Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
- -
- To analyze differences in the concentration of early biomarkers of metabolic syndrome between childhood brain tumor cancer survivors and healthy controls [adiponectin, leptin, TNF-α, IL-1, IL-6, IL-10, endothelin, apoB, and Lp(a)].
- -
- To analyze differences in CPET results between childhood brain tumor cancer survivors and healthy controls.
- -
- To evaluate the correlation between early biomarkers of metabolic syndrome and the results of CPET.
- -
- To evaluate the correlation between the results of CPET and the treatments used (chemotherapy, radiotherapy, and steroid therapy).
- -
- To evaluate the correlation between early biomarkers of metabolic syndrome and the treatments used (chemotherapy, radiotherapy, and steroid therapy).
2.2. Study Design, Patients’ Characteristics, Ethical Approval, and Inclusion and Exclusion Criteria
2.3. Disease and Treatment Related Data
- -
- Age, weight, height, BMI, and relative percentile at diagnosis;
- -
- data related to the neoplastic disease (date of diagnosis, histology, presence of metastasis at diagnosis, and primarily affected site);
- -
- type and duration of the treatment [radiotherapy (site and dose), chemotherapy, or high-dose chemotherapy with autologous transplantation (ASCT)];
- -
- data on steroid supportive therapy during treatment;
- -
- data relating to any endocrinological defects developed during oncological treatment or subsequently.
2.4. Personal and Family History, Anthropometric Characteristics, and Information Relating to the Level of Physical Activity
- -
- Height, height-for-age percentile, and Z-score. Percentiles were calculated with a percentile calculator available on Internet, based on CDC growth charts (https://peditools.org/, accessed on 13 November 2023).
- -
- Weight, weight-for-age percentile, and Z-score. Weight was measured in the absence of clothing (except undergarments) with electronic scales. Percentiles were calculated with a percentile calculator available on the Internet, based on CDC growth tables (https://peditools.org/, accessed on 13 November 2023).
- -
- BMI, BMI-for-age percentile, and Z-score. BMI was calculated using the formula (weight in kg)/(height in m)2. The BMI percentile and Z-score were calculated by percentile calculator available on the Internet, based on CDC growth tables (https://peditools.org/, accessed on 13 November 2023).
- -
- Waist circumference, measured at the point of smallest circumference between the last rib and the top of the iliac crest and reported in cm.
- -
- Hip circumference, measured at the major circumference point at the posterior extension of the buttocks and reported in cm.
- -
- Waist to hip ratio (WHR), measured using the formula (waist circumference in cm)/(hip circumference in cm); based on the value obtained.
- -
- Waist to height ratio (WHtR), measured by the formula (waist circumference in cm)/(height in cm); a value of WHtR > 0.5 was considered indicative of central obesity [25].
- -
- − Systolic and diastolic blood pressures, reported in mmHg and pressure-per-age percentiles, calculated for patients > 18 years by comparison of CDC tables [26] and for patients <18 years by calculator available on the Internet (https://www.mdcalc.com/calc/4052/aap-pediatric-hypertension-guidelines, accessed on 13 November 2023).
2.5. Metabolic and Cardiovascular Risk Biomarkers
2.6. Cardiopulmonary Tests
2.7. Simple Size and Statistical Analysis
3. Results
3.1. Disease and Treatment Related Data
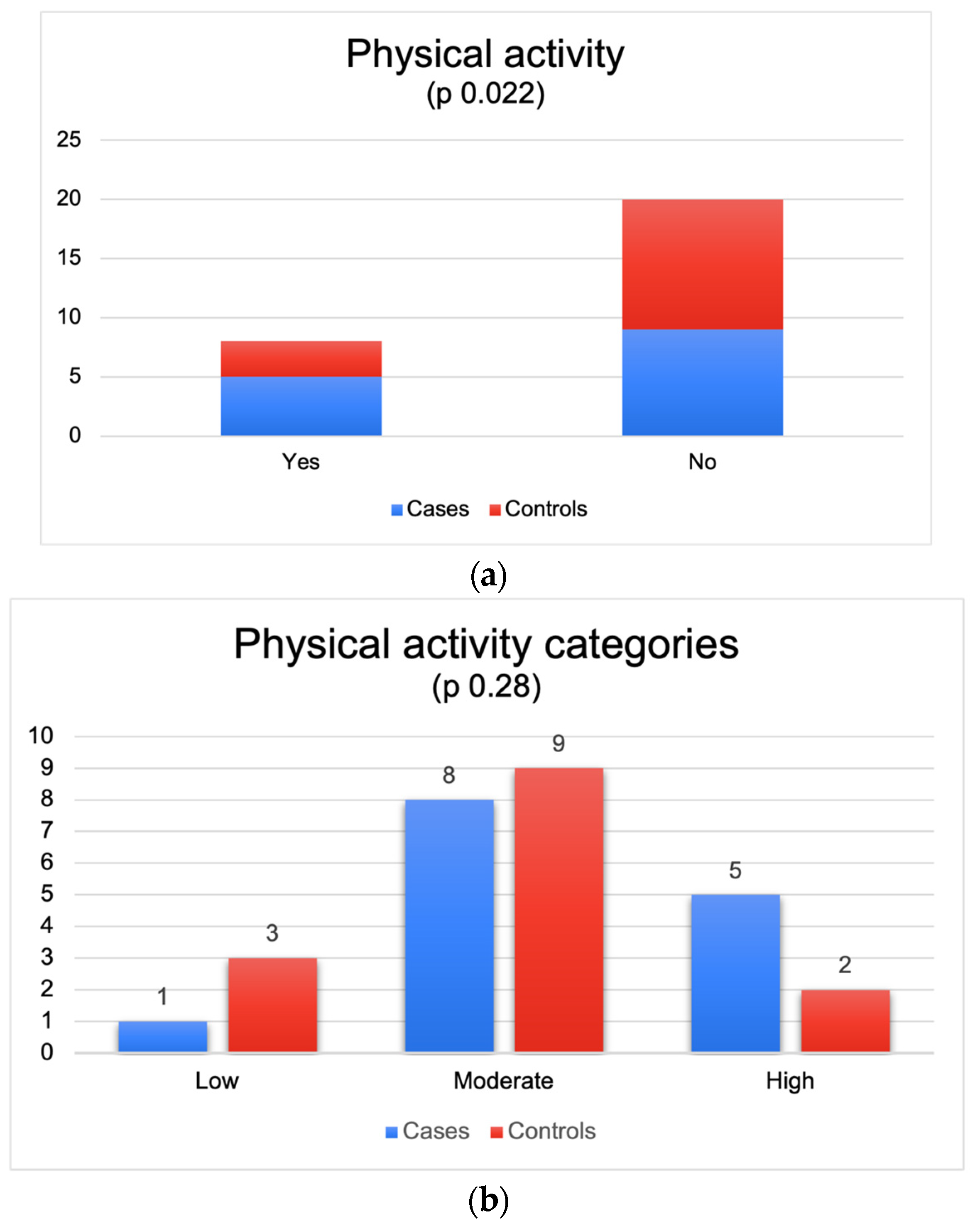
3.2. Personal and Family History, Anthropometric Characteristics and Information Relating to the Level of Physical Activity of Patients and Controls
