Establishment of Different Intraoperative Monitoring and Mapping Techniques and Their Impact on Survival, Extent of Resection, and Clinical Outcome in Patients with High-Grade Gliomas—A Series of 631 Patients in 14 Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
- Patients under 18 years at time of diagnosis;
- Primary surgery at external hospital;
- Loss of follow-up < 3 months;
- Incomplete clinical data (NIHSS, KPS, MRI).
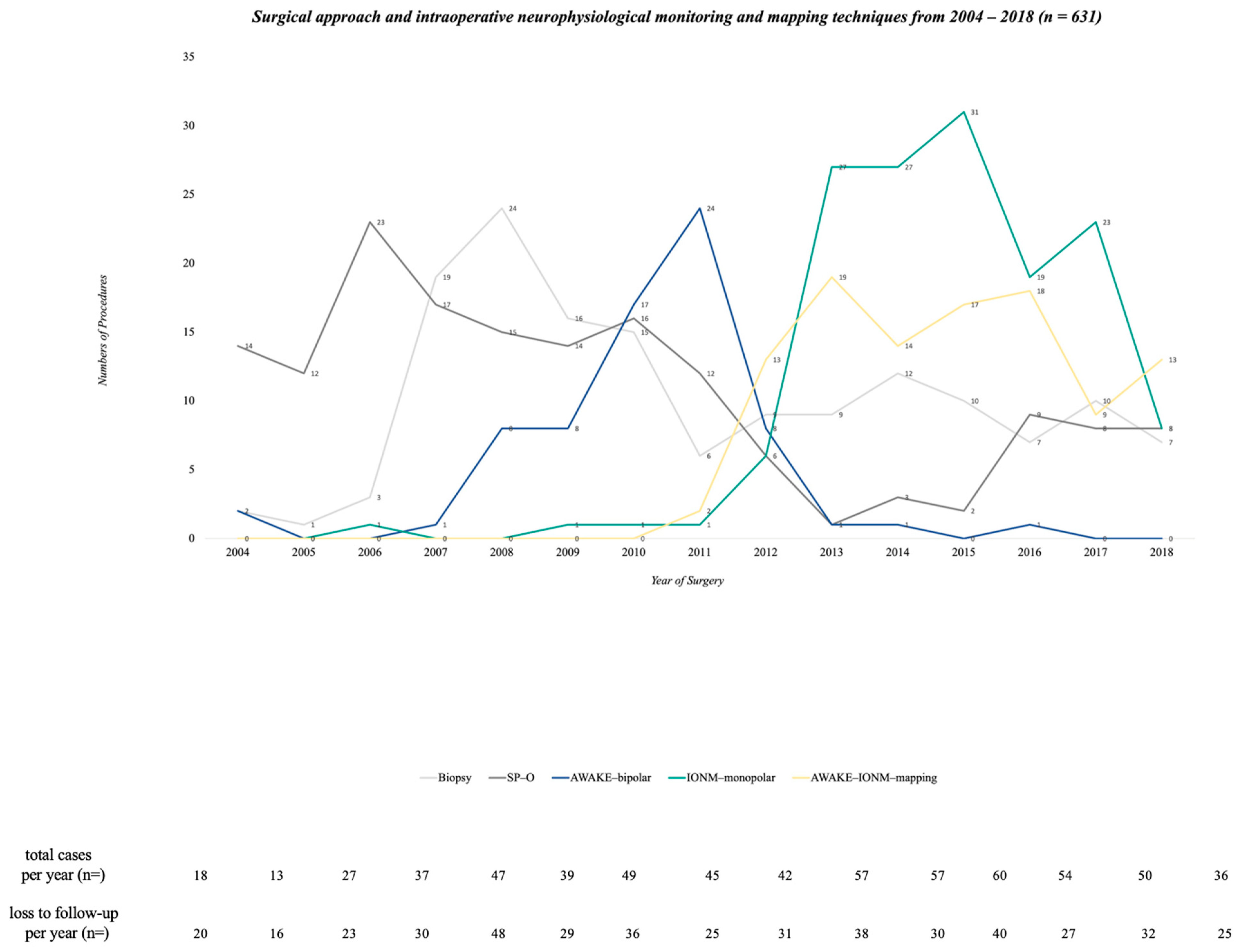
2.1. Surgical Procedure—Resection vs. Biopsy and Categories of Surgical Monitoring and Mapping Approaches
2.2. PFS and OS
2.3. Further Variables of Data Collection
2.3.1. IDH Status and Adjuvant Therapy of Resection Group
2.3.2. Eloquence of Tumor Localization
2.3.3. Extent of Resection and Residual Tumor Volume
2.3.4. Neurological Outcome
2.4. Statistical Analyses
3. Results
3.1. Surgical Characteristics and their Changes from 2004 to 2018
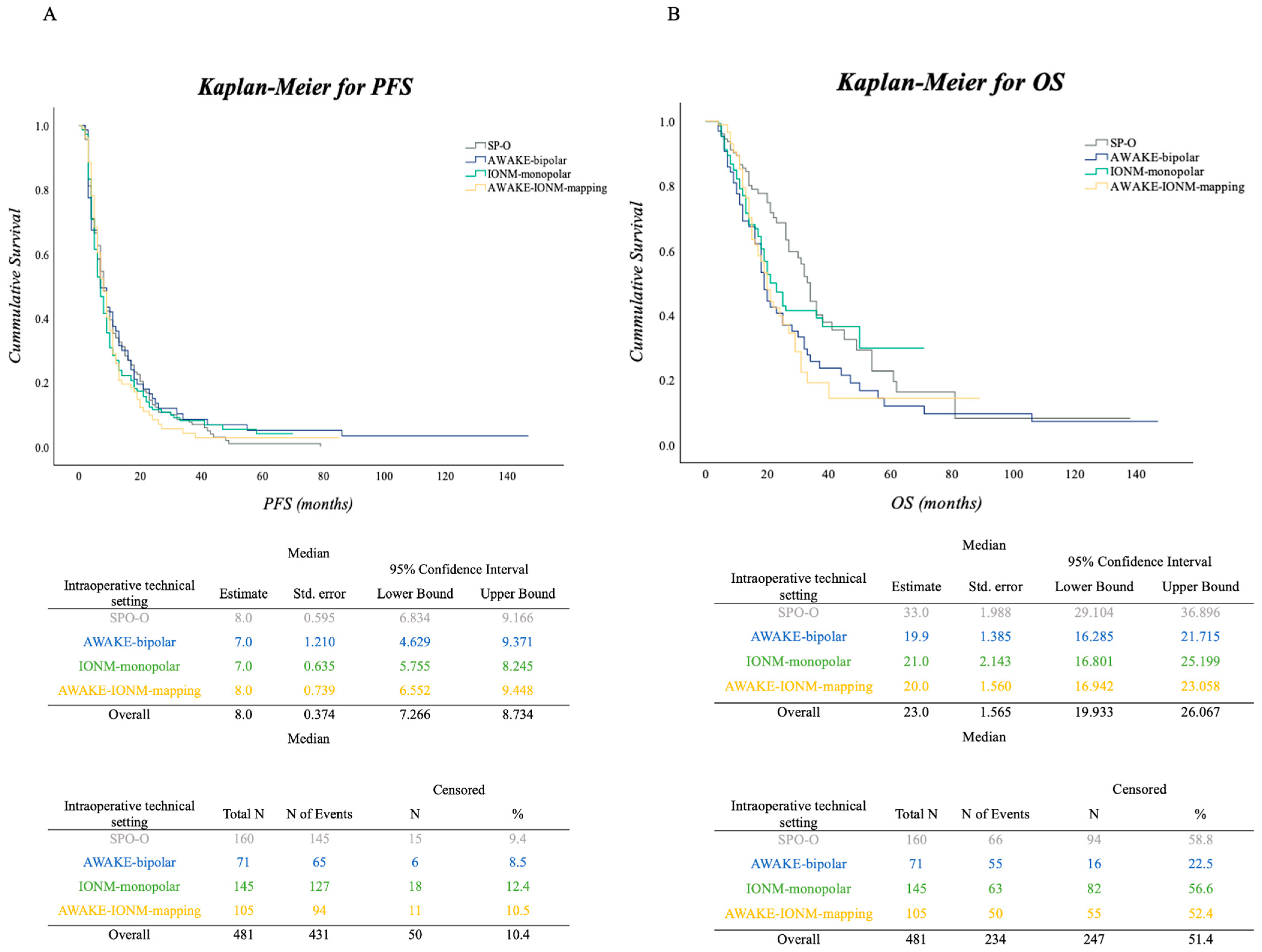
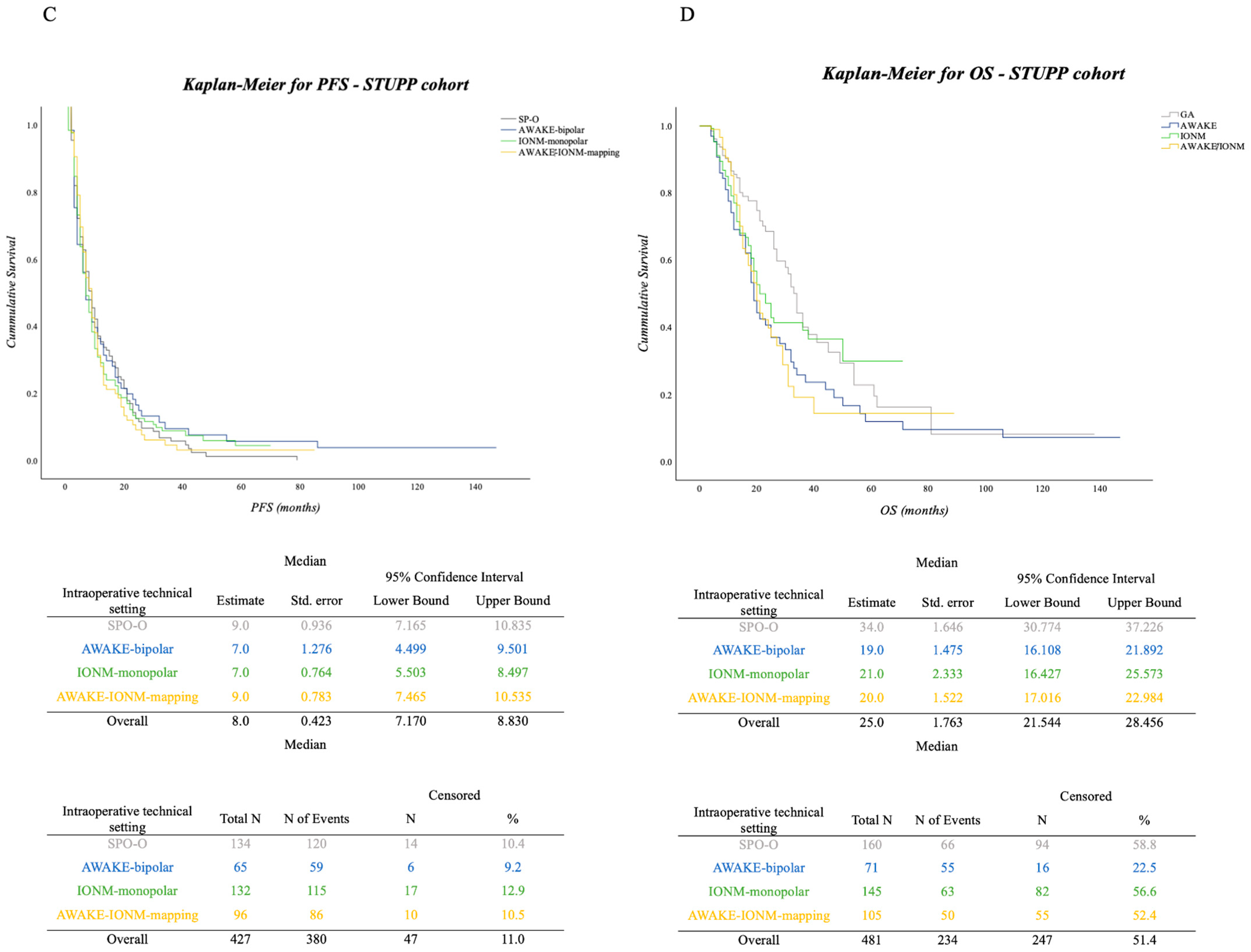
3.2. PFS and OS in the Surgical Cohort
Impact of Adjuvant Therapy in Surgical Approaches concerning PFS and OS
3.3. Tumor Localization and Eloquence
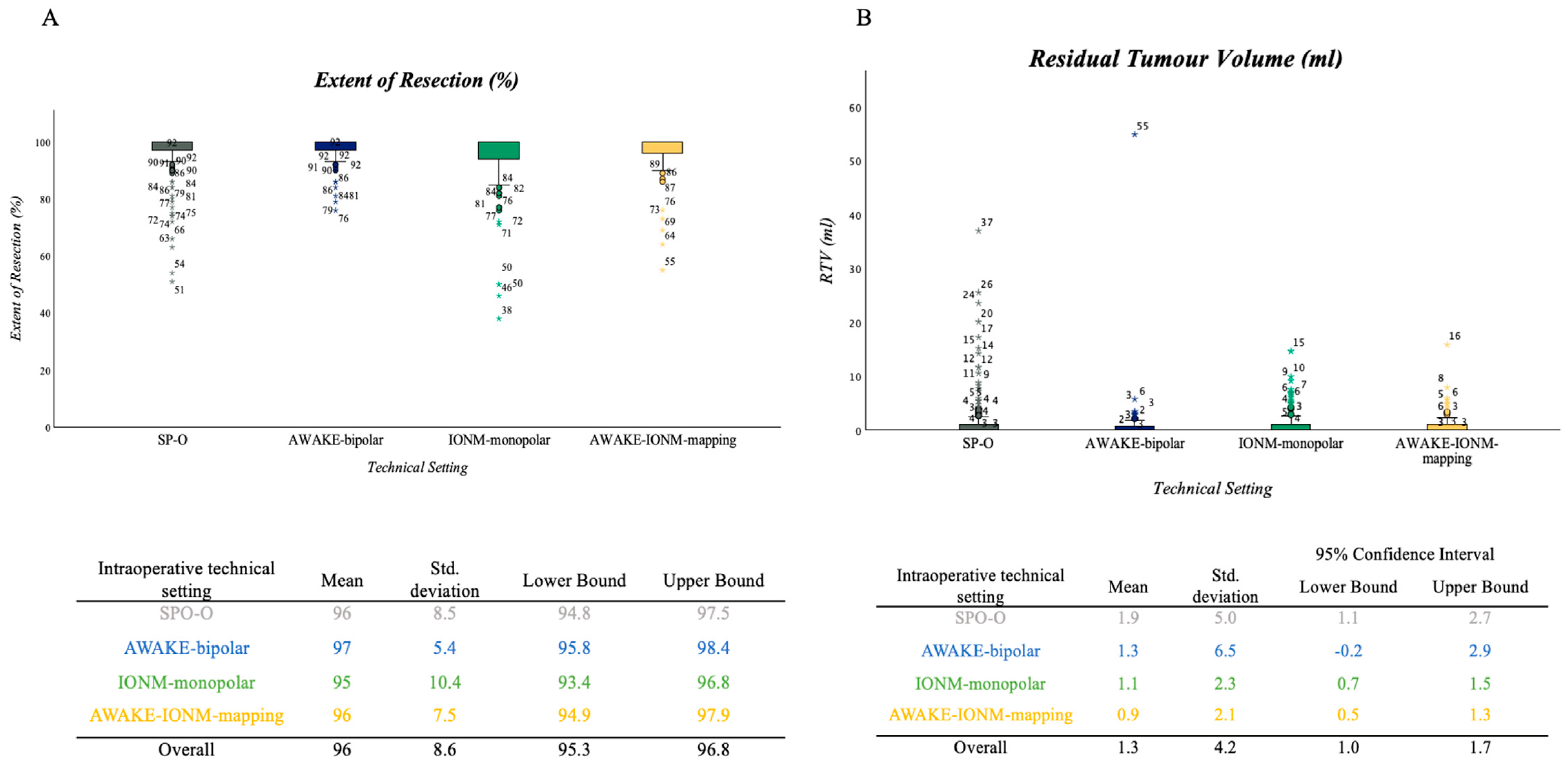
3.4. Extent of Resection and RTV
3.5. Neurological Outcome in Resection Group: NIHSS and KPS
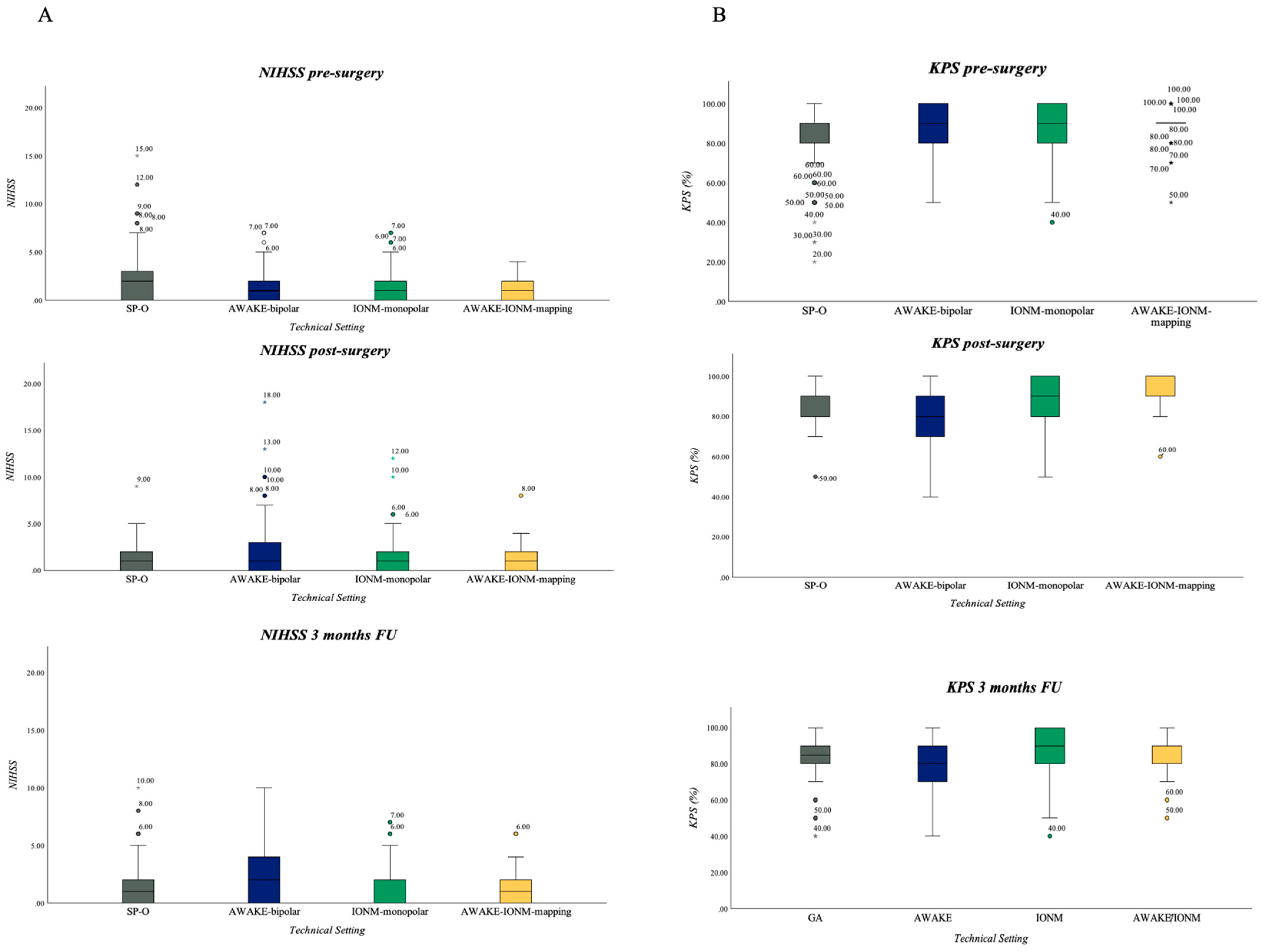
3.5.1. NIHSS
3.5.2. KPS
4. Discussion
Implementation of Different Surgical Monitoring and Mapping Techniques and Their Impacts on Short-Time and Long-Time Outcomes
