Simple Summary
This study investigates the potential of exosomal profiles as a tool for early detection of relapse and long-term outcomes in oral squamous cell carcinoma (OSCC) patients undergoing conventional therapy. Examining 27 OSCC patients, this study found a significant reduction in presurgical exosome levels after surgery. Patients experiencing relapse showed higher postsurgical exosome concentrations and larger sizes compared to disease-free individuals. Lower presurgical exosome levels were correlated with better disease-free survival. Receiver operating characteristic analysis indicated the promising diagnostic efficacy of presurgical exosome concentrations in identifying relapse (AUC = 0.82). The findings suggest that presurgical exosomal plasma levels independently predict early recurrence and survival in OSCC, proposing peripheral exosome detection as a novel tool for clinical management with implications for prognosis assessment.
Abstract
Background: Oral squamous cell carcinoma (OSCC) is characterized by an immunosuppressive tumor microenvironment. Their plasma-derived exosomes deliver immunomodulatory molecules and cargo that correlate significantly with clinical parameters. This study aims to assess the exosomal profile as a potential tool for early detection of relapse and long-term outcomes in OSCC patients undergoing conventional therapy. Methods: 27 OSCC patients with a median 38-month follow-up were included in this study. The relationship between NTA-derived parameters and clinical pathological parameters was examined, and receiver operating characteristic (ROC) curves were utilized to evaluate the diagnostic efficacy of these values in detecting cancer relapse. Results: Plasmatic levels of exosomes prior to surgery showed a drastic reduction after surgical intervention (8.08E vs. 1.41 × 109 particles/mL, p = 0.006). Postsurgical concentrations of exosomes were higher in patients who experienced relapse compared to those who remained disease-free (2.97 × 109 vs. 1.11 × 109 particles/mL, p = 0.046). Additionally, patients who relapsed exhibited larger exosome sizes after surgery (141.47 vs. 132.31 nm, p = 0.03). Patients with lower concentrations of exosomes prior to surgery demonstrated better disease-free survival compared to those with higher levels (p = 0.012). ROC analysis revealed an area under the curve of 0.82 for presurgical exosome concentration in identifying relapse. Conclusions: Presurgical exosomal plasmatic levels serve as independent predictors of early recurrence and survival in OSCC. All in all, our findings indicate that the detection of peripheral exosomes represents a novel tool for the clinical management of OSCC, with potential implications for prognosis assessment.
1. Introduction
Oral squamous cell carcinoma (OSCC) is an aggressive form of cancer that represents a growing public health problem, globally accounting for 377,713 new cases and 48,143 deaths annually [1]. Even with relevant progress in surgical treatment, chemoradiotherapy, radiotherapy, and immunotherapy, the OSCC patients’ overall survival remains low as compared to other tumors [2,3]. Risk factors for OSCC are broadly classified into three categories: chemical, physical, and viral mechanisms [4]. However, from a molecular perspective, OSCC exhibits several hallmarks of cancer, such as sustained proliferative signaling, evasion of growth suppressors, resistance to cell death, and the ability to induce angiogenesis, invasion, and metastasis. Additionally, OSCC cells exhibit deregulated cellular energetics, a high level of immune escape associated with tumor-promoting inflammation, and rates of both genome instability and mutations [5,6].
Tissue biopsy is the gold standard for the diagnosis of oral cancer as well as all solid tumors. Liquid biopsies have the potential to help in identifying molecular features related to tumor behavior, such as invasive capacity, relapse rate, and metastasis. This technique is being used with increasing force in other types of cancers of the digestive system [7]. Until a decade ago, liquid biopsy was confined to circulating tumor cells and nucleic acids, either DNA or RNA. Although today they continue to be useful for the prognosis of oral squamous cell carcinoma [8]. Circulating exosomes are emerging as a very promising new noninvasive tool for the clinical management of tumor patients [9,10,11].
Exosomes are nanosized extracellular vehicles having origins in the re-cycling of intracellular vesicles. They have demonstrated that they exert a key role in intercellular communication in both physiological and pathological scenarios [12,13,14,15]. Exosomes are the natural cargo vesicles for proteins, DNAs, RNAs, lipids, and metabolites that are essential in a myriad of physiological and pathological functions [16,17]. Exosomes play a Janus-faced role in head and neck cancers [18,19,20,21]. It has been shown that exosomes can deliver tumor-associated antigens to dendritic cells, inducing anticancer immunity, preserving homeostasis, and playing cytoprotective roles [22]. Conversely, recent clinical data have shown that a distinguishing and relevant hallmark of cancer patients is the increased plasmatic exosome levels that are not tumor-specific, being common in many cancers but distinguishing cancer from a healthy condition [23,24,25]. Exosome cargo is primarily involved in tumor progression and metastasis [26,27]. Exosomes are also involved in multidrug resistance, being responsible for the extracellular elimination of chemotherapeutics [28,29,30] in the epithelial–mesenchymal transition and in extracellular matrix remodeling [31]. On the other hand, and as a consequence of the aforementioned, it is crucial to highlight the role of exosomes as an oral delivery vehicle for cancer therapies. Various reviews debate and analyze strategies in which the use of exosomes has yielded encouraging results in exploring diagnostic and treatment options for various types of cancer in humans [32]. Notably, tumor exosomes have a key role in the development of metastasis in both setting a sort of “premetastatic niche” and in transformingmesenchymal stem cells (MSC), contained in target organs, into tumor-like cells [33,34]. Similarly, in the opposite direction, recent studies have indicated a crucial involvement of exosomes derived from MSC-derived exosomes in conferring resistance to chemotherapy agents, targeted therapy drugs, radiotherapy, and immunotherapy in cancer [35,36]. Therefore, the presence of a high level of tumor exosomes in the plasma of tumor patients is a sign that predicts the development of metastasis.
Recently, a pilot clinical study from our group showed different levels of plasmatic exosomes in patients with OSCC before and after surgical treatment. In fact, our study showed a dramatic reduction in the plasmatic levels of exosomes expressing CD63 and of the surrogate tumor marker Caveolin-1 (cav-1) after ablative surgery [23]. This previous study used a pioneering method to analyze and characterize exosomes by means of immunocapture-based ELISA previously reported by our group and further validated by benchmarking against two existing gold standards, namely nanoparticle tracking analysis (NTA) and nanoscale flow cytometry [23,37], and summarized it as a technique in a recent article [38]. The potential use of exosomes as a natural delivery for anticancer molecules is under investigation [15,25]. However, much still needs to be understood about the source of exosomes to deliver therapeutic molecules. The use of human exosomes has raised many problems, and at the moment, they are not allowed for clinical use. An interesting new approach could be the use of plant-derived exosomes, particularly those derived from organic agriculture, that are entirely free of toxic molecules [39]. Moreover, very recently, the ability of plant-derived exosomes to exert clear antitumor activity has been shown [40]. The ensemble of these reports highly supports the revolutionary role of exosomes in the clinical management of tumor patients.
Building upon these preliminary results, we embarked on a prospective cohort study aiming to assess the clinical relevance of plasmatic exosome number and size distribution using NTA in a larger cohort with an extended follow-up period to refine our estimates.
2. Materials and Methods
2.1. Study Design
This study was a prospective cohort study aimed at assessing the population of exosomes before and after surgery and their correlation with the long-term outcomes of patients. The study protocol was approved by the Clinical Research Ethics Committee of Galicia under ethical code 2018/435, and it was designed in accordance with the STROBE recommendations [41]. All procedures were conducted in accordance with the Helsinki Declaration and its subsequent modifications, with the full understanding and written consent of all participants. No identifying data were recorded to ensure the anonymity of participants.
2.2. Patient Selection and Clinical Data
This study included 27 subjects who were prospectively recruited and diagnosed with OSCC. The inclusion criteria were as follows: patients over the age of 18, of both genders, diagnosed with OSCC in stages T1–T4, and who underwent tumor resection and lymph node resection surgery if necessary. The follow-up period started in 2014 and lasted until mid-2022. The exclusion criteria consisted of patients with tumors of any other origin in any part of their body, immunological diseases, a previous history of radiotherapy or chemotherapy treatment, or those using proton pump inhibitors or any other antacid drug.
Each patient underwent a comprehensive clinical examination, including a medical history assessment, detailed clinical assessment, tumor biopsy, complementary radiology techniques, and sentinel lymph node evaluation. Staging was performed according to the 8th edition of the American Joint Committee on Cancer’s Cancer Staging Manual. The established protocol of the University Hospital of Santiago de Compostela (Santiago de Compostela, Corunna, Spain) was followed [41,42]. The decision to perform surgery was made by the maxillofacial surgeon based on a comprehensive examination and deliberation by the CHUS committee for tumors.
Patients were followed up for an average of 38.56 ± 23.36 months. During this period, data on clinicopathological features (i.e., tumor location, TNM stating, T status, N status, presence of local metastasis, and tumor differentiation), relapse, overall survival (OS), and disease-free survival (DFS) were collected.
2.3. Sample Collection and Plasmatic Exosomes Characterization and Quantification
Before surgery, two plasma tubes of 1 mL each were obtained from each patient and stored at −80 degrees Celsius until transfer to the molecular oncology laboratory of the Istituto Superiore di Sanità (Rome, Italy). The same procedure was repeated 7 days after surgery [21].
To obtain plasma from blood samples, EDTA-treated blood from patients was centrifuged at 400× g for 20 min. Plasma was collected and stored at −80 °C until analysis. Upon thawing, 1 mL of plasma underwent a centrifugal procedure to remove cell debris, organelles, microvesicles, and pellet exosomes. In the final step, plasma samples were centrifuged for 1 h and 30 min at 110,000× g using a Fiberlite™ F50L-24 × 1.5 fixed-angle rotor, K-Factor: 33 (Thermo Fisher Scientific, Waltham, MA, USA) in the Sorvall WX Ultracentrifuge Series (Thermo Fisher Scientific) to obtain the exosomal pellet, which was then washed in PBS and resuspended in an appropriate buffer for subsequent analysis. Specifically, the exosomal pellet was resuspended in PBS forNTA.
NTA from Malvern (NanoSight NS300, Worcestershire, UK) was used to measure the size distribution and concentration of exosome samples in liquid suspension in the range of 10–1000 nm based on the analysis of Brownian motion [43]. After laser beam illumination, light scattering allowed for the visualization, recording, and tracking of particles with a CCD or CMOS camera. Five videos of typically 60 s duration were taken. Data were analyzed using the NTA 3.0 software (Malvern Instruments, Malvern, UK), which was optimized to detect and track each particle on a frame-by-frame basis. NTA is based on the phenomenon of the random movement (diffusion) of small particles when dispersed in a liquid, enabling direct and precise measurement of particle concentration and size. The Brownian motion of each particle was tracked using the Stokes–Einstein equation: D° = kT/6πηr, where D° is the diffusion coefficient, kT/6πηr = f0 is the frictional coefficient of the particle, for the special case of a spherical particle of radius r moving with a uniform velocity in a continuous fluid of viscosity η, k is Boltzmann’s constant, and T is the absolute temperature.
The evaluation of the particle size distribution (PSD) was performed using the parameters mean, mode, and standard deviation (SD), which indicate the average, most frequent particle class size, and variability of the analyzed particles, respectively. The same statistical descriptors were adopted to summarize the number of particle distributions.
2.4. Statistical Analysis
The statistical analysis was performed using patients affected by OSCC as basic units. Data were analyzed using IBM SPSS Statistics 20.4 (SPSS Inc., Chicago, IL, USA) and R v.4.1.0 software. Descriptive statistics were reported for each variable based on its distribution, determined by the Shapiro–Wilk test. Gaussian distributed variables were reported as mean ± SD with 95% confidence intervals (CIs), while continuous non-normally distributed variables were reported as median (interquartile range). Categorical, normally distributed variables were reported as percentages.
Given the inherent pairing of the data, involving observations before and after surgery, inferential tests were conducted in their corresponding mode. A paired t-test (or equivalently, a Mann–Whitney or Chi-square test) was used to compare pre- and postsurgery measurements. Univariate survival analysis was conducted using Kaplan–Meier and log-rank tests for both OS and DFS, which were used to compute hazard ratios (HRs). Multinomial logistic regression models were constructed to determine the risk of relapse based on exosome quantification and characterization. The cutoff point for classifying patients as having a high or low concentration and size of exosomes was based on the arithmetic mean of these values, thus considering these values per patient as over or underexpressed.
The best discriminant cut-off thresholds of the mean presurgical and postsurgical exosomal concentrations, as well as their difference, to predict OSCC relapse, were computed using a receiver operating characteristic (ROC) curve. A bootstrap resampling method (k = 1000) was used for output. The criterion chosen for model selection was the Akaike information criterion to limit the possibility of collinearity effects [44]. A logarithmic transformation of exosome concentrations was used for these analyses due to the violation of normality in the residuals of the regression model using raw data at the lower and upper tails.
To address the possibility of residual confounding by age on presurgical exosome concentration and PSD, a Pearson correlation was performed separately for cases with and without cancer recurrence. No statistically significant correlations were found (Figure S1). A p-value of 0.05 was considered statistically significant for all tests.
3. Results
3.1. Sample Description
The sample for this study consisted of 27 patients, with 14 males and 13 females. The mean age of the patients was 70.35 (15.79) years. All the tumors included in this study were from the oral cavity, with six located in the gingiva, five in the tongue, and six in the hard palate, which were the most frequent locations. Among the patients, 11 were smokers, with men smoking an average of 18 ± 16.7 cigarettes compared to women who smoked an average of 5.3 ± 9.7 cigarettes (p = 0.024). In terms of tumor stage, 15 patients were diagnosed in the initial stages (I and II), while 12 patients were diagnosed in the advanced stages (III and IV). Most of the tumors (n = 14) exhibited moderate differentiation. For detailed information, refer to Table 1.

Table 1.
Characteristics of the studied population.
3.2. Concentration and Size Distribution of Plasmatic Exosomes
The concentration of exosomes was evaluated both presurgically and after oncological surgery. The mean presurgical exosome concentration was 1.02 × 109 ± 5.89 × 108 particles/mL, ranging from 1 × 108 to 2 × 109 particles/mL. The mean postsurgical exosome concentration was 1.76 × 109 ± 1.98 × 109 particles/mL, ranging from 3 × 108 to 9 × 109 particles/mL. Regarding exosome size, the presurgical distribution had a mean of 133.57 ± 17.46 nm, ranging from 107.1 to 171.7 nm. The postsurgical distribution had a mean of 125.52 ± 10.36 nm, ranging from 111.2 to 154 nm.
While there was a positive correlation between pre- and postsurgical concentrations, the correlation did not reach statistical significance (r = 0.398; p = 0.091). In the overall analysis, no significant differences were observed in the concentrations (p = 0.152) or dimensions (p = 0.933) of exosomes before and after surgery (Figure 1). However, when analyzing the sample based on relapse, it was observed that patients who experienced relapse had higher concentrations of exosomes presurgically but a significant reduction in exosome number after surgery (8.08 × 108 [95%CI 5.52 × 108–1.06 × 109] vs. 1.41 × 109 [95%CI 9.77 × 108–1.85 × 109] particles/mL, p = 0.006) (Table S1).
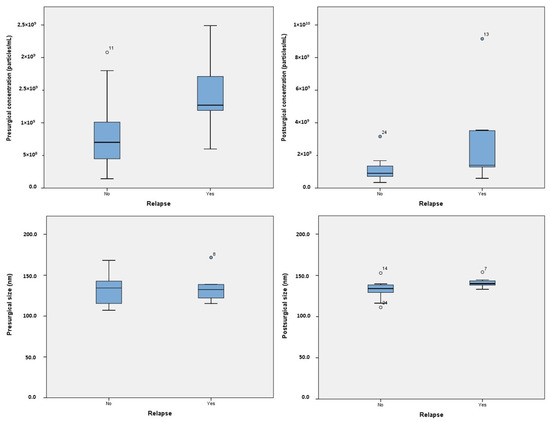
Figure 1.
Boxplot representing the exosome levels and sizes, both presurgical and postsurgical.
A violin plot illustrating the distribution of presurgical exosome concentration in patients with and without relapse is presented in Figure 2. Postsurgical concentrations were statistically higher in patients who experienced relapse (2.97 × 109 [95%CI 2.3 × 108–5.7 × 108] vs. 1.11 × 109 [95%CI 6.66 × 108–1.55 × 109] particles/mL, p = 0.046). In terms of dimensions, patients who experienced relapse had larger postsurgical exosome sizes (141.47 nm [95%CI 135.39–147.55] vs. 132.31 nm [95%CI 125.78–138.84], p = 0.03). No significant differences in exosomal concentration or size were found based on gender, tumor location, or tobacco use.
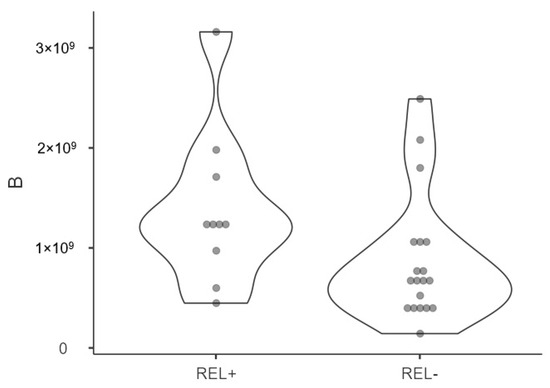
Figure 2.
Violin plot displaying NTA distribution and quantification of patients affected by relapse (REL+) and not (REL-) of presurgical plasmatic exosome concentrations (nm) showing density probability with kernel smoothing.
3.3. Survival Analysis
The mean follow-up time for this study was 38.56 ± 23.26 months. DFS was 26.44 ± 26.46 months, while the OS was 18.22 ± 11.71 months. Kaplan–Meier survival models were constructed for DFS and OS (Table S2). Patients with low concentrations of exosomes prior to surgery had a significantly higher DFS (92.49 [95%CI 61.06–123.93] months) compared to patients with high levels (41.42 [95%CI 24.31–58.53] months, p = 0.012). Refer to Figure 3 for the corresponding survival curves.
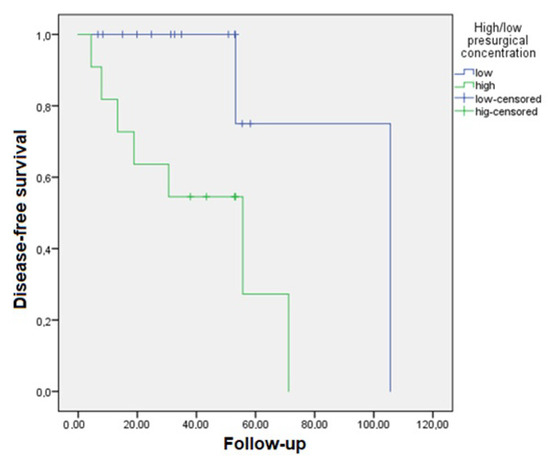
Figure 3.
Kaplan–Meier curve for disease-free survival in patients with a lower presurgical plasmatic exosome concentration (particles/mL) who survived longer than those with higher concentrations (p = 0.012).
3.4. Relapse Analysis
Multinomial logistic regression models were constructed to assess the risk of recurrence associated with different parameters of plasmatic exosomes and clinicopathological variables. It should be noted that the tumor stage and related variables did not yield the most optimal models. Despite the direct association between stage as N status and DFS and OS, incorporating these variables into the models did not enhance the estimations for the compute. The most accurate models included adjustments for gender (Table 2). The results indicated that patients with elevated presurgical plasma levels of exosomes had increased risk of recurrence, both in the univariate model (HR = 11.37 [95%CI 1.65–78.37], p = 0.014) and the adjusted model (HR = 18.35 [95%CI 1.61–208.35], p = 0.019). The minimum and maximum log10 exosome concentration values, both before and after surgery, and their differences were analyzed to establish an approximate range for patients with relapse compared to those who remained disease-free at the end of the follow-up period. ROC analysis for presurgical exosome concentration yielded an area under the curve (AUC) of 0.82 for identifying OSCC relapse (Figure 4A), with a sensitivity of 50.0% and a specificity of 91.7% (Figure 4B). For postsurgical concentration, the AUC was 0.79 (Figure 4C), with a sensitivity of 33.3% and a specificity of 88.2% (Figure 4D). The AUC for the difference in exosome concentrations before and after surgery was 0.665 (Figure 4E), with a sensitivity of 0% and a specificity of 100% (Figure 4F).

Table 2.
Relapse risk according to low/high concentrations of exosomes.
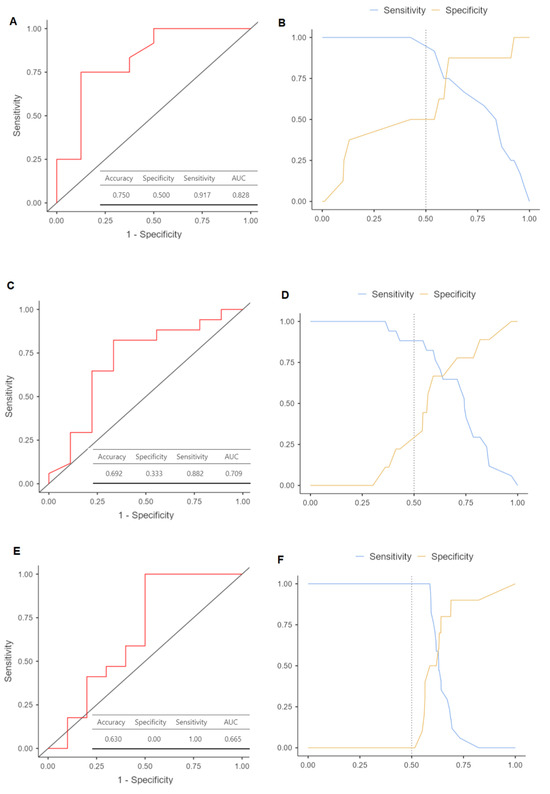
Figure 4.
Receiver operating characteristic (ROC) curves to predict oral squamous cell carcinoma relapse based on log10 levels of plasmatic exosome concentrations: (A) ROC plot with its area under the curve (AUC) for presurgical exosomes (p = 0.012); (B) cut-off plot representing the intersection that allows maximizing the level of sensitivity and specificity of the model used; (C) ROC plot with its AUC for postsurgical exosomes (p = 0.035); (D) cut-off plot representing the sensitivity and specificity of the model; (E) ROC plot with its AUC for the difference of concentrations between pre- and postsurgial exosomes (p = 0.043); (F) cut-off plot representing the sensitivity and specificity of the model.
4. Discussion
In a comprehensive study involving 27 patients, the concentration and size distribution of plasmatic exosomes in OSCC were extensively explored. Through meticulous quantitative analysis, the mean presurgical exosome concentration was identified as 1.02 × 109 ± 5.89 × 108 particles/mL, while the postsurgical concentration averaged 1.76 × 109 ± 1.98 × 109 particles/mL. Intriguingly, a positive correlation was observed between pre- and postsurgical concentrations, with a significant reduction in exosome number after surgery for patients experiencing relapse. Survival analysis revealed compelling results, indicating a substantial impact of exosome concentrations on disease-free and overall survival. These findings not only contribute valuable insights into OSCC prognosis but also underscore the potential significance of exosome concentrations in predicting relapse, offering avenues for further exploration in cancer research.
The current gold standard for early detection of OSCC is tissue biopsy and subsequent histopathological analysis. Moreover, tumor staging classification according to the TNM system is mandatory for treatment decision-making and prognosis assessment [45,46]. However, it lacks the ability to reliably predict the biological characteristics of tumors, rendering it of minimal significance when determining treatment approaches influenced by the tumor’s biological behavior [47].
The trigger for this study stemmed from the observation that, despite the increasing number of potentially useful biomarkers for OSCC, significant challenges remain in their clinical translation [48,49]. Traditionally, tissue-based biomarkers, particularly immuno-histochemical analysis, have dominated this field [50,51,52].
This study contributes to the growing body of evidence supporting the utility of exosomes as new noninvasive diagnostic/prognostic tools for OSCC [53,54,55,56,57]. In the case of head and neck cancer, Bergmann et al. were the first to reveal the capacity of serum exosomes to induce T-cell apoptosis, linking this molecular machinery with poor locoregional control and the presence of distant metastases [58]. Exosomes in liquid biopsies capture the dynamic landscape of oral neoplasms, making them potential vehicles for OSCC biomarkers [59]. Additionally, in the context of these solid neoplasms, exosome cargo has proven abilities to create a particular hypoxic tumor microenvironment, enhancing tumor aggressiveness and metastasic potential [60].
Here, through the implementation ofNTA, plasmatic exosome ability proved to be linked with the survival and clinicopathological characteristics of OSCC patients. A lack of correlation in terms of age between subgroups was preliminarily demonstrated [61]. Consequently, the data were analyzed to stratify patients based on both the number and size of peripheral exosomes and their clinicopathological and long-term outcomes.
This investigation has revealed a noteworthy association between pre- and postsurgical exosome levels and a statistically significant risk of disease recurrence, as previously reported [62,63,64,65]. Interestingly, a study comparing disease-free intervals in ovarian cancer patients reported a negative correlation with cav-1 exosomes, revealing a paradoxical relationship between exosome expression and cancer prognosis [66]. Jiang et al. have recently confirmed these phenomena in liver tumors [67]. On the other hand, multiple studies support the notion that the surrogate marker used in our pilot study, i.e., cav-1 overexpression, is directly related to disease progression and has an outstanding predictive ability to stratify affected patients in terms of prognosis, distinguishing it from other tumor types [68,69]. Moreover, a correlation between patient survival and the number of exosomes prior to surgery was ascertained, consistent with our preliminary study. Other studies have attempted to investigate the exosome cargo related to this nexus in OSCC. He et al. demonstrated the greatest power of a reduced concentration of plasmatic exosomes prior to surgery in predicting the absence of recurrence through microRNA-130a exosome cargo [70]. Deng et al., examining peripheral concentrations of exosome hsa_circRNA_047733 in OSCC, created a dynamic nomogram integrating five other clinical variables to predict recurrence with a powerful diagnostic yield (AUC = 0.87) [71]. On the other hand, several in vitro and in vivo reports have described various molecular pathways related to the effect of exosomes in OSCC, such as the p38, Akt, SAPK/JNK, Wnt/β-catenin, or ERK1/2 signaling pathways [72,73,74]. Moreover, in vitro research on OSCC cell lines has shown the ability of certain exosome cargo to disrupt the proper function of some cells. In this vein, Wang et al. showed that exosomal NAP1 enhances the cytotoxicity of natural killer cells [75]. Pan et al. showed that oral cancer cell-secreted exosomal CMTM6 induced M2-like macrophage polarization as PD-L1 expression [74].
Regarding exosome size, measured in nanometers, our findings have revealed a significant correlation between larger exosome size following surgical intervention and disease recurrence in affected patients. This observation is particularly intriguing given the growing body of literature highlighting the role of exosome content in promoting tumor progression through diverse mechanisms [76,77,78]. In this vein, Deng et al. demonstrated an association between STIM1, a protein involved in tumor angiogenesis, and larger EBV-LMP1-containing exosomes using nasopharyngeal carcinoma as a model [74].
The relapse analysis showed a more than 10 times greater probability of disease recurrence in those patients with a higher concentration of exosomes prior to surgery. ROC curve analysis, particularly for exosome concentration prior to surgery, demonstrated a significant discriminative value in this study, with an AUC of 0.82, a sensitivity of 50.0%, and a specificity of 91.7%. Tests examining diagnostic yield confirmed that the measurement of presurgical exosome concentration was an effective method for prospectively distinguishing between OSCC patients affected by relapse or not after conventional treatment. This corroborates data from a previous study by Li et al., although in our case, a superior AUC was obtained, probably due to the exosome analysis being built from a different pipeline based on ELISA analysis [79].
He et al. obtained similar results when investigating plasma-derived exosome microRNA-130a, inferring through a multivariate analysis that patients with high levels of these extracellular vesicles had a value nearly threefold higher than those with low levels in terms of overall survival [70]. Ju et al. also verified that the relapse-free survival time of tongue carcinoma patients with low and high expression of exosomes derived from LINC00152 was significantly higher in patients with a lower exosome concentration [80]. This study significantly contributes to the clinical application of plasma exosome measurements in the follow-up of OSCC patients. Moreover, this prospective clinical study is the first one in OSCC comparing the plasma exosome levels before and after surgery, providing a significant relationship with exosomes both before and after surgery and relapse risk, time free of disease, and life expectancy of patients for nearly 5 years. An interesting finding was that exosome parameters returned to normal shortly after ablative surgery, aligning with a previous effort of our group related to Hsp60-related exosomes in bowel cancer [81]. The data presented herein encourages the belief that exosome concentration is a candidate for theranostics in OSCC.
Based on clinical evidence, it was observed that variation in exosome levels represents a tumor biomarker that enables the prediction of patient outcomes, mandatorily needing closer follow-up in patients with higher plasmatic levels [82,83]. Furthermore, the detection of a possible recurrence can be identified in a timely manner. The term “circulating tumor mass”, previously discussed in the pilot article, is becoming increasingly relevant due to its practical association with disease prognosis [84,85,86]. Previous studies have correlated the increased plasmatic levels of exosomes in tumor patients to the acidic microenvironment [37,87,88].
As limitations of our study and in acknowledging potential confounding issues related to cancer progression, metastases, and therapeutic interventions, we believe in the need to conduct broader studies in the future. However, due to the scale of our study, there is a risk of overfitting or limited stratification. In this context, the identified confounding factors may diminish the already limited statistical power of this study, steering the results in a conservative direction and increasing the difficulty of achieving significance. It is crucial to recognize that, in a naturalistic setting, a prognostic marker must withstand variations in contextual conditions.
In summary, the findings from this study have great potential in cancer clinics by implementing current clinical indicators of tumor progression such as tumor size, overall survival, and disease-free interval. From these results, it appears conceivable that measuring exosome plasmatic levels before tumor resection may help in determining the best surgical approach.
5. Conclusions
This study sheds light on the potential use of exosomes as a noninvasive biomarker for OSCC patients. Surgical treatment was found to induce a significant reduction in plasma levels of exosomes, and high concentrations of exosomes were associated with an increased risk of disease recurrence. On the other hand, patients with low concentrations of exosomes prior to surgery demonstrated better survival.
These findings have important clinical implications, as they provide a potential new strategy for improving the clinical follow-up of OSCC patients. The noninvasive nature of exosome quantification and characterization makes it a valuable tool for monitoring disease progression and detecting recurrence in a timely manner, thus hopefully improving patient prognosis. However, further studies are needed to confirm the results of this study and explore the potential clinical applications of exosomes as a tumor biomarker for OSCC. This study emphasizes the importance of characterizing and quantifying exosomes in tumor patients’ body fluids, most of all in the plasma [89]. Of course, larger multicenter clinical studies are mandatory in order to validate the results of this study in a larger number of patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15235693/s1, Figure S1: Correlation between age (years) and A serum exosomes concentration (particles/mL); B exosomal size (nm); Table S1: Results in relapse and non-relapse patients; Table S2: Survival models by Kaplan Meier for disease free survival (DFS) and overall survival (OS).
Author Contributions
All the authors contributed to the preparation of this manuscript. A.G.-G. performed the tumor resection surgery and collected blood samples. S.F. and M.A.L. performed the laboratory analysis and collected data. A.I.L.-P., A.G. and M.P.-S. performed the statistical analysis. S.R.-Z., A.I.L.-P., M.P.-S. and S.F. wrote the article. D.M., R.D.R., A.P.-J., K.L.O. and Á.M.-G. were responsible for correcting the text. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the Clinical Research Ethics Committee of Galicia under code 2018/683.
Informed Consent Statement
Informed consent for publication was obtained from all participants.
Data Availability Statement
The data will be shared by requesting the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.M.; Reade, P.C. Mechanisms of carcinogenesis with particular reference to the oral mucosa. J. Oral Pathol. Med. 1988, 17, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.Q.; Inchakalody, V.P.; Mestiri, S.; Yoosuf, Z.S.K.; Bedhiafi, T.; El-Ella, D.M.A.; Taib, N.; Hydrose, S.; Akbar, S. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 99. [Google Scholar] [CrossRef]
- Balachander, K.; Roy, A.; Priyadharsini, J.V.; Murugan, S.; Paramasivam, A. Mitochondrial DNA in circulating exosomes: A novel biomarker and potential therapeutic target for oral cancer. Oral Oncol. 2022, 128, 105857. [Google Scholar] [CrossRef]
- Tadimety, A.; Closson, A.; Li, C.; Yi, S.; Shen, T.; Zhang, J.X. Advances in liquid biopsy on-chip for cancer management: Technologies, biomarkers, and clinical analysis. Crit. Rev. Clin. Lab. Sci. 2018, 55, 140–162. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, R.; Li, Z.; Li, J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017, 8, 55632. [Google Scholar] [CrossRef]
- Mason, T.E.; Ricks-Santi, L.; Chen, W.; Apprey, V.; Joykutty, J.; Ahaghotu, C.; Kittles, R.; Bonney, G.; Dunston, G.M. Association of CD14 variant with prostate cancer in African American men. Prostate 2010, 70, 262–269. [Google Scholar] [CrossRef]
- Li, Y.; Gao, S.; Hu, Q.; Wu, F. Functional Properties of Cancer Epithelium and Stroma-Derived Exosomes in Head and Neck Squamous Cell Carcinoma. Life 2022, 12, 757. [Google Scholar] [CrossRef]
- Shi, S.; Yu, Z.; Jia, J. The Roles of Exosomes in the Diagnose, Development and Therapeutic Resistance of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 1968. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; O’Driscoll, L.; Borras, F.E.; Buzas, E.; Camussi, G.; Cappello, F.; Carvalho, J.; Cordeiro da Silva, A.; Del Portillo, H.; El Andaloussi, S.; et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 2016, 10, 3886–3899. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C.; Biology, D. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Gong, H.; Luo, S.; Cui, Y. The role of exosomes and their applications in cancer. Int. J. Mol. Sci. 2021, 22, 12204. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.; Tang, J.; Shiau, J.; Yen, C.; Chang, F.; Yang, K.; Hou, M.; Farooqi, A.A.; Chang, H. Modulating Effects of Cancer-Derived Exosomal miRNAs and Exosomal Processing by Natural Products. Cancers 2023, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Guo, J.; Fan, Z.; Yang, S.; Zhang, C.; Cheng, B.; Xia, J. Exosomal miR-146b-5p derived from cancer-associated fibroblasts promotes progression of oral squamous cell carcinoma by downregulating HIPK3. Cell. Signal. 2023, 106, 110635. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiao, P.; Wang, Z.; Chen, M.; Du, H.; Xu, L.; Xu, J.; Dai, Y.; Wu, F.; Zhang, Y. Endoplasmic reticulum stress promotes the release of exosomal PD-L1 from head and neck cancer cells and facilitates M2 macrophage polarization. Cell Commun. Signal. 2022, 20, 12. [Google Scholar] [CrossRef]
