.; Hodivala-Dilke, K.; Capasso, M.; et al. Pancreatic Cancer Chemotherapy Is Potentiated by Induction of Tertiary Lymphoid Structures in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1543–1565. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Watanabe, T. Gel-Trapped Lymphorganogenic Chemokines Trigger Artificial Tertiary Lymphoid Organs and Mount Adaptive Immune Responses In Vivo. Front. Immunol. 2016, 7, 316. [Google Scholar] [CrossRef]
- Zhu, G.; Nemoto, S.; Mailloux, A.W.; Perez-Villarroel, P.; Nakagawa, R.; Falahat, R.; Berglund, A.E.; Mule, J.J. Induction of Tertiary Lymphoid Structures with Antitumor Function by a Lymph Node-Derived Stromal Cell Line. Front. Immunol. 2018, 9, 1609. [Google Scholar] [CrossRef]
- Hussain, B.; Kasinath, V.; Ashton-Rickardt, G.P.; Clancy, T.; Uchimura, K.; Tsokos, G.; Abdi, R. High endothelial venules as potential gateways for therapeutics. Trends Immunol. 2022, 43, 728–740. [Google Scholar] [CrossRef]
- Maldonado, L.; Teague, J.E.; Morrow, M.P.; Jotova, I.; Wu, T.C.; Wang, C.; Desmarais, C.; Boyer, J.D.; Tycko, B.; Robins, H.S.; et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med. 2014, 6, 221ra13. [Google Scholar] [CrossRef]
- Lutz, E.R.; Wu, A.A.; Bigelow, E.; Sharma, R.; Mo, G.; Soares, K.; Solt, S.; Dorman, A.; Wamwea, A.; Yager, A.; et al. Immunotherapy Converts Nonimmunogenic Pancreatic Tumors into Immunogenic Foci of Immune Regulation. Cancer Immunol. Res. 2014, 2, 616–631. [Google Scholar] [CrossRef] [PubMed]
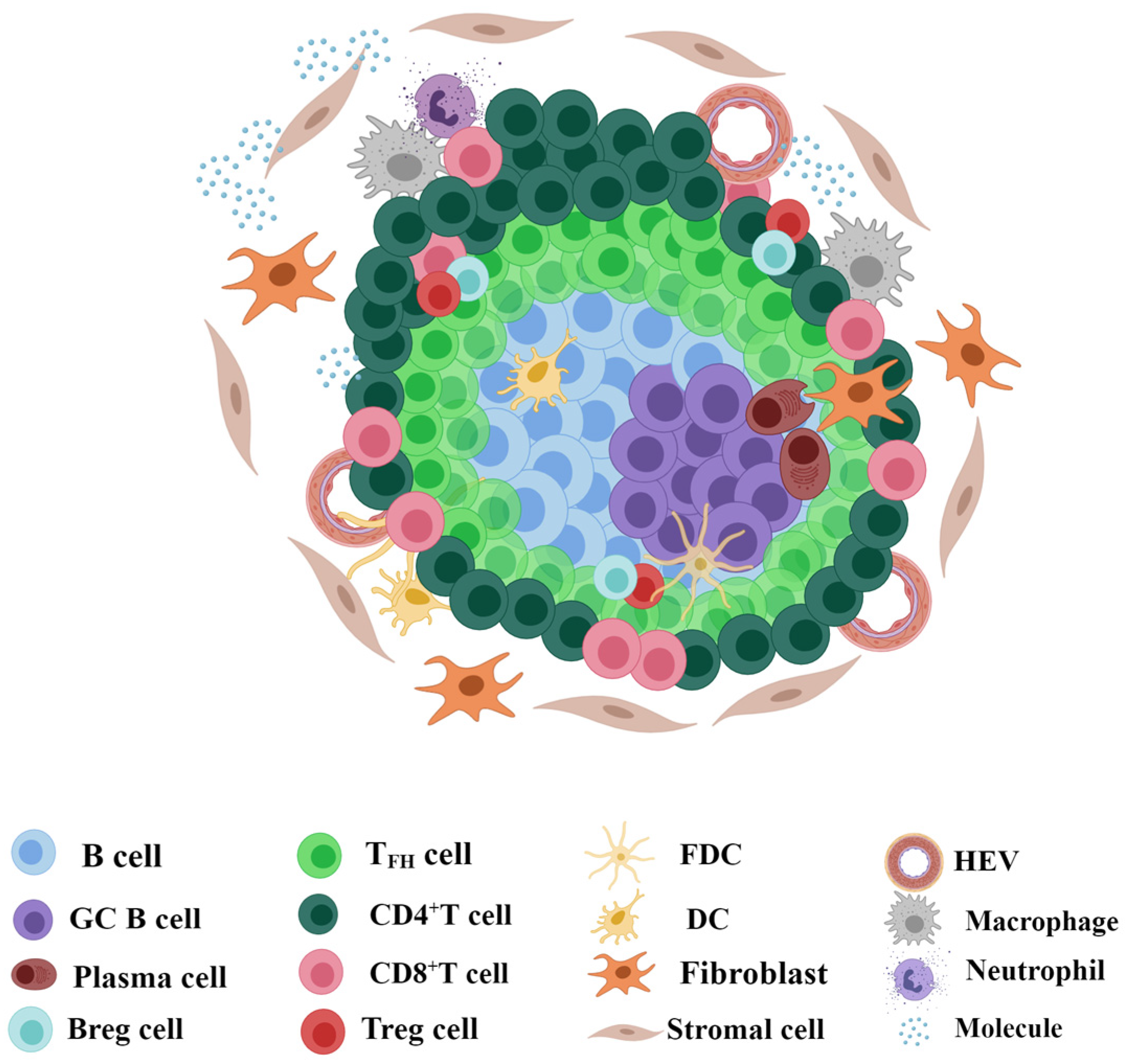
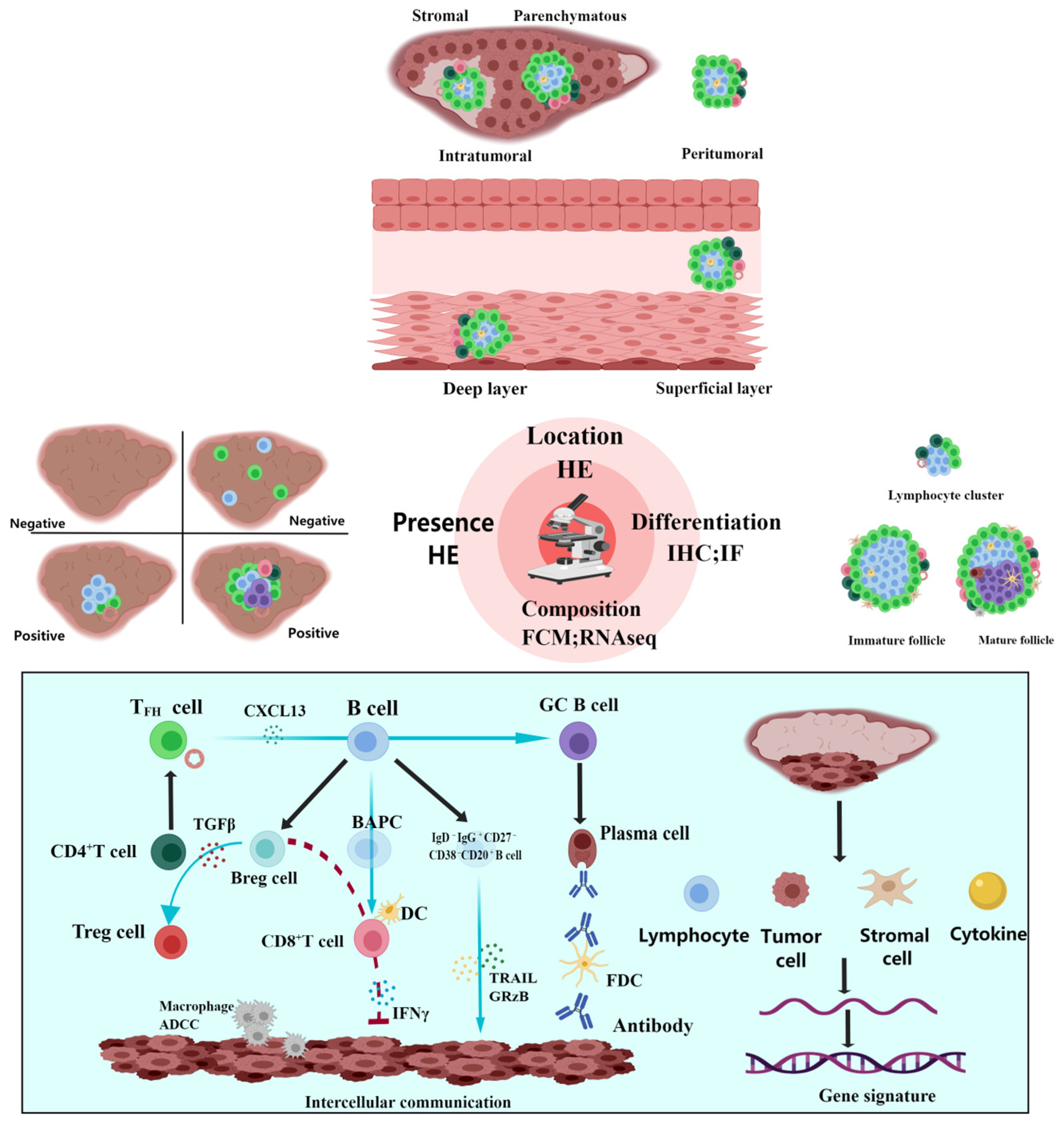

| Categories | Components | Immune Markers | Gene Signatures | Ref. | |
|---|---|---|---|---|---|
| Immune cells (CD45+) | T cells (CD3+) | TFH cells | CD4+, CXCR5+, CD40L+ | CXCL13, CD200, FBLN7, ICOS, SGPP2, SH2D1A, TIGIT, PDCD1 | [23] |