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro. Oncol. 2017, 19, 1553–1564. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro. Oncol. 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neuro. Oncol. 2021, 151, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Zwarthoed, R.H.; Kilgallon, J.L.; Nawabi, N.L.; Versyck, G.; Jessurun, C.A.C.; Pruijn, K.P.; Fisher, F.L.; Larivière, E.; Solie, L.; et al. Impact of maximal extent of resection on postoperative deficits, patient functioning, and survival within clinically important glioblastoma subgroups. Neuro. Oncol. 2023, 25, 958–972. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro. Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Oppenlander, M.E.; Wolf, A.B.; Snyder, L.A.; Bina, R.; Wilson, J.R.; Coons, S.W.; Ashby, L.S.; Brachman, D.; Nakaji, P.; Porter, R.W.; et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 2014, 120, 846–853. [Google Scholar] [CrossRef]
- Esquenazi, Y.; Friedman, E.; Liu, Z.; Zhu, J.J.; Hsu, S.; Tandon, N. The Survival Advantage of “Supratotal” Resection of Glioblastoma Using Selective Cortical Mapping and the Subpial Technique. Neurosurgery 2017, 81, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- McGirt, M.J.; Mukherjee, D.; Chaichana, K.L.; Than, K.D.; Weingart, J.D.; Quinones-Hinojosa, A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 2009, 65, 463–469, discussion 469–470. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Shiban, E.; Droese, D.; Gempt, J.; Buchmann, N.; Pape, H.; Ryang, Y.M.; Meyer, B.; Ringel, F. Predictive value and safety of intraoperative neurophysiological monitoring with motor evoked potentials in glioma surgery. Neurosurgery 2012, 70, 1060–1070, discussion 1070–1061. [Google Scholar] [CrossRef]
