Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Current Standard of Care Treatments in BTC
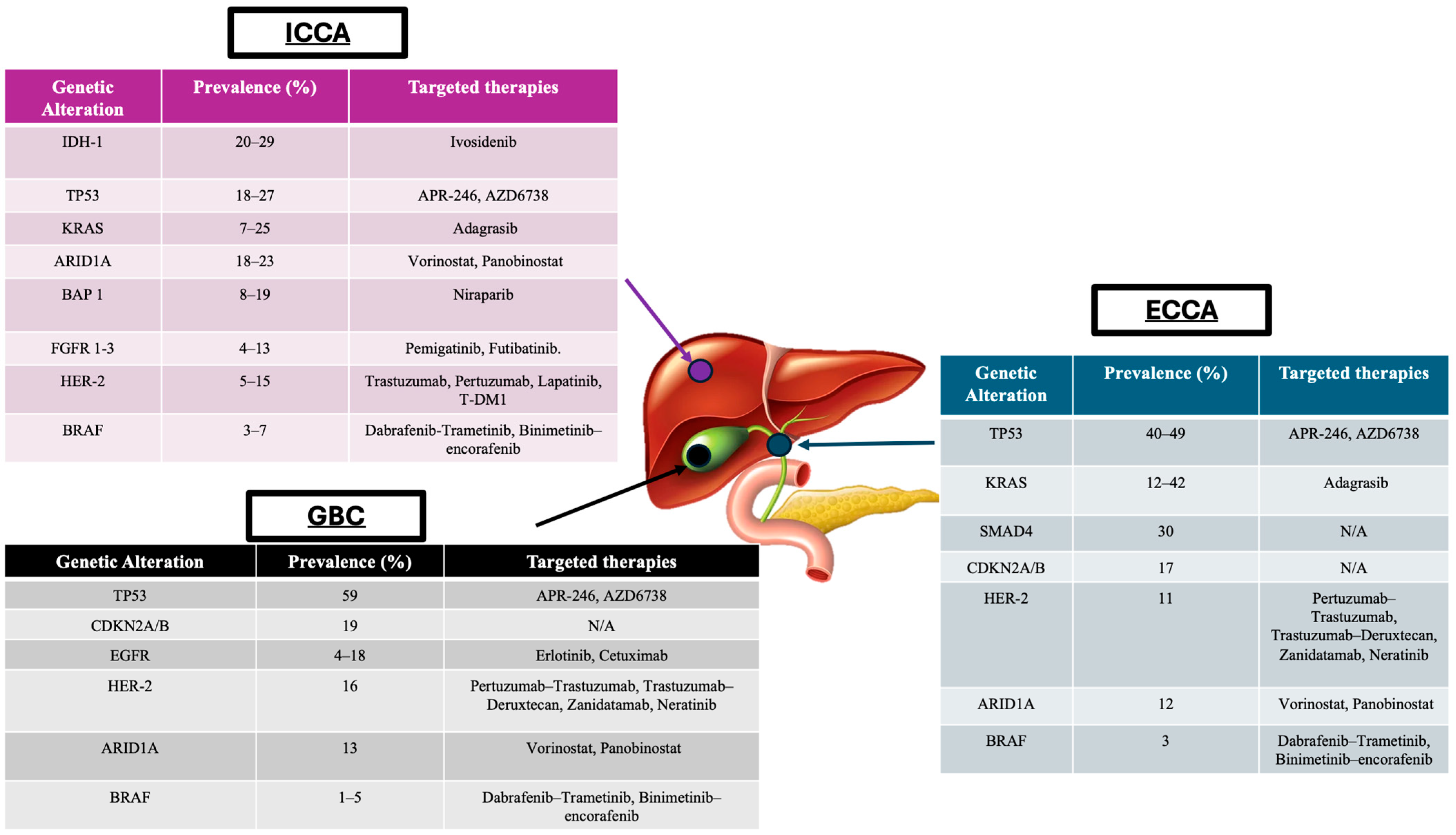
4. