Prevention of Post-Hepatectomy Liver Failure in Cirrhotic Patients Undergoing Minimally Invasive Liver Surgery for HCC: Has the Round Ligament to Be Preserved?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preoperative Assessment
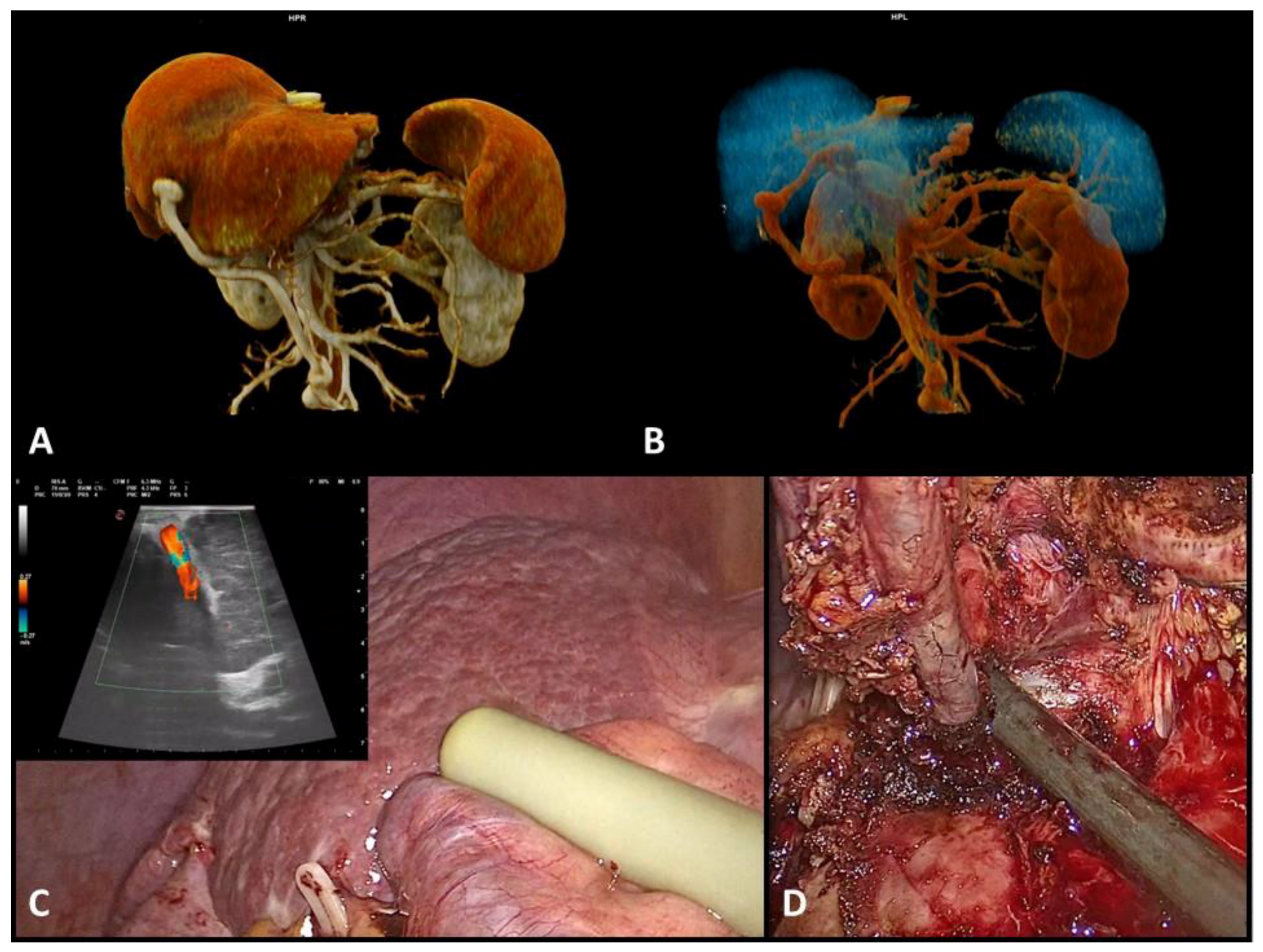
2.3. Surgical Technique and Definitions
2.4. Statistical Analysis
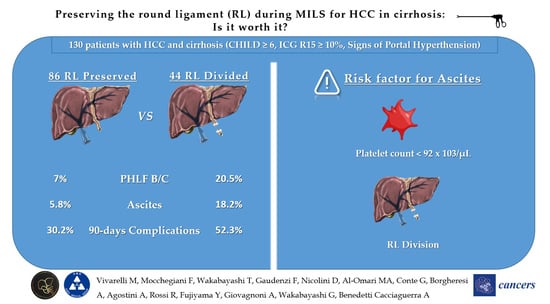
3. Results
3.1. Preoperative and Intraoperative Characteristics
3.2. Postoperative Outcomes
3.3. Risk Factors for Ascites
3.4. Sensitivity Analysis Excluding Patients Converted to Open Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Prodeau, M.; Drumez, E.; Duhamel, A.; Vibert, E.; Farges, O.; Lassailly, G.; Mabrut, J.-Y.; Hardwigsen, J.; Régimbeau, J.-M.; Soubrane, O.; et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J. Hepatol. 2019, 71, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.I.; Berardi, G.; Morise, Z.; Cipriani, F.; Ariizumi, S.; Sposito, C.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: Multicentre propensity score-matched study. Br. J. Surg. 2021, 108, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Calthorpe, L.; Rashidian, N.; Cacciaguerra, A.B.; Conroy, P.C.; Hibi, T.; Hilal, M.A.; Hoffman, D.; Park, K.M.; Wang, J.; Adam, M.A.; et al. Using the Comprehensive Complication Index to Rethink the ISGLS Criteria for Post-hepatectomy Liver Failure in an International Cohort of Major Hepatectomies. Ann. Surg. 2023, 277, e592–e596. [Google Scholar] [CrossRef]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Tanabe, M.; Wakabayashi, G.; Cheung, T.T.; Lo, C.M.; et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Sugioka, A.; Kawabe, N.; Umemoto, S.; Nagata, H.; Ohshima, H.; Kawase, J.; Aeakawa, S.; Yoshida, R. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J. Endosc. Surg. 2011, 4, 143–146. [Google Scholar] [CrossRef]
- Benedetti Cacciaguerra, A.; Görgec, B.; Lanari, J.; Cipriani, F.; Russolillo, N.; Mocchegiani, F.; Zimmitti, G.; Alseidi, A.; Ruzzenente, A.; Edwin, B.; et al. Outcome of major hepatectomy in cirrhotic patients; does surgical approach matter? A propensity score matched analysis. J. Hepatobiliary Pancreat. Sci. 2022, 29, 1226–1239. [Google Scholar] [CrossRef]
- Berardi, G.; Morise, Z.; Sposito, C.; Igarashi, K.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; Kubo, S.; Tonaka, S.; et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2020, 72, 75–84. [Google Scholar] [CrossRef]
- Coletta, D.; De Padua, C.; Parrino, C.; De Peppo, V.; Oddi, A.; Frigieri, C.; Grazi, G.L. Laparoscopic Liver Surgery: What Are the Advantages in Patients with Cirrhosis and Portal Hypertension? Systematic Review and Meta-Analysis with Personal Experience. Journal of laparoendoscopic & advanced surgical techniques. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1054–1065. [Google Scholar] [CrossRef]
- Allard, M.A.; Adam, R.; Bucur, P.O.; Termos, S.; Cunha, A.S.; Bismuth, H.; Castaing, D.; Vibert, E. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann. Surg. 2013, 258, 822–829, discussion 29–30. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Ranaivojaona, S.; Livideanu, C.; Mery, C.; Gaches, F. Umbilical Vein Recanalization. Am. J. Med. Sci. 2021, 361, e41. [Google Scholar] [CrossRef]
- Koliogiannis, D.; Nieß, H.; Koliogiannis, V.; Ilmer, M.; Angele, M.; Werner, J.; Guba, M. Preservation of the round ligament to accommodate transient portal hypertension after major hepatectomy. Langenbecks Arch. Surg. 2022, 407, 2393–2397. [Google Scholar] [CrossRef]
- Gray, B.H.; Cooke, R.A.; Tannenbaum, A.S. Research involving human subjects. Science 1978, 201, 1094–1101. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef]
- Strasberg, S.M. Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Surg. 2005, 12, 351–355. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Cacciaguerra, A.B.; Görgec, B.; Cipriani, F.; Aghayan, D.; Borelli, G.; Aljaiuossi, A.; Dagher, I.; Gayet, B.; Fuks, D.; Rotellar, F.; et al. Risk Factors of Positive Resection Margin in Laparoscopic and Open Liver Surgery for Colorectal Liver Metastases: A New Perspective in the Perioperative Assessment: A European Multicenter Study. Ann. Surg. 2022, 275, e213–e221. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Hanley, J.A. Receiver operating characteristic (ROC) methodology: The state of the art. Crit. Rev. Diagn. Imaging 1989, 29, 307–335. [Google Scholar]
- Ray, S.; Mehta, N.N.; Golhar, A.; Nundy, S. Post hepatectomy liver failure—A comprehensive review of current concepts and controversies. Ann. Med. Surg. 2018, 34, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ocak, I.; Topaloglu, S.; Acarli, K. Posthepatectomy liver failure. Turk. J. Med. Sci. 2020, 50, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Riddiough, G.E.; Christophi, C.; Jones, R.M.; Muralidharan, V.; Perini, M.V. A systematic review of small for size syndrome after major hepatectomy and liver transplantation. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2020, 22, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Ohkohchi, N.; Orii, T.; Koyamada, N.; Tsukamoto, S.; Sato, M.; Enomoto, Y.; Usuda, M.; Satomi, S. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2003, 16, 376–382. [Google Scholar] [CrossRef]