- Gogos, A.J.; Young, J.S.; Morshed, R.A.; Avalos, L.N.; Noss, R.S.; Villanueva-Meyer, J.E.; Hervey-Jumper, S.L.; Berger, M.S. Triple motor mapping: Transcranial, bipolar, and monopolar mapping for supratentorial glioma resection adjacent to motor pathways. J. Neurosurg. 2020, 134, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Szelényi, A.; Bello, L. Chapter 8—Intraoperative mapping and monitoring during brain tumor surgeries. In Handbook of Clinical Neurology; Nuwer, M.R., MacDonald, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 186, pp. 133–149. [Google Scholar]
- Staub-Bartelt, F.; Rapp, M.; Sabel, M. Feasibility of intraoperative neuromonitoring and cortical/subcortical mapping in patients with cerebral lesions of highly functional localizations-pathway to case adapted monitoring and mapping procedures. Front. Oncol. 2023, 13, 1235212. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Callipo, V.; Lamperti, M.; Rossi, M.; Conti Nibali, M.; Sciortino, T.; Gay, L.; Puglisi, G.; Leonetti, A.; Cerri, G.; et al. Transcranial versus direct electrical stimulation for intraoperative motor-evoked potential monitoring: Prognostic value comparison in asleep brain tumor surgery. Front. Oncol. 2022, 12, 963669. [Google Scholar] [CrossRef] [PubMed]
