Incidence and Risk Assessment of Capsular Contracture in Breast Cancer Patients following Post-Mastectomy Radiotherapy and Implant-Based Reconstruction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Collection and Follow-Up
2.2. Statistical Analysis
3. Results
3.1. Patient and Radiotherapy Characteristics
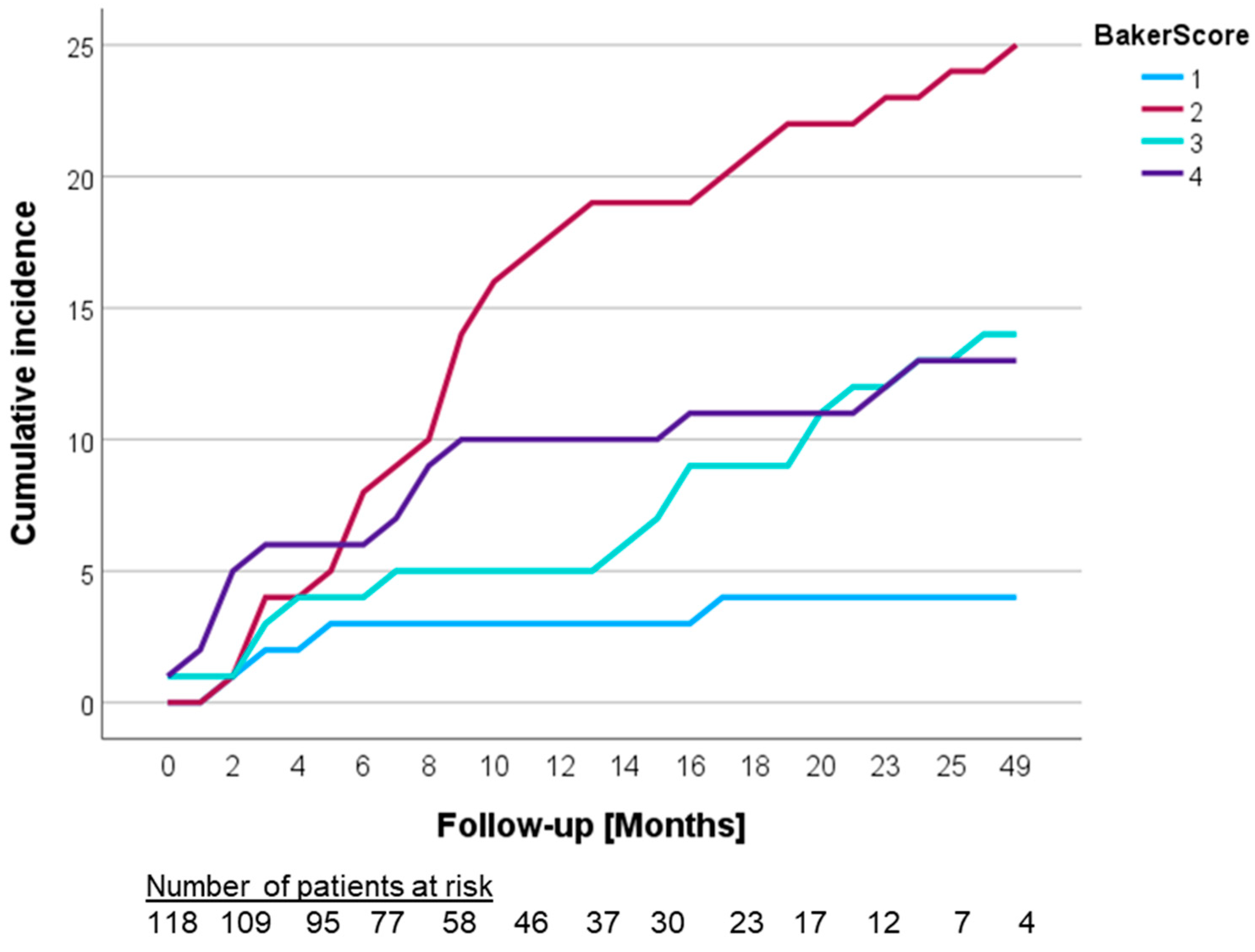
3.2. Development of Capsular Contracture
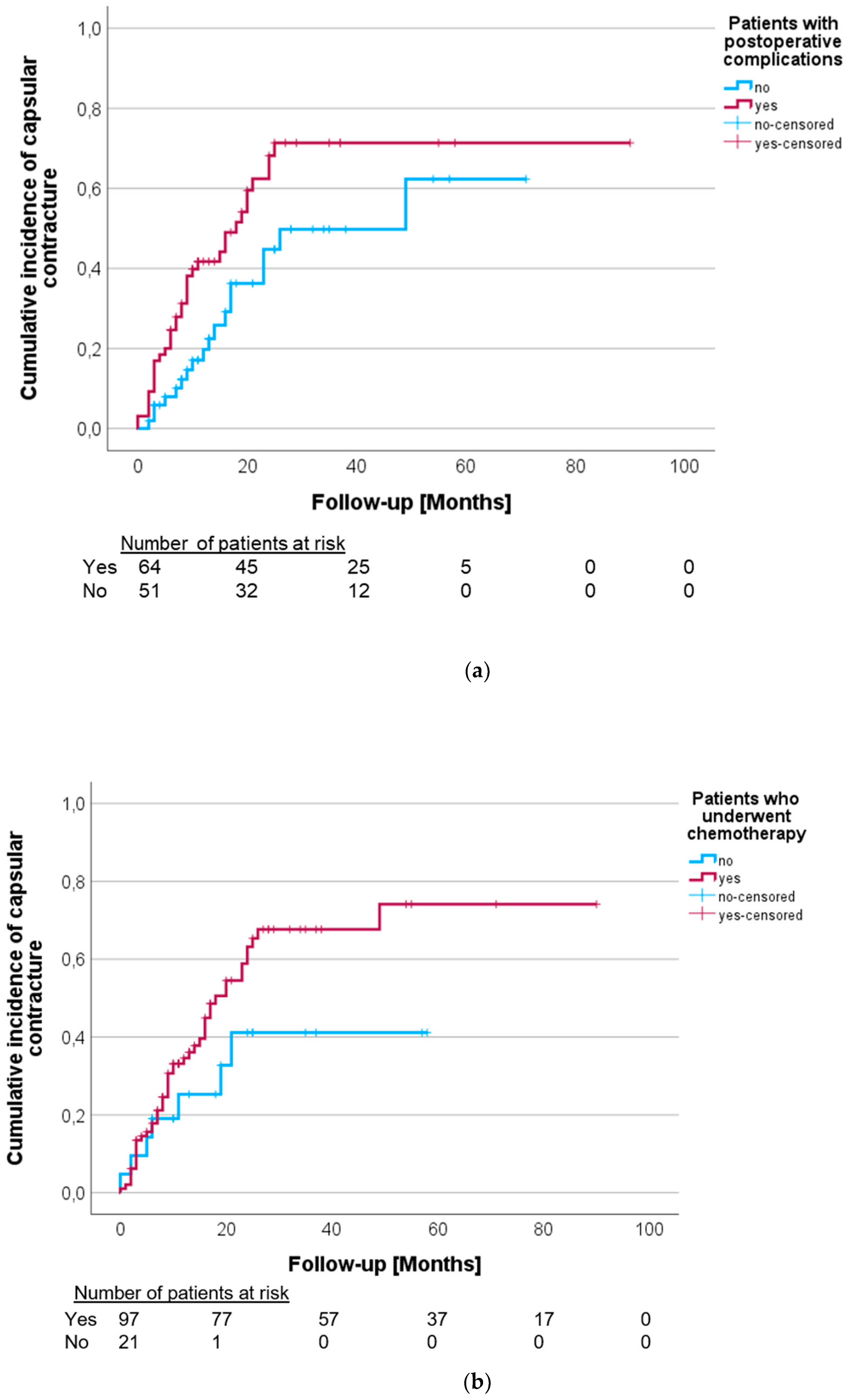
3.3. Analysis of Potential Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ghazal, S.K.; Fallowfield, L.; Blamey, R.W. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Mao, T.C.; Zhang, Y.M.; Wang, S.L.; Fan, D.L. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: A meta-analysis. Medicine 2018, 97, e9548. [Google Scholar] [CrossRef] [PubMed]
- McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; Whelan, T.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Safran, T.; Nepon, H.; Chu, C.K.; Winocour, S.; Murphy, A.M.; Davison, P.G.; Dionisopolos, T.; Vorstenbosch, J. Current Concepts in Capsular Contracture: Pathophysiology, Prevention, and Management. Semin. Plast. Surg. 2021, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Haran, O.; Bracha, G.; Tiosano, A.; Menes, T.; Madah, E.; Gur, E.; Barnea, Y.; Arad, E. Postirradiation Capsular Contracture in Implant-Based Breast Reconstruction: Management and Outcome. Plast. Reconstr. Surg. 2021, 147, 11–19. [Google Scholar] [CrossRef]