3.3. Metabolic and Cardiovascular Risk Biomarkers
3.4. Cardiopulmonary Tests Results
3.5. Relation between CPET Results, Metabolic and Cardiovascular Biomarkers and Treatment
4. Discussion
5. Conclusions
- small sample size;
- impossibility to establish with certainty whether the results observed are linked exclusively to the effect of long-term radiotherapy or to other factors linked to the disease or to the other treatments carried out.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood Cancer Survival in Europe 1999–2007: Results of EUROCARE-5—A Population-Based Study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Vassal, G.; Schrappe, M.; Pritchard-Jones, K.; Arnold, F.; Basset, L.; Biondi, A.; Bode, G.; Eggert, A.; Hjorth, L.; Kamerić, L.; et al. The SIOPE Strategic Plan: A European Cancer Plan for Children and Adolescents. J. Cancer Policy 2016, 8, 17–32. [Google Scholar] [CrossRef]
- Brock, P.R.; Knight, K.R.; Freyer, D.R.; Campbell, K.C.; Steyger, P.S.; Blakley, B.W.; Rassekh, S.R.; Chang, K.W.; Fligor, B.J.; Rajput, K.; et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012, 30, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Sofia, R.; Melita, V.; De Vita, A.; Ruggiero, A.; Romano, A.; Attinà, G.; Birritella, L.; Lamendola, P.; Lombardo, A.; Lanza, G.A.; et al. Cardiac Surveillance for Early Detection of Late Subclinical Cardiac Dysfunction in Childhood Cancer Survivors after Anthracycline Therapy. Front. Oncol. 2021, 11, 624057. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Sommer, G.; Kasteler, R.; Scheinemann, K.; Grotzer, M.; Kompis, M.; Kuehni, C.E.; Swiss Pediatric Oncology Group (SPOG). Long-term auditory complications after childhood cancer: A report from the Swiss Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2017, 64, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G. Cisplatin-induced nephrotoxicity in children: What is the best protective strategy? J. Oncol. Pharm. Pract. 2021, 27, 180–186. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Attina, G.; Triarico, S.; Romano, A.; Maurizi, P.; Ruggiero, A. The DNA-topoisomerase Inhibitors in Cancer Therapy. Biomed. Pharmacol. J. 2022, 15, 553–562. [Google Scholar] [CrossRef]
- Di Iorgi, N.; Morana, G.; Cappa, M.; D’Incerti, L.; Garrè, M.L.; Grossi, A.; Iughetti, L.; Matarazzo, P.; Parpagnoli, M.; Pozzobon, G.; et al. Expert Opinion on the Management of Growth Hormone Deficiency in Brain Tumor Survivors: Results from an Italian Survey. Front. Endocrinol. 2022, 13, 920482. [Google Scholar] [CrossRef]
- Talvensaari, K.K.; Lanning, M.; Tapanainen, P.; Knip, M. Long-Term Survivors of Childhood Cancer Have an Increased Risk of Manifesting the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3051–3055. [Google Scholar]
- Romano, A.; Rigante, D.; Cipolla, C. Autoimmune phenomena involving the pituitary gland in children: New developing data about diagnosis and treatment. Autoimmun. Rev. 2019, 18, 102363. [Google Scholar] [CrossRef]
- Sklar, C.A.; Antal, Z.; Chemaitilly, W.; Cohen, L.E.; Follin, C.; Meacham, L.R.; Murad, M.H. Hypothalamic-Pituitary and Growth Disorders in Survivors of Childhood Cancer: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 2761–2784. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.O.L.; Vestergaard, E.; Gormsen, L.; Jessen, N.; Nørrelund, H.; Christiansen, J.S.; Møller, N. Metabolic Consequences of GH Deficiency. J. Endocrinol. Investig. 2005, 28, 47–51. [Google Scholar]