- Shiban, E.; Krieg, S.M.; Haller, B.; Buchmann, N.; Obermueller, T.; Boeckh-Behrens, T.; Wostrack, M.; Meyer, B.; Ringel, F. Intraoperative subcortical motor evoked potential stimulation: How close is the corticospinal tract? J. Neurosurg. 2015, 123, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Riva, M.; Fava, E.; Ferpozzi, V.; Castellano, A.; Raneri, F.; Pessina, F.; Bizzi, A.; Falini, A.; Cerri, G. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro-Oncol. 2014, 16, 1110–1128. [Google Scholar] [CrossRef]
- Bello, L.; Gallucci, M.; Fava, M.; Carrabba, G.; Giussani, C.; Acerbi, F.; Baratta, P.; Songa, V.; Conte, V.; Branca, V.; et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 2007, 60, 67–80, discussion 80–62. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Li, J.; Lau, D.; Molinaro, A.M.; Perry, D.W.; Meng, L.; Berger, M.S. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J. Neurosurg. 2015, 123, 325–339. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Sreenivasan, S.A.; Madhugiri, V.S.; Sasidharan, G.M.; Kumar, R.V. Measuring glioma volumes: A comparison of linear measurement based formulae with the manual image segmentation technique. J. Cancer Res. Ther. 2016, 12, 161–168. [Google Scholar] [CrossRef]
- Karschnia, P.; Vogelbaum, M.A.; van den Bent, M.; Cahill, D.P.; Bello, L.; Narita, Y.; Berger, M.S.; Weller, M.; Tonn, J.C. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur. J. Cancer 2021, 149, 23–33. [Google Scholar] [CrossRef]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Zwarthoed, R.H.; Kilgallon, J.L.; Nawabi, N.L.; Jessurun, C.A.C.; Versyck, G.; Pruijn, K.P.; Fisher, F.L.; Larivière, E.; Solie, L.; et al. Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): A propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncol. 2022, 23, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.A.; Rincon-Torroella, J.; Sattari, A.R.; Feghali, J.; Yang, W.; Kim, J.E.; Xu, R.; Jackson, C.M.; Mukherjee, D.; Lin, S.C.; et al. Awake versus Asleep Craniotomy for Patients with Eloquent Glioma: A Systematic Review and Meta-Analysis. Neurosurgery 2023, 94, 38–52. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Nibali, M.C.; Viganò, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2019, 133, 642–654. [Google Scholar] [CrossRef]
- Schucht, P.; Seidel, K.; Jilch, A.; Beck, J.; Raabe, A. A review of monopolar motor mapping and a comprehensive guide to continuous dynamic motor mapping for resection of motor eloquent brain tumors. Neurochirurgie 2017, 63, 175–180. [Google Scholar] [CrossRef]
- Fukui, A.; Muragaki, Y.; Saito, T.; Nitta, M.; Tsuzuki, S.; Asano, H.; Kawamata, T. Impact of awake mapping on overall survival and extent of resection in patients with adult diffuse gliomas within or near eloquent areas: A retrospective propensity score-matched analysis of awake craniotomy vs. general anesthesia. Acta Neurochir. 2022, 164, 395–404. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chen, J.P.; Cheng, W.Y.; Lee, H.T.; Shen, C.C. The role of tailored intraoperative neurophysiological monitoring in glioma surgery: A single institute experience. J. Neuro. Oncol. 2020, 146, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764, discussion 264–756. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]

| Characteristic | SP–O | AWAKE–Bipolar | IONM–Monopolar | AWAKE–IONM–Mapping | Overall Resection Cohort | |