- Cowen, D.; Gross, E.; Rouannet, P.; Teissier, E.; Ellis, S.; Resbeut, M.; Tallet, A.; Cowen, V.V.; Azria, D.; Hannoun-Levi, J.M. Immediate post-mastectomy breast reconstruction followed by radiotherapy: Risk factors for complications. Breast Cancer Res. Treat. 2010, 121, 627–634. [Google Scholar] [CrossRef]
- Hammond, J.B.; Kosiorek, H.E.; Cronin, P.A.; Rebecca, A.M.; Casey, W.J., 3rd; Wong, W.W.; Vargas, C.E.; Vern-Gross, T.Z.; McGee, L.A.; Pockaj, B.A. Capsular contracture in the modern era: A multidisciplinary look at the incidence and risk factors after mastectomy and implant-based breast reconstruction. Am. J. Surg. 2021, 221, 1005–1010. [Google Scholar] [CrossRef]
- Reinders, F.C.J.; Young-Afat, D.A.; Batenburg, M.C.T.; Bruekers, S.E.; van Amerongen, E.A.; Macaré van Maurik, J.F.M.; Braakenburg, A.; Zonnevylle, E.; Hoefkens, M.; Teunis, T.; et al. Higher reconstruction failure and less patient-reported satisfaction after post mastectomy radiotherapy with immediate implant-based breast reconstruction compared to immediate autologous breast reconstruction. Breast Cancer 2020, 27, 435–444. [Google Scholar] [CrossRef]
- Adams, W.P., Jr. Capsular contracture: What is it? What causes it? How can it be prevented and managed? Clin. Plast. Surg. 2009, 36, 119–126. [Google Scholar] [CrossRef]