| Th1 cells | CD45RO+ | CD4, CCR5, CXCR3, CSF2, IGSF6, IL2RA, CD38, CD40, CD5, MS4A1, SDC1, GFI1, IL1R1, IL1R2, IL10, CCL20, IRF4, TRAF6, STAT5A | [24] | ||
| Treg cells | CD4+ Foxp3+ | NM | [15] | ||
| CTLs | CD8+, CXCL-9,10,11,13 | NM | [25] | ||
| Dysfunctional/ Exhausted cells | CD8+PD1+ | NM | [26] | ||
| B cells (CD20+) | BAPCs | CD86highCD21low | NM | [27] | |
| GC B cells | CD38+BCL6+ AID+ Ki67+ | NM | [16] | ||
| Breg cells | CD19+ | NM | [18] | ||
| Plasma cells | CD38+/CD138+ | TNFRSF17, IGJ | [28] | ||
| Myeloid cells | DCs | CD83+DC-LAMP | NM | [16] | |
| pDCs | BDCA-2+ IRF7+ | NM | [20] | ||
| Macrophage | CD68+ | NM | [22] | ||
| Neutrophilic granulocyte | CD66b, Myeloperoxidase | NM | [21] | ||
| Non-immune cells (CD45−) | Stromal cells | FDCs | CD21+/CD23+ | NM | [16] |
| Fibroblast | PDPN+ | NM | [18] | ||
| Endothelial cells | HEVs | MECA79+ PNAd+ | NM | [19] | |
| Other molecular components | Chemokine and cytokine | CXCL13, CCL2, IL7, IL17 | CXCL13, CCL21, IL7, IL17 | CCL2, CCL3, CCL4, CCL5, CCL8, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11, CXCL13 | [29] |
| Method | Source | Target | Single Cell | Characterization | Quantification | Localization |
|---|---|---|---|---|---|---|
| HE | FFPE | Tissue | No | Yes | Yes | Yes |
| IHC/mIHC | FFPE | Protein | No | Yes | Yes | Yes |
| IF/mIF | Fresh tissues FFPE | Protein | No | Yes | Yes | Yes |
| FCM | Fresh tissues Peripheral blood | Protein | Yes | Yes | Yes | No |
| RNA-seq | Fresh tissues FFPE Peripheral blood | mRNA | No | Yes | Yes | No |
| scRNA-seq | Fresh tissues Peripheral blood | mRNA | Yes | Yes | Yes | No |
| ST | Frozen tissue FFPE | mRNA | No | Yes | No | Yes |
| Tumor Type | Detection of TLSs | Presence and Prognosis | Quantity, Density, and Prognosis | Location and Prognosis | Differentiation and Prognosis | Composition and Prognosis | Predictive Value | Ref. |