|---|---|---|---|---|---|---|
| n = 160 | n = 71 | n = 145 | n = 105 | n = 481 | ||
| Sex | ||||||
| male | 97 | 50 | 88 | 68 | 303 (63%) | |
| female | 63 | 21 | 57 | 37 | 178 (37%) | |
| Age at diagnosis, y | ||||||
| (p = 0.281) | Median (IQR) | 62.1 (17.4) | 60 (17.7) | 60 (19.0) | 64 (15.0) | 61.0 (52.8-70) |
| range | 23.0–89 | 24–80 | 24–85 | 29–86 | 23–89 | |
| ALA | ||||||
| (p = 0.281) | Administered | 142 | 70 | 136 | 104 | 452 (94%) |
| Not administered | 18 | 1 | 9 | 1 | 29 (6%) | |
| IDH-Status | ||||||
| (p < 0.001) | Wildtype | 50 | 36 | 134 | 100 | 320 (66.5%) |
| Mutant | 3 | 2 | 6 | 3 | 14 (2.9%) | |
| Unknown | 107 | 33 | 5 | 2 | 147 (30.6%) | |
| MGMT-Status | ||||||
| (p < 0.001) | MGMT + | 22 | 7 | 60 | 50 | 139 (28.9%) |
| MGMT - | 30 | 15 | 75 | 46 | 166 (34.5% | |
| Unknown | 107 | 49 | 10 | 9 | 176 (36.6% | |
| KPS (mean) | ||||||
| preoperative | 82 | 87 | 89 | 90 | 86.78 (±12.34) | |
| postoperative | 85 | 77 (p < 0.001) | 90 | 91 | 86.78 (±11.08) | |
| 3 months | 82 | 77 (p = 0.012) | 88 (p = 0.004) | 88 (p = 0.021) | 84.57 (±13.22) | |
| NIHSS (mean) | ||||||
| preoperative | 1.9 | 1.6 | 1.4 | 1 | 1.4 | |
| postoperative | 1.6 | 2.5 (p = 0.005) | 1.2 | 1 | 1.3 | |
| 3 months | 1.6 | 2.3 (p = 0.044) | 1.3 | 1.4 | 1.6 | |
| Pre- OP Tumour volume (mL) | ||||||
| (p = 0.288) | Mean (SD) | 37.99 (±28.14) | 36.83 (±43.32) | 35.97 (±33.21) | 29.09 (±24.64) | 35.2 (±31.8) |
| Extent of resection, % by volume | ||||||
| (p = 4.04) | Mean | 96% | 97% | 95% | 96% | 96.1 (8.6%) |
| Median | 100% | 100% | 100% | 100% | ||