- Larsen, A.; Rasmussen, L.E.; Rasmussen, L.F.; Weltz, T.K.; Hemmingsen, M.N.; Poulsen, S.S.; Jacobsen, J.C.B.; Vester-Glowinski, P.; Herly, M. Histological Analyses of Capsular Contracture and Associated Risk Factors: A Systematic Review. Aesthetic Plast. Surg. 2021, 45, 2714–2728. [Google Scholar] [CrossRef]
- Polo, M.; Smith, P.D.; Kim, Y.J.; Wang, X.; Ko, F.; Robson, M.C. Effect of TGF-beta2 on proliferative scar fibroblast cell kinetics. Ann. Plast. Surg. 1999, 43, 185–190. [Google Scholar] [CrossRef]
- Kuhn, A.; Singh, S.; Smith, P.D.; Ko, F.; Falcone, R.; Lyle, W.G.; Maggi, S.P.; Wells, K.E.; Robson, M.C. Periprosthetic breast capsules contain the fibrogenic cytokines TGF-beta1 and TGF-beta2, suggesting possible new treatment approaches. Ann. Plast. Surg. 2000, 44, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Brazin, J.; Malliaris, S.; Groh, B.; Mehrara, B.; Hidalgo, D.; Otterburn, D.; Silver, R.B.; Spector, J.A. Mast cells in the periprosthetic breast capsule. Aesthetic Plast. Surg. 2014, 38, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Onkologie, L. Interdisziplinäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom (accessed on 20 November 2023).
- A.A.G.O. e.V. Diagnosis and Treatment of Patients with Early and Advanced Breast Cancer: Adjuvant Radiotherapy. Available online: https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen (accessed on 20 November 2023).
- National Comprehensive Cancer Network. Breast Cancer Version 4. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 20 November 2023).