|---|---|---|---|---|---|---|---|---|
| Melanoma | HE; IHC; mIF; scRNA-seq | Favorable | NM | Favorable (intra- tumoral) | No impact | NM | Gene signature associated with efficacy of ICB | [29] |
| Metastatic melanoma | HE; mIF | Favorable | NM | NM | No impact | Favorable (low fractions of CD21+ B cells) | NM | [46] |
| Lung squamous-cell carcinoma | HE; IHC; IF | NM | Favorable | Favorable | Favorable | NM | NM | [47] |
| HER2-positive breast cancer | HE; IHC | Favorable | NM | NM | NM | NM | NM | [39] |
| Endometrial cancer | HE; IHC | Favorable | No impact | No impact | Favorable | Favorable (number of CD20+ B cell) | NM | [41] |
| Clear-cell renal-cell carcinoma | HE; IHC | NM | NM | NM | NM | Poor (abundance of CXCL13+ CD8+ T cells) | NM | [36] |
| Kidney clear-cell carcinoma | HE; IHC | Poor | NM | NM | NM | NM | NM | [48] |
| Bladder cancer | HE; IHC | Favorable | NM | NM | NM | NM | NM | [48] |
| Stage II and III colorectal cancer | HE; mIF | NM | Favorable | NM | Favorable | NM | NM | [49] |
| Hepatocellular carcinoma | HE | NM | NM | Favorable (intra- tumoral) | Favorable | NM | NM | [50] |
| Pancreatic cancer | HE; IHC; IF | Favorable | NM | NM | Favorable | NM | NM | [40] |
| Gastrointestinal stromal tumors | HE; mIHC | Favorable | Favorable | No impact | NM | NM | NM | [43] |
| Glioblastoma | HE; mIF | NM | NM | NM | NM | Favorable (intratumoral densities of proliferating CD8+ T cells and higher CD8/CD4 ratios) | NM | [51] |
| Non-functional pancreatic neuroendocrine tumors | HE; IHC; mIF | Favorable | No impact | NM | NM | NM | NM | [44] |
| Epithelioid pleural mesothelioma | HE; IHC | Favorable | NM | NM | NM | Favorable (number of B cells) | NM | [52] |