| Range | 51–100% | 76–100% | 38–100% | 55–100% | 38.2–226 | |
| Residual volume (mL) | ||||||
| (p = 0.186) | Mean (±SD) | 1.95 mL (±5.1) | 1.36 mL (±6.58) | 1.11 mL (±2.26) | 0.91 mL (±2.06) | 1.37 (±4.16) |
| Range | 0–37 mL | 0–54.91 mL | 0–14.64 mL | 0–15.83 mL | 0–54.91 | |
| Tumour location by hemisphere | ||||||
| (p = 0.001) | Bilateral | 4 | 0 | 4 | 1 | 15 (3.1%) |
| Left | 60 | 42 | 40 | 82 | 223 (46.4%) | |
| Right | 96 | 29 | 96 | 21 | 243 (50.5%) | |
| Eloquence | ||||||
| (p < 0.001) | Eloquent | 84 | 71 | 145 | 105 | 405 (84.2%) |
| Not eloquent | 76 | 0 | 0 | 0 | 76 (15.8%) | |
| Before 2010 | ||||||
| Eloquent | 75 (64.7%) | |||||
| Not eloquent | 41 (35.3%) | |||||
| After 2010 | ||||||
| Eloquent | 330 (90.4%) | |||||
| Not eloquent | 35 (9.6%) | |||||
| Adjuvant Therapy (n = 427) | SP–O | AWAKE–BIPOLAR | IONM–Monopolar | AWAKE–IONM-Mapping |
|---|---|---|---|---|
| Total Number | n = 134 | n = 65 | n = 132 | n = 96 |
| Details Concerning Adjuvant Therapy Available from | n = 75 | n = 52 | n = 113 | n = 81 |
| Stupp complete (60 Gy radiation + concomitant TMZ + adjuvant 6 cycles Temodal | 22 | 14 | 16 | 14 |
| Radiation + concomitant Chemotherapy applied, adjuvant TMZ cancelled after initiation | 23 | 15 | 52 | 35 |
| Radiation + concomitant Chemotherapy finalised, adjuvant TMZ not initiated | 18 | 22 | 33 | 25 |
| Radiation + concomitant Chemotherapy cancelled, adjuvant TMZ not initiated | 3 | 0 | 2 | 2 |
| Radiation + concomitant Chemotherapy cancelled, adjuvant TMZ finalised (at least 6 cycles) | 1 | 0 | 2 | 0 |
| Herrlinger scheme | 8 | 1 | 7 | 5 |