- Perez, A.; Jansen-Chaparro, S.; Saigi, I.; Bernal-Lopez, M.R.; Miñambres, I.; Gomez-Huelgas, R. Glucocorticoid-Induced Hyperglycemia. J. Diabetes 2014, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Landy, D.C.; Lopez-Mitnik, G.; Lipsitz, S.R.; Hinkle, A.S.; Constine, L.S.; French, C.A.; Rovitelli, A.M.; Proukou, C.; Adams, M.J.; et al. Cardiovascular Status of Childhood Cancer Survivors Exposed and Unexposed to Cardiotoxic Therapy. J. Clin. Oncol. 2012, 30, 1050–1057. [Google Scholar] [CrossRef]
- Friedman, D.N.; Tonorezos, E.S.; Cohen, P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019, 91, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Schaffer, W.L.; Steingart, R.M. Late Cardiovascular Toxicity Following Chemotherapy for Germ Cell Tumors. J. Natl. Compr. Cancer Netw. JNCCN 2012, 10, 537–544. [Google Scholar] [CrossRef][Green Version]
- Rossi, F.; Di Paola, A.; Pota, E.; Argenziano, M.; Di Pinto, D.; Marrapodi, M.M.; Di Leva, C.; Di Martino, M.; Tortora, C. Biological Aspects of Inflamm-Aging in Childhood Cancer Survivors. Cancers 2021, 13, 4933. [Google Scholar] [CrossRef]
- Attinà, G.; Romano, A.; Maurizi, P.; D’Amuri, S.; Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Ruggiero, A. Management of Oral Mucositis in Children with Malignant Solid Tumors. Front Oncol. 2021, 11, 599243. [Google Scholar] [CrossRef]
- Triarico, S.; Agresti, P.; Rinninella, E.; Mele, M.C.; Romano, A.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer. Pathogens 2022, 11, 448. [Google Scholar] [CrossRef]
- Posteraro, B.; Bruno, S.; Boccia, S.; Ruggiero, A.; Sanguinetti, M.; Romano Spica, V.; Ricciardi, G.; Fadda, G. Candida parapsilosis bloodstream infection in pediatric oncology patients: Results of an epidemiologic investigation. Infect. Control Hosp. Epidemiol. 2004, 25, 641–645. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith Jr, S.C.; Cleeman, J.I.; Kahn, R.A. American Heart Association, National Heart, Lung, and Blood Institute, American Diabetes Association. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [PubMed]
- Romano, A.; Del Vescovo, E.; Rivetti, S.; Triarico, S.; Attinà, G.; Mastrangelo, S.; Maurizi, P.; Ruggiero, A. Biomarkers Predictive of Metabolic Syndrome and Cardiovascular Disease in Childhood Cancer Survivors. J. Pers. Med. 2022, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Kernizan, D.; Glass, A.; D’Aloisio, G.; Hossain, J.; Quillen, J. Cardiopulmonary Exercise Testing Characterizes Silent Cardiovascular Abnormalities in Asymptomatic Pediatric Cancer Survivors. Pediatr. Cardiol. 2022, 44, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. Principles of Exercise Testing and Interpretation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Yoo, E.G. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016, 59, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Hughes, J.P.; Ostchega, Y.; Yoon, S.S.; Nwankwo, T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl. Health Stat. Rep. 2011, 35, 24. [Google Scholar]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Casaburi, R. Principles of Exercise Testing and Interpretation, 3rd ed.; Lea & Fabiger: Philadelphia, PA, USA, 1999. [Google Scholar]
- Burstein, D.S.; McBride, M.G.; Min, J.; Paridon, A.A.; Perelman, S.; Huffman, E.M.; O’Malley, S.; Del Grosso, J.; Groepenhoff, H.; Paridon, S.M.; et al. Normative values for cardiopulmonary exercise stress testing using ramp cycle ergometry in children and adolescents. J. Pediatr. 2021, 229, 61–69.e5. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Chow, E.J.; Goodman, P.J.; Leisenring, W.M.; Dietz, A.C.; Perkins, J.L.; Chow, L.; Sinaiko, A.; Moran, A.; Petryk, A.; et al. Impact of Treatment Exposures on Cardiovascular Risk and Insulin Resistance in Childhood Cancer Survivors. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1954–1963. [Google Scholar] [CrossRef]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef]
- Piqueras, P.; Ballester, A.; Durá-Gil, J.V.; Martinez-Hervas, S.; Redón, J.; Real, J.T. Anthropometric Indicators as a Tool for Diagnosis of Obesity and Other Health Risk Factors: A Literature Review. Front. Psychol. 2021, 12, 631179. [Google Scholar] [CrossRef]
- Steinberger, J.; Sinaiko, A.R.; Kelly, A.S.; Leisenring, W.M.; Steffen, L.M.; Goodman, P.; Mulrooney, D.A.; Dietz, A.C.; Moran, A.; Perkins, J.L.; et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J. Pediatr. 2012, 160, 494–499. [Google Scholar] [CrossRef]
- Dashwood, M.; Tsui, J. Endothelin-1 and atherosclerosis: Potential complications associated with endothelin-receptor blockade. Atherosclerosis 2002, 160, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.; Schiffrin, E. Role of endothelin in human hypertension. Can. J. Physiol. Pharmacol. 2003, 81, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, F.; Iantorno, M.; Campia, U.; Mores, N.; Rovella, V.; Tesauro, M.; Di Daniele, N.; Cardillo, C. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am. J. Physiol. Metab. 2015, 309, E787–E792. [Google Scholar] [CrossRef]
- Jenkins, H.N.; Rivera-Gonzalez, O.; Gibert, Y.; Speed, J.S. Endothelin-1 in the pathophysiology of obesity and insulin resistance. Obes. Rev. 2020, 21, e13086. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.J.; Templeton, D.L.; Ma, J.; Weil, B.R.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Impaired fasting blood glucose is associated with increased endothelin-1 vasoconstrictor tone. Atherosclerosis 2013, 229, 130–133. [Google Scholar] [CrossRef]
- Weil, B.R.; Westby, C.M.; Van Guilder, G.P.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Enhanced endothelin-1 system activity with overweight and obesity. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H689–H695. [Google Scholar] [CrossRef]
- Rocha, N.G.; Templeton, D.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Metabolic syndrome and endothelin-1 mediated vasoconstrictor tone in overweight/obese adults. Metabolism 2014, 63, 951–956. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Albers, J.J.; Kennedy, H.; Marcovina, S.M. Evidence that Lp[a] contains one molecule of apo[a] and one molecule of apoB: Evaluation of amino acid analysis data. J. Lipid Res. 1996, 37, 192–196. [Google Scholar] [CrossRef]
- Virani, S.S.; Saeed, A. Lipoprotein(a) and cardiovascular disease current state and future directions for an enigmatic lipoprotein. Front. Biosci. 2018, 23, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Waldeyer, C.; Makarova, N.; Zeller, T.; Schnabel, R.B.; Brunner, F.J.; Jørgensen, T.; Linneberg, A.; Niiranen, T.; Salomaa, V.; Jousilahti, P.; et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: Results from the BiomarCaRE consortium. Eur. Hear. J. 2017, 38, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, V.; Rojas, J.; Salazar, J.; Bello, L.; Áñez, R.; Toledo, A.; Chacín, M.; Aguirre, M.; Villalobos, M.; Chávez, M.; et al. Variations of Lipoprotein(a) Levels in the Metabolic Syndrome: A Report from the Maracaibo City Metabolic Syndrome Prevalence Study. J. Diabetes Res. 2013, 2013, 416451. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.; Fonseca, L.; Ribeiro, L.; Ramos, H.; Oliveira, J.C.; Palma, I. Novel and traditional lipid profiles in Metabolic Syndrome reveal a high atherogenicity. Sci. Rep. 2019, 9, 11792. [Google Scholar] [CrossRef]
- Wabitsch, M.; Blum, W.F.; Muche, R.; Braun, M.; Hube, F.; Rascher, W.; Heinze, E.; Teller, W.; Hauner, H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Investig. 1997, 100, 808–813. [Google Scholar] [CrossRef]