- Spear, S.L.; Baker, J.L., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast. Reconstr. Surg. 1995, 96, 1119–1123; discussion 1124. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.G.; Calobrace, M.B.; Alizadeh, K.; Zeidler, K.R.; Harrington, J.L.; d’Incelli, R.C. Ten-year Core Study Data for Sientra’s Food and Drug Administration-Approved Round and Shaped Breast Implants with Cohesive Silicone Gel. Plast. Reconstr. Surg. 2018, 141, 7s–19s. [Google Scholar] [CrossRef] [PubMed]
- Bachour, Y.; Bargon, C.A.; de Blok, C.J.M.; Ket, J.C.F.; Ritt, M.; Niessen, F.B. Risk factors for developing capsular contracture in women after breast implant surgery: A systematic review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, e29–e48. [Google Scholar] [CrossRef] [PubMed]
- Serritzlev, M.S.; Lorentzen, A.K.; Matthiessen, L.W.; Hölmich, L.R. Capsular contracture in patients with prior breast augmentation undergoing breast conserving therapy and irradiation. J. Plast. Surg. Hand Surg. 2020, 54, 225–232. [Google Scholar] [CrossRef]
- Gross, E.; Hannoun-Levi, J.M.; Rouanet, P.; Houvenaeghel, G.; Teissier, E.; Ellis, S.; Resbeut, M.; Tallet, A.; Vaini Cowen, V.; Azria, D.; et al. Evaluation of immediate breast reconstruction and radiotherapy: Factors associated with complications. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2010, 14, 704–710. [Google Scholar] [CrossRef]
- Nava, M.B.; Pennati, A.E.; Lozza, L.; Spano, A.; Zambetti, M.; Catanuto, G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast. Reconstr. Surg. 2011, 128, 353–359. [Google Scholar] [CrossRef]
- Mericli, A.F.; Sharabi, S.E. Breast Implants and Radiation. Semin. Plast. Surg. 2019, 33, 240–246. [Google Scholar] [CrossRef]
- Luvsannyam, E.; Patel, D.; Hassan, Z.; Nukala, S.; Somagutta, M.R.; Hamid, P. Overview of Risk Factors and Prevention of Capsular Contracture Following Implant-Based Breast Reconstruction and Cosmetic Surgery: A Systematic Review. Cureus 2020, 12, e10341. [Google Scholar] [CrossRef]
- Doherty, C.; McClure, J.A.; Baxter, N.N.; Brackstone, M. Complications From Postmastectomy Radiation Therapy in Patients Undergoing Immediate Breast Reconstruction: A Population-Based Study. Adv. Radiat. Oncol. 2023, 8, 101104. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Brown, S.A.; Oliveira, I.; Cordeiro, M.; Morales-Helguera, A.; Rodrigues, A.; Amarante, J. Long-term follow-up of breast capsule contracture rates in cosmetic and reconstructive cases. Plast. Reconstr. Surg. 2010, 126, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, P.; Segreto, F.; Carotti, S.; Marangi, G.F.; Tosi, D.; Morini, S. Oestrogen receptor-alpha and -beta expression in breast implant capsules: Experimental findings and clinical correlates. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 308–315. [Google Scholar] [CrossRef]
- Mikulec, A.A.; Hanasono, M.M.; Lum, J.; Kadleck, J.M.; Kita, M.; Koch, R.J. Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch. Facial Plast. Surg. 2001, 3, 111–114. [Google Scholar] [CrossRef]
- Okazaki, M.; Muguruma, M.; Komiya, T.; Miyahara, K.; Kawate, T.; Ueda, A.; Teraoka, S.; Asaoka, M.; Sato, E.; Matsumura, H.; et al. Beneficial effects of transdermal administration of tamoxifen on capsular contracture after breast implantation in murine models. Breast Cancer 2022, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Collette, S.; Collette, L.; Budiharto, T.; Horiot, J.C.; Poortmans, P.M.; Struikmans, H.; Van den Bogaert, W.; Fourquet, A.; Jager, J.J.; Hoogenraad, W.; et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: A study based on the EORTC Trial 22881-10882 ‘boost versus no boost’. Eur. J. Cancer 2008, 44, 2587–2599. [Google Scholar] [CrossRef]
- Lee, K.T.; Bae, J.; Jeon, B.J.; Pyon, J.K.; Mun, G.H.; Bang, S.I. Adjuvant Chemotherapy in Two-Stage Tissue Expander/Implant Breast Reconstruction: Does it Affect Final Outcomes? Ann. Surg. Oncol. 2021, 28, 2191–2198. [Google Scholar] [CrossRef]
- Lam, T.C.; Borotkanics, R.; Hsieh, F.; Salinas, J.; Boyages, J. Immediate Two-Stage Prosthetic Breast Reconstruction Failure: Radiation Is Not the Only Culprit. Plast. Reconstr. Surg. 2018, 141, 1315–1324. [Google Scholar] [CrossRef]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Bachour, Y.; Verweij, S.P.; Gibbs, S.; Ket, J.C.F.; Ritt, M.; Niessen, F.B.; Mullender, M.G. The aetiopathogenesis of capsular contracture: A systematic review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Angele, P.; Schreml, S.; Ulrich, D.; Pöppl, N.; Eisenmann-Klein, M. Determination of serum fibrosis indexes in patients with capsular contracture after augmentation with smooth silicone gel implants. Plast. Reconstr. Surg. 2006, 118, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Schreml, S.; Fichtner-Feigl, S.; Pöppl, N.; Eisenmann-Klein, M.; Schwarze, H.; Füchtmeier, B. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast. Reconstr. Surg. 2007, 120, 275–284. [Google Scholar] [CrossRef]
- Wolfram, D.; Rainer, C.; Niederegger, H.; Piza, H.; Wick, G. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Mun, G.H. Optimal Sequencing of Postmastectomy Radiotherapy and Two Stages of Prosthetic Reconstruction: A Meta-analysis. Ann. Surg. Oncol. 2017, 24, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Santosa, K.B.; Chen, X.; Qi, J.; Ballard, T.N.S.; Kim, H.M.; Hamill, J.B.; Bensenhaver, J.M.; Pusic, A.L.; Wilkins, E.G. Postmastectomy Radiation Therapy and Two-Stage Implant-Based Breast Reconstruction: Is There a Better Time to Irradiate? Plast. Reconstr. Surg. 2016, 138, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Albornoz, C.R.; McCormick, B.; Hudis, C.A.; Hu, Q.; Heerdt, A.; Matros, E. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast. Reconstr. Surg. 2015, 135, 1509–1517. [Google Scholar] [CrossRef]
- Largent, J.A.; Reisman, N.R.; Kaplan, H.M.; Oefelein, M.G.; Jewell, M.L. Clinical Trial Outcomes of High- and Extra High–Profile Breast Implants. Aesthetic Surg. J. 2013, 33, 529–539. [Google Scholar] [CrossRef]
- Spear, S.L.; Murphy, D.K. Natrelle round silicone breast implants: Core Study results at 10 years. Plast. Reconstr. Surg. 2014, 133, 1354–1361. [Google Scholar] [CrossRef]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Wimmer, K.; Fitzal, F. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann. Surg. Oncol. 2023, 30, 126–136. [Google Scholar] [CrossRef]
- Sobti, N.; Weitzman, R.E.; Nealon, K.P.; Jimenez, R.B.; Gfrerer, L.; Mattos, D.; Ehrlichman, R.J.; Gadd, M.; Specht, M.; Austen, W.G.; et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci. Rep. 2020, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Araco, A.; Caruso, R.; Araco, F.; Overton, J.; Gravante, G. Capsular contractures: A systematic review. Plast. Reconstr. Surg. 2009, 124, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Malahias, M.; Jordan, D.J.; Hughes, L.C.; Hindocha, S.; Juma, A. A literature review and summary of capsular contracture: An ongoing challenge to breast surgeons and their patients. Int. J. Surg. Open 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Vrou Offersen, B.; Hol, S.; Arenas, M.; Aristei, C.; Bourgier, C.; Cardoso, M.J.; Chua, B.; Coles, C.E.; Engberg Damsgaard, T.; et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 137, 159–166. [Google Scholar] [CrossRef]