- Yagi, S.; Iida, T.; Taniguchi, K.; Hori, T.; Hamada, T.; Fujii, K.; Mizuno, S.; Uemoto, S. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2005, 11, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Cescon, M.; Vetrone, G.; Grazi, G.L.; Ramacciato, G.; Ercolani, G.; Ravaioli, M.; Del Gaudio, M.; Pinna, A.D. Trends in perioperative outcome after hepatic resection: Analysis of 1500 consecutive unselected cases over 20 years. Ann. Surg. 2009, 249, 995–1002. [Google Scholar] [CrossRef]
- Bruix, J.; Castells, A.; Bosch, J.; Feu, F.; Fuster, J.; Garcia-Pagan, J.C.; Visa, J.; Bru, C.; Rodes, J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology 1996, 111, 1018–1022. [Google Scholar] [CrossRef]
- Azoulay, D.; Ramos, E.; Casellas-Robert, M.; Salloum, C.; Lladó, L.; Nadler, R.; Busquets, J.; Caula-Frexia, C.; Mils, K.; Lopez-Ben, S.; et al. Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep. 2021, 3, 100190. [Google Scholar] [CrossRef]
- Görgec, B.; Cacciaguerra, A.B.; Lanari, J.; Russolillo, N.; Cipriani, F.; Aghayan, D.; Zimmitti, G.; Efanov, M.; Alseidi, A.; Mocchegaini, F.; et al. Assessment of Textbook Outcome in Laparoscopic and Open Liver Surgery. JAMA Surg. 2021, 156, e212064. [Google Scholar] [CrossRef]
- Afdhal, N.; McHutchison, J.; Brown, R.; Jacobson, I.; Manns, M.; Poordad, F.; Weksler, B.; Esteban, R. Thrombocytopenia associated with chronic liver disease. J. Hepatol. 2008, 48, 1000–1007. [Google Scholar] [CrossRef]
- Gangireddy, V.G.; Kanneganti, P.C.; Sridhar, S.; Talla, S.; Coleman, T. Management of thrombocytopenia in advanced liver disease. Can. J. Gastroenterol. Hepatol. 2014, 28, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Levi Sandri, G.B.; Lai, Q.; Ravaioli, M.; Di Sandro, S.; Balzano, E.; Pagano, D.; Magistri, P.; Di Benedetto, F.; Rossi, M.; Gruttadauria, S.; et al. The Role of Salvage Transplantation in Patients Initially Treated with Open Versus Minimally Invasive Liver Surgery: An Intention-to-Treat Analysis. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2020, 26, 878–887. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | RL Divided (n = 44) | RL Preserved (n = 86) | p |
|---|---|---|---|
| Preoperative Characteristics | |||
| Age, years (IQR) | 65 (56–71) | 67 (59–76) | 0.104 |
| Sex, male (%) | 32 (72.7%) | 65 (75.6%) | 0.723 |
| BMI, kg/m2 (IQR) | 24 (23–28) | 25 (23–29) | 0.438 |
| ASA grade | 0.281 | ||
| ASA 1 (%) | 0 | 0 | |
| ASA 2 (%) | 26 (59.1%) | 59 (68.6%) | |
| ASA 3 (%) | 18 (40.9%) | 27 (31.4%) | |
| ASA 4 (%) | 0 | 0 | |
| Grade of Cirrhosis | 0.370 | ||
| Child–Pugh A (%) | 34 (77.3%) | 72 (83.7%) | |
| Child–Pugh B (%) | 10 (22.7%) | 14 (16.3%) | |
| Etiology of Cirrhosis | 0.255 | ||
| Alcohol (%) | 2 (4.5%) | 15 (17.4%) | |
| HCV (%) | 17 (38.6%) | 35 (40.7%) | |