- Rodríguez Zorrilla, S.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Gallas Torreira, M.; García García, A. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Logozzi, M.; De Milito, A.; Lugini, L.; Borghi, M.; Calabrò, L.; Spada, M.; Perdicchio, M.; Marino, M.L.; Federici, C.; Iessi, E.; et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 2009, 4, e5219. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Giuliani, A.; Maggi, M.; Sciarra, A.; Fais, S. Plasmatic exosome number and size distinguish prostate cancer patients from healthy individuals: A prospective clinical study. Front. Oncol. 2021, 11, 4258. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Fais, S. Extracellular vesicles in cancer pros and cons: The importance of the evidence-based medicine. Semin. Cancer Biol. 2022, 86, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef]
- Słomka, A.; Kornek, M.; Cho, W.C. Small Extracellular Vesicles and Their Involvement in Cancer Resistance: An Up-to-Date Review. Cells 2022, 11, 2913. [Google Scholar] [CrossRef]
- Khan, M.I.; Alsayed, R.K.; Choudhry, H.; Ahmad, A. Exosome-mediated response to cancer therapy: Modulation of epigenetic machinery. Int. J. Mol. Sci. 2022, 23, 6222. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.; Goh, B. Exosome-mediated metastasis: From epithelial–mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, R.C. Exosomes in cancer therapy. Cancers 2022, 14, 500. [Google Scholar] [CrossRef]
- Xiang, X.; Poliakov, A.; Liu, C.; Liu, Y.; Deng, Z.; Wang, J.; Cheng, Z.; Shah, S.V.; Wang, G.; Zhang, L.; et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 2009, 124, 2621–2633. [Google Scholar] [CrossRef]
- Lugini, L.; Sciamanna, I.; Federici, C.; Iessi, E.; Spugnini, E.P.; Fais, S. Antitumor effect of combination of the inhibitors of two new oncotargets: Proton pumps and reverse transcriptase. Oncotarget 2017, 8, 4147. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. Meth Enzymol. 2020, 645, 155–180. [Google Scholar]
- Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Logozzi, M.; Fais, S. Purposing plant-derived exosomes-like nanovesicles for drug delivery: Patents and literature review. Expert Opin. Ther. Pat. 2023, 33, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Cerio, A.; Dolo, V.; Pasquini, L.; Screnci, M.; Ottone, T.; Testa, U.; et al. Ex Vivo Anti-Leukemic Effect of Exosome-like Grapefruit-Derived Nanovesicles from Organic Farming-The Potential Role of Ascorbic Acid. Int. J. Mol. Sci. 2023, 24, 15663. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strobe Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Ajuria, M.; García-García, A.; Suárez-Peñaranda, J.M.; Garrido-Pumar, M.; Chamorro-Petronacci, C.M.; Somoza-Martín, J.M.; Pérez-Sayáns, M. Analysis of the Efficiency and Prognostic Value of the Sentinel Node Technique in Oral Squamous Cell Carcinoma after Seven Years. Medicina 2021, 57, 1092. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, M.H.; Kim, S.H.; Lee, G.; Song, D.H. Prognostic role of extracellular vesicles in squamous cell carcinoma of the lung. Thorac. Cancer 2020, 11, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Heckler, C.E. Applied Multivariate Statistical Analysis. Technometrics 2005, 47, 517. [Google Scholar] [CrossRef]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Muzio, L.L.; Santarelli, A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th Edition oral cavity squamous cell carcinoma staging–Is it an improvement on the AJCC 7th Edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef]
- Farah, C.S. Molecular landscape of head and neck cancer and implications for therapy. Ann. Transl. Med. 2021, 9, 915. [Google Scholar] [CrossRef]
- Rivera, C.; Oliveira, A.K.; Costa, R.A.P.; De Rossi, T.; Leme, A.F.P. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral Oncol. 2017, 72, 38–47. [Google Scholar] [CrossRef]
- Gleber-Netto, F.O.; Braakhuis, B.J.; Triantafyllou, A.; Takes, R.P.; Kelner, N.; Rodrigo, J.P.; Strojan, P.; Vander Poorten, V.; Rapidis, A.D.; Rinaldo, A. Molecular events in relapsed oral squamous cell carcinoma: Recurrence vs secondary primary tumor. Oral Oncol. 2015, 51, 738–744. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; López-Ansio, M.; Ramos-García, P. Hallmarks of Cancer Applied to Oral and Oropharyngeal Carcinogenesis: A Scoping Review of the Evidence Gaps Found in Published Systematic Reviews. Cancers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Søland, T.M.; Brusevold, I.J. Prognostic molecular markers in cancer–quo vadis? Histopathology 2013, 63, 297–308. [Google Scholar] [CrossRef]
- Peterson, E.B.; Chou, W.S.; Gaysynsky, A.; Krakow, M.; Elrick, A.; Khoury, M.J.; Kaphingst, K.A. Communication of cancer-related genetic and genomic information: A landscape analysis of reviews. Transl. Behav. Med. 2018, 8, 59–70. [Google Scholar] [CrossRef]
- Bano, A.; Vats, R.; Yadav, P.; Bhardwaj, R. Exosomics in Oral Cancer Diagnosis, Prognosis, and Therapeutics-An emergent and imperative non-invasive natural nanoparticle-based approach. Crit. Rev. Oncol. 2022, 178, 103799. [Google Scholar] [CrossRef]
- Li, S.; Man, Q.; Gao, X.; Lin, H.; Wang, J.; Su, F.; Wang, H.; Bu, L.; Liu, B.; Chen, G. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 2021, 10, e12175. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Patil, K.; Ramm, G.A.; Ochiya, T.; Soekmadji, C. Extracellular vesicles in the development of organ-specific metastasis. J. Extracell. Vesicles 2021, 10, e12125. [Google Scholar] [CrossRef] [PubMed]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Fais, S.; Iero, M.; Lugini, L.; Canese, P.; Squarcina, P.; Zaccheddu, A.; Colone, M.; Arancia, G.; Gentile, M. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology 2005, 128, 1796–1804. [Google Scholar] [CrossRef]
- Bergmann, C.; Strauss, L.; Wieckowski, E.; Czystowska, M.; Albers, A.; Wang, Y.; Zeidler, R.; Lang, S.; Whiteside, T.L. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck 2009, 31, 371–380. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kalluri, R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef]