- Shumway, D.A.; Momoh, A.O.; Sabel, M.S.; Jagsi, R. Integration of Breast Reconstruction and Postmastectomy Radiotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2329–2340. [Google Scholar] [CrossRef]
- Zerella, M.A.; Dicuonzo, S.; Zaffaroni, M.; Gerardi, M.A.; Rojas, D.P.; Morra, A.; Nicolò, D.; Cambria, R.; Luraschi, R.; Cattani, F.; et al. Halfmoon Radiotherapy: A Real-World Experience in a Single Institution; Abstract ESTRO 2023; Presentation Number: PO-1278; ESTRO: Brussels, Belgium, 2023. [Google Scholar]
- Wang, S.L.; Fang, H.; Song, Y.W.; Wang, W.H.; Hu, C.; Liu, Y.P.; Jin, J.; Liu, X.F.; Yu, Z.H.; Ren, H.; et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 352–360. [Google Scholar] [CrossRef]
- Whitfield, G.A.; Horan, G.; Irwin, M.S.; Malata, C.M.; Wishart, G.C.; Wilson, C.B. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 90, 141–147. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, E.; Heo, C.Y.; Jin, U.S.; Kim, E.K.; Han, W.; Shin, K.H.; Kim, I.A. Hypofractionated versus conventional fractionated radiotherapy for breast cancer in patients with reconstructed breast: Toxicity analysis. Breast 2021, 55, 37–44. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, E.; Heo, C.Y.; Jin, U.S.; Kim, E.K.; Han, W.; Shin, K.H.; Kim, I.A. Influence of Hypofractionated Versus Conventional Fractionated Postmastectomy Radiation Therapy in Breast Cancer Patients With Reconstruction. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 445–456. [Google Scholar] [CrossRef]
- Rojas, D.P.; Leonardi, M.C.; Frassoni, S.; Morra, A.; Gerardi, M.A.; La Rocca, E.; Cattani, F.; Luraschi, R.; Fodor, C.; Zaffaroni, M.; et al. Implant risk failure in patients undergoing postmastectomy 3-week hypofractionated radiotherapy after immediate reconstruction. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 163, 105–113. [Google Scholar] [CrossRef]
- Khan, A.J.; Poppe, M.M.; Goyal, S.; Kokeny, K.E.; Kearney, T.; Kirstein, L.; Toppmeyer, D.; Moore, D.F.; Chen, C.; Gaffney, D.K.; et al. Hypofractionated Postmastectomy Radiation Therapy Is Safe and Effective: First Results From a Prospective Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Becherini, C.; Boersma, L.; Kaidar-Person, O.; Marta, G.N.; Montero, A.; Offersen, B.V.; Aznar, M.C.; Belka, C.; Brunt, A.M.; et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022, 23, e21–e31. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Capsular Contracture No. (%) | Total No. (%) | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Patients (No., %) | 56 (47.5) | 62 (52.5) | 118 (100.0) | |
| Age, years (No., %) | 0.338 | |||
| Age ≤ 50 | 39 (69.6) | 48 (77.4) | 87 (73.7) | |
| Age > 50 | 17 (30.4) | 14 (22.6) | 31 (26.3) | |
| BMI (Mean, SD) | 24.5 (4.0) | 24.6 (4.7) | 0.834 | |
| Tumor stage (pT/ypT stage) | 0.500 | |||
| 0 | 10 (17.9) | 12 (19.4) | 22 (18.6) | |
| is | 4 (7.1) | 3 (4.8) | 7 (5.9) | |
| 1 | 14 (25.0) | 19 (30.6) | 33 (28.0) | |
| 2 | 17 (30.4) | 23 (37.1) | 40 (33.9) | |
| 3 | 10 (17.9) | 5 (8.1) | 15 (12.7) | |
| 4 | 1 (1.8) | 0 (0.0) | 1 (0.8) | |