- Wasim, M.; Awan, F.R.; Najam, S.S.; Khan, A.R.; Khan, H.N. Role of Leptin Deficiency, Inefficiency, and Leptin Receptors in Obesity. Biochem. Genet. 2016, 54, 565–572. [Google Scholar] [CrossRef]
- Dornbush, S.; Aeddula, N.R. Physiology, Leptin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Franks, P.W.; Brage, S.; Luan, J.; Ekelund, U.; Rahman, M.; Farooqi, I.S.; Halsall, I.; O’Rahilly, S.; Wareham, N.J. Leptin Predicts a Worsening of the Features of the Metabolic Syndrome Independently of Obesity. Obes. Res. 2005, 13, 1476–1484. [Google Scholar] [CrossRef]
- Galletti, F.; D’Elia, L.; Barba, G.; Siani, A.; Cappuccio, F.P.; Farinaro, E.; Iacone, R.; Russo, O.; De Palma, D.; Ippolito, R.; et al. High-Circulating Leptin Levels Are Associated with Greater Risk of Hypertension in Men Independently of Body Mass and Insulin Resistance: Results of an Eight-Year Follow-up Study. J. Clin. Endocrinol. Metab. 2008, 93, 3922–3926. [Google Scholar] [CrossRef]
- Pluimakers, V.G.; van Waas, M.; Looman, C.W.N.; de Maat, M.P.; de Jonge, R.; Delhanty, P.; Huisman, M.; Mattace-Raso, F.U.S.; van den Heuvel-Eibrink, M.M.; Neggers, S.J.C.M.M. Metabolic Syndrome Detection with Biomarkers in Childhood Cancer Survivors. Endocr. Connect. 2020, 9, 676–686. [Google Scholar] [CrossRef]
- Follin, C.; Erfurth, E.M. Long-Term Effect of Cranial Radiotherapy on Pituitary-Hypothalamus Area in Childhood Acute Lymphoblastic Leukemia Survivors. Curr. Treat. Options Oncol. 2016, 17, 50. [Google Scholar] [CrossRef]
- Sims, E.D.; Jennings, W.J.; Empringham, B.; Fleming, A.; Portwine, C.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Thabane, L.; et al. Circulating Leptin Levels Are Associated with Adiposity in Survivors of Childhood Brain Tumors. Sci. Rep. 2020, 10, 4711. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Mohlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef]
- Testa, R.; Olivieri, F.; Bonfigli, A.R.; Sirolla, C.; Boemi, M.; Marchegiani, F.; Marra, M.; Cenerelli, S.; Antonicelli, R.; Dolci, A.; et al. Interleukin-6-174 G > C Polymorphism Affects the Association between IL-6 Plasma Levels and Insulin Resistance in Type 2 Diabetic Patients. Diabetes Res. Clin. Pract. 2006, 71, 299–305. [Google Scholar] [CrossRef]
- Cifra, B.; Chen, C.K.; Fan, C.S.; Slorach, C.; Manlhiot, C.; McCrindle, B.W.; Dragulescu, A.; Redington, A.N.; Friedberg, M.K.; Nathan, P.C.; et al. Dynamic Myocardial Response to Exercise in Childhood Cancer Survivors Treated with Anthracyclines. J. Am. Soc. Echocardiogr. 2018, 31, 933–942. [Google Scholar] [CrossRef]
- Ness, K.K.; Plana, J.C.; Joshi, V.M.; Luepker, R.V.; Durand, J.B.; Green, D.M.; Partin, R.E.; Santucci, A.K.; Howell, R.M.; Srivastava, D.K.; et al. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J. Clin. Oncol. 2020, 38, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Scheede-Bergdahl, C.; Jagoe, R.T. After the chemotherapy: Potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front. Pharmacol. 2013, 4, 49. [Google Scholar] [CrossRef]
- Gavin, T.P. Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc. Sport Sci. Rev. 2009, 37, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, G.; Hartz, J.; Lubans, D.R.; Takken, T.; Wiltz, J.L.; Mietus-Snyder, M.; Perak, A.M.; Baker-Smith, C.; Pietris, N.; Edwards, N.M.; et al. Cardiorespiratory fitness in youth: An important marker of health: A scientific statement from the American Heart Association. Circulation 2020, 142, E101–E118. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.W.; Nagarajan, R.; Mays, W.A.M.; Chin, C.; Knilans, T.K.; Knecht, S.K.; Amos, M.A.; Gerdes, Y.M.; Ryan, T.D. Cardiopulmonary Aerobic Fitness Assessment During Maximal and Submaximal Exercise Testing in Pediatric Oncology Patients After Chemotherapy. Am. J. Clin. Oncol. 2018, 41, 1058–1061. [Google Scholar] [CrossRef]