| HBV (%) | 7 (15.9%) | 11 (12.8%) | |
| Metabolic (%) | 9 (20.5%) | 14 (16.3%) | |
| Other (%) | 9 (20.5%) | 11 (12.8%) | |
| ICG-R15 (IQR) | 15.0 (12–18) | 16.0 (13–19.5) | 0.650 |
| Platelets count ×103/µL (IQR) | 105 (87–122) | 108 (90–120) | 0.218 |
| Previous abdominal surgery (%) | 26 (59.1%) | 38 (44.2%) | 0.108 |
| Previous liver surgery (%) | 9 (20.5%) | 15 (17.4%) | 0.675 |
| Number of nodules (IQR) | 1 (1–1) | 1 (1–1) | 0.904 |
| Max. nodule size, mm (IQR) | 30 (15–39) | 25 (15–35) | 0.305 |
| Intraoperative Characteristics | |||
| Type of Resection | 0.494 | ||
| Non-anatomic resection (%) | 19 (43.2%) | 43 (50.0%) | |
| Segmentectomy (%) | 17 (38.6%) | 34 (39.5%) | |
| Left lateral sectionectomy (%) | 6 (13.6%) | 5 (5.8%) | |
| Right posterior sectionectomy (%) | 2 (4.5%) | 4 (4.7%) | |
| Conversion to Open (%) | 13 (29.5%) | 1 (1.6%) | <0.001 |
| Pringle maneuver (%) | 34 (77.3%) | 74 (86.0%) | 0.207 |
| Duration of Pringle man, min (IQR) | 37 (18–68) | 33 (15–41) | 0.785 |
| Operative time, min (IQR) | 297 (216–380) | 264 (195–300) | 0.064 |
| Blood loss, cc (IQR) | 100 (30–200) | 100 (20–190) | 0.696 |
| Postoperative Outcomes | RL Divided (n = 44) | RL Preserved (n = 86) | p |
|---|---|---|---|
| Postoperative complication—30 days ¥ (%) | 23 (52.3%) | 24 (27.9%) | 0.006 |
| Severe, grade 3–5 (%) | 5 (11.4%) | 10 (11.6%) | 0.964 |
| Postoperative complication—90 days ¥ (%) | 23 (52.3%) | 26 (30.2%) | 0.014 |
| Post Hepatectomy Liver Failure * (%) | 13 (29.5%) | 10 (11.6%) | 0.011 |
| Severe, grade B–C (%) | 9 (20.5%) | 6 (7.0%) | 0.023 |
| Ascites (%) | 8 (18.2%) | 5 (5.8%) | 0.026 |
| 30-days readmission (%) | 2 (4.5%) | 5 (5.8%) | 0.762 |
| ICU Stay, days (IQR) | 1 (0–1) | 1 (0–1) | 0.215 |
| Hospital stays, days (IQR) | 5 (4–7) | 5 (4–5) | 0.551 |
| Ascites Predictive Factors | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Preoperative Characteristics | ||||
| Age, years | 0.980 (0.931 to 1.031) | 0.433 | ||
| Sex, male | 1.149 (0.296 to 4.457) | 0.840 | ||
| BMI, kg/m2 | 1.008 (0.875 to 1.161) | 0.912 | ||
| ASA grade | ||||
| ASA 2 | ||||
| ASA 3 | 3.459 (1.059 to 11.296) | 0.040 | 2.753 (0.694 to 10.921) | 0.150 |
| Grade of Cirrhosis | ||||
| Child–Pugh A | ||||
| Child–Pugh B | 6.863 (2.056 to 22.912) | 0.002 | 1.715 (0.383 to 7.679) | 0.481 |
| Etiology of Cirrhosis | ||||
| Alcohol | ||||
| HCV | 0.726 (0.165 to 3.187) | 0.671 | ||
| HBV | 0.275 (0.026 to 2.940) | 0.285 | ||
| Metabolic | 0.444 (0.066 to 3.010) | 0.406 | ||
| Other | 1.000 (0.999 to 1.002) | 0.998 | ||
| ICG-R15 (IQR) | 1.008 (0.989 to 1.027) | 0.418 | ||
| Platelets count ×103/µL | 0.977 (0.961 to 0.992) | 0.004 | 0.971 (0.954 to 0.989) | 0.002 |