- Ye, B.; Duan, Y.; Zhou, M.; Wang, Y.; Lai, Q.; Yue, K.; Cao, J.; Wu, Y.; Wang, X.; Jing, C. Hypoxic tumor-derived exosomal miR-21 induces cancer-associated fibroblast activation to promote head and neck squamous cell carcinoma metastasis. Cell. Signal. 2023, 108, 110725. [Google Scholar] [CrossRef]
- Xiao, C.; Song, F.; Zheng, Y.L.; Lv, J.; Wang, Q.F.; Xu, N. Exosomes in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Medyany, V.; Ezić, J.; Lotfi, R.; Niesler, B.; Röth, R.; Engelhardt, D.; Laban, S.; Schuler, P.J.; Hoffmann, T.K.; et al. Cargo and Functional Profile of Saliva-Derived Exosomes Reveal Biomarkers Specific for Head and Neck Cancer. Front. Med. 2022, 9, 2030. [Google Scholar] [CrossRef]
- Lou, C.; Shi, J.; Xu, Q. Exosomal miR-626 promotes the malignant behavior of oral cancer cells by targeting NFIB. Mol. Biol. Rep. 2022, 49, 4829–4840. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Oliveira, C.; Dourado, M.; Macedo, C.; Winck, F.; Paes Leme, A.; Salo, T.; Coletta, R.; Almeida Freitas, R.; Galvão, H. Extracellular vesicles from oral squamous carcinoma cells display pro- and anti-angiogenic properties. Oral Dis. 2018, 24, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, H.; Zhu, Y.; Chen, X.; Chen, Y. Plasma exosomal caveolin-1 predicts Poor Prognosis in Ovarian Cancer. J. Cancer 2021, 12, 5005–5012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, S.; Hu, C. A narrative review of the role of exosomes and caveolin-1 in liver diseases and cancer. Int. Immunopharmacol. 2023, 120, 110284. [Google Scholar] [CrossRef]
- Fu, P.; Chen, F.; Pan, Q.; Zhao, X.; Zhao, C.; Cho, W.C.; Chen, H. The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther. 2017, 10, 819–835. [Google Scholar] [CrossRef]
- Xue, J.; Chen, H.; Diao, L.; Chen, X.; Xia, D. Expression of caveolin-1 in tongue squamous cell carcinoma by quantum dots. Eur. J. Histochem. 2010, 54, e20. [Google Scholar] [CrossRef]
- He, T.; Guo, X.; Li, X.; Liao, C.; Wang, X.; He, K. Plasma-Derived Exosomal microRNA-130a Serves as a Noninvasive Biomarker for Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. J. Oncol. 2021, 2021, 5547911. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, Y.; Lin, L.; Lin, J.; Wang, H.; Qiu, Y.; Pan, L.; Zheng, X.; Wei, L.; Wang, J. Exosomal hsa_circRNA_047733 integrated with clinical features for preoperative prediction of lymph node metastasis risk in oral squamous cell carcinoma. J. Oral Pathol. Med. 2023, 52, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, J.; Chen, W.; Chen, W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 143. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Jin, N.; Bu, W.; Li, X.; Liu, L.; Wang, Z.; Tong, J.; Li, D. Long non-coding RNA TIRY promotes tumor metastasis by enhancing epithelial-to-mesenchymal transition in oral cancer. Exp. Biol. Med. 2020, 245, 585–596. [Google Scholar] [CrossRef]
- Pang, X.; Wang, S.; Zhang, M.; Jiang, J.; Fan, H.; Wu, J.; Wang, H.; Liang, X.; Tang, Y. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 2021, 70, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, X.; Zhu, X.; Chen, W.; Zhang, J.; Chen, W. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncol. 2018, 76, 34–41. [Google Scholar] [CrossRef]
- Zorrilla, S.R.; García, A.G.; Carrión, A.B.; Vila, P.G.; Martín, M.S.; Torreira, M.G.; Sayans, M.P. Exosomes in head and neck cancer. Updating and revisiting. J. Enzym. Inhib. Med. Chem. 2019, 34, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zheng, Z.; Yuan, Y.; Pathak, J.L.; Yang, X.; Wang, L.; Ye, Z.; Cho, W.C.; Zeng, M.; Wu, L. The Emerging Role of Exosomes in Oral Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 324. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, E.; Shao, Z.; Shang, Z. Long noncoding RNAs in the metastasis of oral squamous cell carcinoma. Front. Oncol. 2021, 10, 616717. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Liu, J.; Su, X.; Qin, H.; Huang, S.; Huang, X.; Zhou, N. Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1668–1681. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Guo, C.; Zhang, S.; Gong, Z.; Tang, Y.; Yang, L.; He, Y.; Lian, Y.; Li, X. Upregulated long non-coding RNA LINC00152 expression is associated with progression and poor prognosis of tongue squamous cell carcinoma. J. Cancer 2017, 8, 523. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Bunduc, S.; Gede, N.; Váncsa, S.; Lillik, V.; Kiss, S.; Juhász, M.F.; Erőss, B.; Szakács, Z.; Gheorghe, C.; Mikó, A.; et al. Exosomes as prognostic biomarkers in pancreatic ductal adenocarcinoma–a systematic review and meta-analysis. Transl. Res. 2022, 244, 126–136. [Google Scholar] [CrossRef]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2017, 96, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Valtieri, M.; Federici, C.; Cecchetti, S.; Meschini, S.; Condello, M.; Signore, M.; Fais, S. Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget 2016, 7, 50086–50098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, L.; Baddour, J.; Achreja, A.; Bernard, V.; Moss, T.; Marini, J.C.; Tudawe, T.; Seviour, E.G.; San Lucas, F.A.; et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 2016, 5, e10250. [Google Scholar] [CrossRef] [PubMed]
- Cossetti, C.; Lugini, L.; Astrologo, L.; Saggio, I.; Fais, S.; Spadafora, C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: Possible transport by exosomes. PLoS ONE 2014, 9, e101629. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and exosome levels interplay in human cancer cell lines of different histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef]
- Logozzi, M.; Capasso, C.; Di Raimo, R.; Del Prete, S.; Mizzoni, D.; Falchi, M.; Supuran, C.T.; Fais, S. Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 272–278. [Google Scholar] [CrossRef]
- Logozzi, M.; Orefice, N.S.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Importance of Detecting, Quantifying, and Characterizing Exosomes as a New Diagnostic/Prognostic Approach for Tumor Patients. Cancers 2023, 15, 2878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).