- Berkman, A.M.; Lakoski, S.G. A Review of Cardiorespiratory Fitness in Adolescent and Young Adult Survivors of Childhood Cancer: Factors that Affect its Decline and Opportunities for Intervention. J. Adolesc. Young Adult Oncol. 2016, 5, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Abdelwahab, E.H.M.M.; Steib, A.; Papp, E.; Torok, Z.; Jakab, L.; Smuk, G.; Sarosi, V.; Pongracz, J.E. Cisplatin treatment induced interleukin 6 and 8 production alters lung adenocarcinoma cell migration in an oncogenic mutation dependent manner. Respir. Res. 2020, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Lazzareschi, I.; Ruggiero, A.; Riccardi, R.; Attinà, G.; Colosimo, C.; Lasorella, A. Hypersensitivity reactions to carboplatin in children. J. Neurooncol. 2002, 58, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Nickenig, G. From fat fighter to risk factor: The zigzag trek of leptin. Arter.-Oscler. Thromb. Vasc. Biol. 2004, 24, 7–9. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
| Number of Patients (%) or Mean (SD) | |
|---|---|
| Age at diagnosis (years) | 9.6 (5) |
| Weight (kg) * | 43.8 (22.3) |
| Weight percentile * | 71.8 (33.9) |
| Weight Z-score * | 0.9 (1.2) |
| Height (m) * | 1.4 (0.3) |
| Height percentile * | 52.8 (33.3) |
| Height Z-score * | 0.1 (1.2) |
| BMI (kg/cm2) * | 21.1 (4.8) |
| BMI percentile * | 87.9 (17.3) |
| BMI Z-score * | 0.8 (1.6) |
| Histology | |
| Germ cell tumor | 8 (57%) |
| Medulloblastoma | 4 (29%) |
| Ependymoma | 2 (14%) |
| Primary localization | |
| Anterior cranial fossa | 0 (0%) |
| Middle cranial fossa | 8 (57%) |
| Posterior cranial fossa | 6 (43%) |
| Presence of metastasis at diagnosis | 1 (7%) |
| Patients subjected to cranial radiotherapy | 14 (100%) |
| Patients subjected to spinal radiotherapy | 5 (36%) |
| Cranial radiotherapy dose (Gy) | 56.7 (16.6) |
| Spinal radiotherapy dose (Gy) | 30 (0) |
| Patients subjected to chemotherapy | 12 (86%) |
| Patients subjected to steroid therapy (more than 14 days) | 10 (71%) |
| Patients subjected to ASCT | 2 (14%) |
| Relapsed disease | 2 (14%) |
| Endocrinological deficiencies | 9 (64%) |
| Panhypopituitarism | 4 (29%) |
| Hypothyroidism | 2 (14%) |
| GH deficiency | 3 (21%) |
| Age at the enrollement (years) | 24.9 (3.9) |
| Time of follow up at the enrollement (months) | 171 (54) |
| Variables | Cases [Mean (SD) or Number of Patients (%)] | Controls [Mean (SD) or Number of Patients (%)] | p Value |
|---|---|---|---|
| Age (years) | 24.93 (3.89) | 24.64 (2.92) | 0.834 |
| Weight (kg) | 67.89 (10.89) | 76.81 (8.85) | 0.025 * |
| Weight percentile | 48.23 (19.08) | 64.79 (23.91) | 0.015 * |
| Weight Z-score | −0.31 (1.12) | 0.41 (0.70) | 0.347 |
| Height (m) | 1.68 (0.10) | 1.77 (0.08) | 0.015 * |
| Height percentile | 28.67 (19.65) | 51.79 (33.23) | 0.007 * |
| Height Z-score | −1.19 (1.40) | 0.06 (1.13) | 0.434 |
| BMI (kg/m2) | 23.99 (3.22) | 24.59 (3.19) | 0.624 |
| BMI percentile | 57.34 (26.90) | 58.91 (26.29) | 0.877 |
| BMI Z-Score | 0.11 (1.12) | 0.31 (0.83) | 0.607 |
| Waist circumference (cm) | 83.04 (8.31) | 80.36 (9.83) | 0.079 |
| Hip circumference (cm) | 91.07 (7.44) | 87.86 (12.08) | 0.178 |
| WHR | 0.91 (0.05) | 0.92 (0.07) | <0.002 * |
| WHtR | 0.49 (0.05) | 0.45 (0.06) | 0.049 * |
| WHtR > 0.5 | 5 (36%) | 3 (21%) | 0.402 |
| BP | |||
| Systolic (mmHg) | 124.43 (14.55) | 123.50 (7.76) | 0.417 |
| Diastolic (mmHg) | 70.71 (7.44) | 71.71 (7.61) | 0.363 |
| HR (bpm) | 69.07 (11.65) | 73.57 (10.25) | 0.143 |
| Variables | Cases [Mean (SD)] | Controls [Mean (SD)] | p Value |