| Nodal status (pN/ypN stage) | 0.031 | |||
| 0 | 22 (39.3) | 21 (33.9) | 43 (36.4) | |
| 1 | 22 (39.3) | 37 (59.7) | 59 (50.0) | |
| 2 | 8 (14.3) | 4 (6.5) | 12 (10.2) | |
| 3 | 4 (7.1) | 0 (0.0) | 4 (3.4) | |
| Menopausal status (No., %) | 0.618 | |||
| Premenopausal | 33 (58.9) | 41 (66.1) | 74 (62.7) | |
| Perimenopausal | 6 (10.7) | 4 (6.5) | 10 (8.5) | |
| Postmenopausal | 17 (30.4) | 17 (27.4) | 34 (28.8) | |
| Estrogen receptor/ER (No., %) | 0.247 | |||
| Positive | 41 (73.2) | 50 (80.6) | 91 (77.1) | |
| Negative | 14 (25.0) | 10 (16.1) | 24 (20.3) | |
| Unknown | 1 (1.8) | 2 (3.2) | 3 (2.5) | |
| Progesterone receptor/PR (No., %) | 0.149 | |||
| Positive | 33 (58.9) | 43 (69.4) | 76 (64.4) | |
| Negative | 23 (41.1) | 17 (27.4) | 40 (33.9) | |
| Unknown | 0 (0.0) | 2 (3.2) | 2 (1.7) | |
| Her2-targeted Therapy (No., %) | 0.769 | |||
| Yes | 13 (23.2) | 13 (21.0) | 26 (22.0) | |
| No | 43 (76.8) | 49 (79.0) | 92 (78.0) | |
| Perioperative Chemotherapy (No., %) | 0.153 | |||
| Yes | 49 (87.5) | 48 (77.4) | 97 (82.2) | |
| No | 7 (12.5) | 14 (22.6) | 21 (17.8) | |
| Hormone therapy (No., %) | 0.654 | |||
| Yes | 45 (80.4) | 48 (77.4) | 93 (78.8) | |
| No | 11 (19.6) | 14 (22.6) | 25 (21.2) | |
| Surgery technique (No., %) | 0.763 | |||
| Nipple-sparing mastectomy | 26 (46.4) | 27 (43.5) | 53 (44.9) | |
| Skin-sparing mastectomy | 28 (50.0) | 30 (48.4) | 58 (49.2) | |
| Modified radical mastectomy | 2 (3.6) | 4 (6.5) | 6 (5.1) | |
| Unknown | 0 (0.0) | 1 (1.6) | 1 (0.8) | |
| Lymph node surgery (No., %) | 0.744 | |||
| Sentinel node | 13 (23.2) | 16 (25.8) | 29 (24.6) | |
| Axillary dissection | 43 (76.8) | 46 (74.2) | 89 (75.4) | |
| Implant position (No., %) | 0.472 | |||
| Subcutaneous | 51 (91.1) | 53 (85.5) | 104 (88.1) | |
| Subpectoral | 5 (8.9) | 8 (12.9) | 13 (11.0) | |
| Unknown | 0 (0.0) | 1 (1.6) | ||
| Size, mL (median, IQR) | 330.0 (255.0–445.0) | 335.0 (235.0–420.0) | 335.0 (238.8–437.5) | 0.531 |
| Surface (No., %) | 0.622 | |||
| Textured or Polyurethane | 53 (94.6) | 58 (93.5) | 111 (94.1) | |
| Smooth | 1 (1.8) | 2 (3.2) | 3 (2.5) | |
| Unknown | 2 (3.6) | 2 (3.2) | 4 (3.4) | |
| Shape (No., %) | 0.940 | |||
| Round | 1 (1.8) | 1 (1.6) | 2 (1.7) | |
| Anatomical | 53 (94.6) | 59 (95.2) | 112 (94.9) | |
| Unknown | 2 (3.6) | 2 (3.2) | 4 (3.4) | |
| Utilization of Meshes (No., %) | 5 (8.9) | 1 (1.6) | 6 (5.1) | 0.074 |
| Post-surgery complications (No., %) | ||||
| Yes | 38 (67.9) | 28 (45.2) | 66 (55.9) | 0.017 |
| Seroma/haematoseroma | 19 (33.9) | 14 (22.6) | 33 (28.0) | 0.187 |
| Puncture/aspiration of seroma | 7 (12.5) | 8 (12.9) | 15 (12.7) | 0.921 |
| Prolonged wound healing | 3 (5.4) | 0 (0.0) | 3 (2.5) | 0.065 |
| Prolonged pain | 17 (30.4) | 10 (16.1) | 27 (22.9) | 0.073 |
| Swelling | 8 (14.3) | 6 (9.7) | 14 (11.9) | 0.459 |
| None | 18 (32.1) | 33 (53.2) | 51 (43.2) | |
| Unknown | 0 (0.0) | 1 (1.6) | 1 (0.8) | |
| Radiation Specification | Capsular Contracture No. (%) or Median (IQR) or Mean (SD) | Total No. (%) or Median (IQR) or Mean (SD) | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Time between surgery and start of RT, weeks (Median, IQR) | 9.71 (5.79–26.36) | 10.36 (6.13–26.25) | 10.36 (6.39–26.25) | |
| FB or DIBH (No., %) | 0.711 | |||
| FB | 17 (30.4) | 20 (32.2) | 37 (31.4) | |
| DIBH | 39 (69.6) | 42 (67.7) | 81 (68.6) | |
| Doses and fractionation (No., %) | 0.886 | |||
| 50 Gy in 25 × 2 fractions | 47 (83.9) | 52 (83.9) | 99 (83.9) | |