| Radiation only | 0 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staub-Bartelt, F.; Suresh Babu, M.P.; Szelényi, A.; Rapp, M.; Sabel, M. Establishment of Different Intraoperative Monitoring and Mapping Techniques and Their Impact on Survival, Extent of Resection, and Clinical Outcome in Patients with High-Grade Gliomas—A Series of 631 Patients in 14 Years. Cancers 2024, 16, 926. https://doi.org/10.3390/cancers16050926
Staub-Bartelt F, Suresh Babu MP, Szelényi A, Rapp M, Sabel M. Establishment of Different Intraoperative Monitoring and Mapping Techniques and Their Impact on Survival, Extent of Resection, and Clinical Outcome in Patients with High-Grade Gliomas—A Series of 631 Patients in 14 Years. Cancers. 2024; 16(5):926. https://doi.org/10.3390/cancers16050926
Chicago/Turabian StyleStaub-Bartelt, Franziska, Marian Preetham Suresh Babu, Andrea Szelényi, Marion Rapp, and Michael Sabel. 2024. "Establishment of Different Intraoperative Monitoring and Mapping Techniques and Their Impact on Survival, Extent of Resection, and Clinical Outcome in Patients with High-Grade Gliomas—A Series of 631 Patients in 14 Years" Cancers 16, no. 5: 926. https://doi.org/10.3390/cancers16050926
APA StyleStaub-Bartelt, F., Suresh Babu, M. P., Szelényi, A., Rapp, M., & Sabel, M. (2024). Establishment of Different Intraoperative Monitoring and Mapping Techniques and Their Impact on Survival, Extent of Resection, and Clinical Outcome in Patients with High-Grade Gliomas—A Series of 631 Patients in 14 Years. Cancers, 16(5), 926. https://doi.org/10.3390/cancers16050926