| Epithelioid malignant peritoneal mesothelioma | HE | No impact | NM | NM | NM | NM | Associated with NC | [45] |
| Microorganism | Tumor Type | TLSs and Prognosis | Classification of TLSs and Prognostic Value | Microorganisms and Prognosis | TLSs and Microorganisms | Ref. |
|---|---|---|---|---|---|---|
| MCPyV | MCC | Favorable | Presence | Favorable | NC | [37] |
| EBV | Gastric cancer | Favorable | Presence | NC | NM | [24] |
| EBV | NPC | Favorable | Composition (PD-1+ CXCR5− CD4+Th cells) | NM | NM | [60] |
| EBV | GOA | NM | NM | NM | NM | [90] |
| HPV | CESC | Favorable | Presence | NM | PC | [91] |
| HPV | HNSCC | Favorable | Presence; Differentiation; Composition (B cell) | NM | PC | [12] |
| HPV | HNSCC | Favorable | Composition (CD4+ TFH) | Favorable | NM | [72] |
| HBV/HCV | HCC | Favorable | Composition (CD4+ TCM; CD20+ B cells) | NM | PC | [92] |
| HBV | ICC | Favorable | Presence; Location (intratumoral) | Favorable | NM | [93] |
| H. hep | CRC | NM | NM | NM | PC | [94] |
| E. coli | MIBC | Favorable | Composition (CD4+ TFH) | Favorable | NM | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Yang, X.; He, L.; Hou, Y.; Meng, H. Tertiary Lymphoid Structures in Microorganism-Related Cancer. Cancers 2024, 16, 3464. https://doi.org/10.3390/cancers16203464
Deng S, Yang X, He L, Hou Y, Meng H. Tertiary Lymphoid Structures in Microorganism-Related Cancer. Cancers. 2024; 16(20):3464. https://doi.org/10.3390/cancers16203464
Chicago/Turabian StyleDeng, Shuzhe, Xinxin Yang, Lin He, Yunjing Hou, and Hongxue Meng. 2024. "Tertiary Lymphoid Structures in Microorganism-Related Cancer" Cancers 16, no. 20: 3464. https://doi.org/10.3390/cancers16203464
APA StyleDeng, S., Yang, X., He, L., Hou, Y., & Meng, H. (2024). Tertiary Lymphoid Structures in Microorganism-Related Cancer. Cancers, 16(20), 3464. https://doi.org/10.3390/cancers16203464