| 50.4 Gy in 28 × 1.8 fractions | 8 (14.3) | 9 (14.5) | 17 (14.4) | |
| Lower (termination before end of RT, 44–48 Gy) | 1 (1.8) | 1 (1.6) | 2 (1.7) | |
| Tumor-bed boost (No., %) | 3 (5.4) | 2 (3.3) | 5 (4.2) | 0.580 |
| Extent of PMRT (No, %) | 0.183 | |||
| Thoracic wall | 4 (7.1) | 7 (11.3) | 11 (9.3) | |
| Including supra/infraclavicular | 14 (25.0) | 11 (17.7) | 25 (21.2) | |
| Including supra/infraclavicular/IMA | 17 (30.4) | 29 (46.8) | 46 (39.0) | |
| Including supra/infraclavicular/axillary | 15 (26.8) | 8 (12.9) | 23 (19.5) | |
| Including supra/infraclavicular/ axillary/IMA | 6 (10.7) | 7 (11.3) | 13 (11.0) | |
| Radiation technique (No., %) | ||||
| VMAT | 49 (87.5) | 53 (85.5) | 102 (86.4) | |
| 3D | 7 (12.5) | 9 (14.5) | 16 (13.5) | |
| PTV thoracic wall, cm3 (Mean, SD) | 1104.1 (402.2) | 989.5 (372.3) | 1036.2 (385.3) | 0.264 |
| PTV total, cm3 (Mean, SD) | 1255.8 (382.6) | 1185.9 (454.2) | 1205.7 (422.2) | 0.099 |
| PTV-D98%, Gy (Mean, SD) | 37.3 (8.8) | 36.5 (3.9) | 36.9 (6.7) | 0.541 |
| PTV-Dmean, Gy (Mean, SD) | 48.7 (1.5) | 49.1 (1.0) | 48.9 (1.3) | 0.782 |
| PTV-D50%, Gy (Mean, SD) | 49.9 (0.4) | 50.1 (0.8) | 50.0 (0.7) | 0.737 |
| PTV-D2%, Gy (Mean, SD) | 52.7 (1.1) | 53.0 (1.5) | 52.8 (1.3) | 0.360 |
| Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Pathological lymph node status | 0.004 | ||
| N1 | 1.111 | 0.590–2.094 | 0.744 |
| N2 | 2.929 | 1.157–7.415 | 0.023 |
| N3 | 6.739 | 1.979–22.949 | 0.002 |
| Postoperative complications | 2.245 | 1.204–4.187 | 0.011 |
| Chemotherapy | 1.714 | 0.701–4.192 | 0.238 |
| Extent of PMRT | 0.742 | ||
| Chest wall inkl. supra/infra | 1.688 | 9.525–5.421 | 0.379 |
| Inkl. supra/infra/IMA | 1.473 | 0.438–4.954 | 0.531 |
| Inkl. axilla/supra/infra | 2.038 | 0.612–6.781 | 0.246 |
| Inkl. axilla/supra/infra/IMA | 2.164 | 0.553–8.468 | 0.267 |
| PTV volume, cm3 | 1.000 | 1.000–1.001 | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinsensia, M.; Schaub, R.; Meixner, E.; Hoegen, P.; Arians, N.; Forster, T.; Hoeltgen, L.; Köhler, C.; Uzun-Lang, K.; Batista, V.; et al. Incidence and Risk Assessment of Capsular Contracture in Breast Cancer Patients following Post-Mastectomy Radiotherapy and Implant-Based Reconstruction. Cancers 2024, 16, 265. https://doi.org/10.3390/cancers16020265
Vinsensia M, Schaub R, Meixner E, Hoegen P, Arians N, Forster T, Hoeltgen L, Köhler C, Uzun-Lang K, Batista V, et al. Incidence and Risk Assessment of Capsular Contracture in Breast Cancer Patients following Post-Mastectomy Radiotherapy and Implant-Based Reconstruction. Cancers. 2024; 16(2):265. https://doi.org/10.3390/cancers16020265
Chicago/Turabian StyleVinsensia, Maria, Riccarda Schaub, Eva Meixner, Philipp Hoegen, Nathalie Arians, Tobias Forster, Line Hoeltgen, Clara Köhler, Kristin Uzun-Lang, Vania Batista, and et al. 2024. "Incidence and Risk Assessment of Capsular Contracture in Breast Cancer Patients following Post-Mastectomy Radiotherapy and Implant-Based Reconstruction" Cancers 16, no. 2: 265. https://doi.org/10.3390/cancers16020265
APA StyleVinsensia, M., Schaub, R., Meixner, E., Hoegen, P., Arians, N., Forster, T., Hoeltgen, L., Köhler, C., Uzun-Lang, K., Batista, V., König, L., Zivanovic, O., Hennigs, A., Golatta, M., Heil, J., Debus, J., & Hörner-Rieber, J. (2024). Incidence and Risk Assessment of Capsular Contracture in Breast Cancer Patients following Post-Mastectomy Radiotherapy and Implant-Based Reconstruction. Cancers, 16(2), 265. https://doi.org/10.3390/cancers16020265