|---|---|---|---|
| Glucose (mg/dL) | 83.36 (5.80) | 75.21 (14.06) | 0.056 |
| Total cholesterol (mg/dL) | 166.01 (55.78) | 158.57 (29.44) | 0.191 |
| Triglycerides (mg/dL) | 92.64 (50.19) | 85.21 (37.90) | 0.662 |
| HDL (mg/dL) | 49.21 (20.09) | 48.57 (8.30) | 0.913 |
| LDL (mg/dL) | 105.50 (28.05) | 93.00 (25.61) | 0.229 |
| Lp(a) (mg/dL) | 58.52 (71.46) | 35.47 (53.10) | 0.342 |
| Apo B (mg/dL) | 19.98 (8.18) | 20.91 (9.13) | 0.769 |
| Leptin (ng/mL) | 6.22 (4.79) | 4.26 (4.84) | 0.290 |
| TNFα (pg/mL) | 3.13 (1.01) | 3.30 (0.84) | 0.633 |
| IL 1-β (pg/mL) | 0.78 (0.46) | 0.49 (0.29) | 0.179 |
| IL-10 (pg/mL) | 0.36 (0.33) | 0.48 (0.46) | 0.568 |
| IL-6 (pg/mL) | 0.60 (0.64) | 0.22 (0.33) | 0.176 |
| Endothelin-1 (pg/mL) | 1.28 (0.41) | 0.99 (0.17) | 0.025 * |
| Adiponectin (μg/mL) | 10.91 (3.57) | 10.91 (3.47) | 0.998 |
| Variables | Cases [Mean (SD)] | Controls [Mean (SD)] | p Value |
|---|---|---|---|
| VO2 peak (mL) | 1974.4 (451.31) | 2470.9 (384.94) | 0.002 * |
| VO2 pro kg | 29.3 (5.25) | 32.6 (5.39) | 0.054 |
| VO2% | 61.0 (11.2) | 69.7 (12.6) | 0.032 * |
| VO2@AT | 974.8 (238.33) | 1198.8 (271.2) | 0.024 * |
| OUES | 1744.9 (684.16) | 2432.1 (450.12) | 0.002 * |
| OUES% | 62.1 (15.23) | 72.1 (10.7) | 0.027 * |
| VO2/HR | 11.0 (2.63) | 13.6 (2.13) | 0.005 * |
| VO2/hr% | 67.2 (11.9) | 74.3 (12.6) | 0.069 |
| dVO2/WR slope | 10.6 (1.29) | 10.4 (0.9) | 0.611 |
| VE/VCO2 slope | 27.4 (2.85) | 28.1 (4.85) | 0.572 |
| BR | 31.9 (25.1) | 41.2 (15.2) | 0.124 |
| FR peak | 46.0 (10.2) | 40.7 (10.2) | 0.911 |
| VE peak | 85.8 (21.0) | 96.2 (34.3) | 0.172 |
| PCP | 331,341.4 (89,143.1) | 433,262.1 (83,148.8) | 0.002 * |
| Variables | Glucose (mg/dL) | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | |
|---|---|---|---|---|---|---|
| VO2 peak (mL) | r | 0.004 | −0.163 | 0.15 | −0.012 | −0.24 |
| p value | 0.985 | 0.408 | 0.447 | 0.952 | 0.219 | |
| VO2 pro kg | r | 0.039 | −0.06 | −0.039 | 0.177 | −0.175 |
| p value | 0.845 | 0.842 | 0.842 | 0.369 | 0.374 | |
| VO2% | r | 0.073 | −0.079 | 0.021 | 0.198 | −0.231 |
| p value | 0.713 | 0.691 | 0.915 | 0.423 | 0.3 | |
| VO2@AT | r | −0.052 | −0.019 | 0.341 | −0.159 | −0.019 |
| p value | 0.81 | 0.93 | 0.103 | 0.458 | 0.928 | |
| OUES | r | −0.151 | −0.420 * | −0.175 | 0.141 | −0.508 * |
| p value | 0.444 | 0.026 * | 0.372 | 0.474 | 0.006 * | |
| OUES% | r | 0.038 | −0.180 | 0.005 | 0.068 | −0.252 |
| p value | 0.849 | 0.358 | 0.978 | 0.731 | 0.195 | |
| VO2/HR | r | −0.034 | −0.230 | 0.097 | −0.002 | −0.303 |
| p value | 0.862 | 0.240 | 0.623 | 0.994 | 0.117 | |
| VO2/hr% | r | 0.047 | −0.164 | −0.018 | 0.131 | −0.273 |
| p value | 0.813 | 0.405 | 0.928 | 0.507 | 0.159 | |
| dVO2/WR slope | r | 0.011 | −0.131 | −0.380 * | 0.053 | −0.073 |
| p value | 0.955 | 0.506 | 0.046 * | 0.788 | 0.712 | |
| VE/VCO2 slope | r | −0.070 | 0.227 | 0.029 | −0.098 | 0.309 |
| p value | 0.722 | 0.245 | 0.884 | 0.621 | 0.110 | |
| BR | r | −0.269 | −0.076 | −0.466 * | 0.262 | −0.012 |
| p value | 0.167 | 0.701 | 0.012 * | 0.178 | 0.953 | |
| FR peak | r | 0.356 | 0.152 | 0.351 | 0.210 | 0.131 |
| p value | 0.063 | 0.440 | 0.067 | 0.284 | 0.506 | |
| VE peak | r | 0.495 * | 0.106 | 0.365 | 0.005 | −0.021 |
| p value | 0.007 * | 0.591 | 0.056 | 0.982 | 0.917 | |
| PCP | r | 0.014 | −0.147 | 0.182 | −0.012 | −0.224 |
| p value | 0.944 | 0.454 | 0.354 | 0.953 | 0.253 |
| Variables | Lp (a) (mg/dL) | Apo B (mg/dL) | Leptin (ng/mL) | TNF α (pg/mL) | IL−1β (pg/mL) | IL−10 (pg/mL) | IL−6 (pg/mL) | ET−1 (pg/mL) | AN (μg/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|
| VO2 peak (mL) | r | 0.074 | 0.302 | −0.479 * | 0.045 | −0.426 | 0.175 | −0.320 | −0.355 | 0.011 |