| Previous abdominal surgery | 0.614 (0.190 to 1.989) | 0.416 | ||
| Previous liver surgery | 0.785 (0.162 to 3.798) | 0.764 | ||
| Number of nodules | 0.208 (0.018 to 2.377) | 0.207 | ||
| Max. nodule size, mm | 0.980 (0.940 to 1.022) | 0.350 | ||
| Intraoperative Characteristics | ||||
| Round Ligament divided | 3.600 (1.101 to 11.766) | 0.034 | 6.750 (1.730 to 26.336) | 0.006 |
| Type of Resection | ||||
| Non-anatomic resection | ||||
| Segmentectomy | 0.999 (0.998 to 1.001) | 0.998 | ||
| Left lateral sectionectomy | 0.999 (0.999 to 1.000) | 0.999 | ||
| Right posterior sectionectomy | 0.501 (0.145 to 1.735) | 0.275 | ||
| Conversion to Open | 7.500 (2.028 to 27.741) | 0.003 | 3.611 (0.633 to 20.598) | 0.148 |
| Pringle maneuver | 0.646 (0.162 to 2.570) | 0.535 | ||
| Duration of Pringle man, min | 1.000 (0.987 to 1.014) | 0.993 | ||
| Operative time, min | 1.001 (0.995 to 1.006) | 0.851 | ||
| Blood loss, cc | 1.000 (0.998 to 1.002) | 0.930 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivarelli, M.; Mocchegiani, F.; Wakabayashi, T.; Gaudenzi, F.; Nicolini, D.; Al-Omari, M.A.; Conte, G.; Borgheresi, A.; Agostini, A.; Rossi, R.; et al. Prevention of Post-Hepatectomy Liver Failure in Cirrhotic Patients Undergoing Minimally Invasive Liver Surgery for HCC: Has the Round Ligament to Be Preserved? Cancers 2024, 16, 364. https://doi.org/10.3390/cancers16020364
Vivarelli M, Mocchegiani F, Wakabayashi T, Gaudenzi F, Nicolini D, Al-Omari MA, Conte G, Borgheresi A, Agostini A, Rossi R, et al. Prevention of Post-Hepatectomy Liver Failure in Cirrhotic Patients Undergoing Minimally Invasive Liver Surgery for HCC: Has the Round Ligament to Be Preserved? Cancers. 2024; 16(2):364. https://doi.org/10.3390/cancers16020364
Chicago/Turabian StyleVivarelli, Marco, Federico Mocchegiani, Taiga Wakabayashi, Federico Gaudenzi, Daniele Nicolini, Malek A. Al-Omari, Grazia Conte, Alessandra Borgheresi, Andrea Agostini, Roberta Rossi, and et al. 2024. "Prevention of Post-Hepatectomy Liver Failure in Cirrhotic Patients Undergoing Minimally Invasive Liver Surgery for HCC: Has the Round Ligament to Be Preserved?" Cancers 16, no. 2: 364. https://doi.org/10.3390/cancers16020364
APA StyleVivarelli, M., Mocchegiani, F., Wakabayashi, T., Gaudenzi, F., Nicolini, D., Al-Omari, M. A., Conte, G., Borgheresi, A., Agostini, A., Rossi, R., Fujiyama, Y., Giovagnoni, A., Wakabayashi, G., & Benedetti Cacciaguerra, A. (2024). Prevention of Post-Hepatectomy Liver Failure in Cirrhotic Patients Undergoing Minimally Invasive Liver Surgery for HCC: Has the Round Ligament to Be Preserved? Cancers, 16(2), 364. https://doi.org/10.3390/cancers16020364