| p value | 0.708 | 0.126 | 0.010 * | 0.818 | 0.100 | 0.501 | 0.196 | 0.075 | 0.955 | |
| VO2 pro kg | r | 0.278 | 0.187 | −0.761 * | −0.126 | −0.379 | −0.011 | −0.477 * | −0.164 | 0.205 |
| p value | 0.152 | 0.351 | 0.001 * | 0.522 | 0.147 | 0.968 | 0.045 * | 0.422 | 0.295 | |
| VO2% | r | 0.231 | 0.195 | −0.727 * | −0.122 | −0.414 | −0.059 | −0.456 | −0.161 | 0.1 |
| p value | 0.237 | 0.330 | 0.001 * | 0.537 | 0.111 | 0.822 | 0.057 | 0.432 | 0.36 | |
| VO2@AT | r | −0.107 | 0.365− | −0.434 * | −0.180 | −0.412 | 0.313 | −0.030 | −0.262 | −0.091 |
| p value | 0.618 | 0.087 | 0.034 * | 0.401 | 0.184 | 0.256 | 0.915 | 0.228 | 0.471 | |
| OUES | r | 0.047 | 0.288 | −0.361 | 0.121 | −0.363 | 0.143 | −0.115 | −0.256 | 0.266 |
| p value | 0.811 | 0.145 | 0.059 | 0.540 | 0.167 | 0.583 | 0.649 | 0.207 | 0.171 | |
| OUES% | r | 0.117 | 0.219 | −0.575 * | −0.087 | −0.357 | 0.028 | −0.411 | −0.425 * | 0.074 |
| p value | 0.553 | 0.272 | 0.001 * | 0.659 | 0.174 | 0.915 | 0.090 | 0.014 * | 0.71 | |
| VO2/HR | r | −0.000 | 0.270 | −0.407 * | 0.091 | −0.379 | 0.093 | −0.275 | −0.776 * | −0.045 |
| p value | 1000 | 0.174 | 0.031 * | 0.646 | 0.147 | 0.722 | 0.269 | 0.045 * | 0.809 | |
| VO2/hr% | r | 0.124 | 0.176 | −0.692 * | −0.088 | −0.399 | −0.135 | −0.453 | −0.262 | 0.06 |
| p value | 0.529 | 0.380 | 0.001 * | 0.657 | 0.126 | 0.605 | 0.059 | 0.197 | 0.726 | |
| dVO2/WR slope | r | −0.061 | 0.022 | −0.199 | −0.031 | 0.117 | 0.325 | 0.085 | −0.02 | 0.175 |
| p value | 0.757 | 0.913 | 0.310 | 0.876 | 0.665 | 0.203 | 0.738 | 0.994 | 0.37 | |
| VE/VCO2 slope | r | −0.200 | −0.126 | 0.394 * | 0.219 | 0.556 * | 0.059 | 0.275 | 0.279 | −0.098 |
| p value | 0.308 | 0.532 | 0.038 * | 0.263 | 0.025 * | 0.822 | 0.269 | 0.168 | 0.62 | |
| BR | r | −0.377 * | 0.019 | 0.016 | −0.211 | −0.042 | −0.031 | −0.139 | 0.265 | −0.231 |
| p value | 0.048 * | 0.924 | 0.938 | 0.282 | 0.879 | 0.905 | 0.583 | 0.191 | 0.236 | |
| FR peak | r | 0.316 | −0.121 | 0.133 | 0.082 | −0.002 | 0.216 | 0.204 | −0.365 | 0.015 |
| p value | 0.101 | 0.549 | 0.501 | 0.677 | 0.993 | 0.405 | 0.417 | 0.066 | 0.934 | |
| VE peak | r | 0.187 | 0.246 | −0.189 | 0.203 | 0.021 | 0.254 | 0.029 | −0.095 | 0.597 |
| p value | 0.342 | 0.215 | 0.336 | 0.300 | 0.938 | 0.325 | 0.910 | 0.644 | 0.288 | |
| PCP | r | 0.036 | 0.259 | −0.425 * | 0.065 | −0.445 | 0.103 | −0.317 | −0.397 * | 0.635 |
| p value | 0.854 | 0.192 | 0.024 * | 0.741 | 0.084 | 0.694 | 0.200 | 0.045 * | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Sollazzo, F.; Rivetti, S.; Morra, L.; Servidei, T.; Lucchetti, D.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Zovatto, I.C.; et al. Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study. Cancers 2024, 16, 324. https://doi.org/10.3390/cancers16020324
Romano A, Sollazzo F, Rivetti S, Morra L, Servidei T, Lucchetti D, Attinà G, Maurizi P, Mastrangelo S, Zovatto IC, et al. Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study. Cancers. 2024; 16(2):324. https://doi.org/10.3390/cancers16020324
Chicago/Turabian StyleRomano, Alberto, Fabrizio Sollazzo, Serena Rivetti, Lorenzo Morra, Tiziana Servidei, Donatella Lucchetti, Giorgio Attinà, Palma Maurizi, Stefano Mastrangelo, Isabella Carlotta Zovatto, and et al. 2024. "Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study" Cancers 16, no. 2: 324. https://doi.org/10.3390/cancers16020324
APA StyleRomano, A., Sollazzo, F., Rivetti, S., Morra, L., Servidei, T., Lucchetti, D., Attinà, G., Maurizi, P., Mastrangelo, S., Zovatto, I. C., Monti, R., Bianco, M., Palmieri, V., & Ruggiero, A. (2024). Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study. Cancers, 16(2), 324. https://doi.org/10.3390/cancers16020324









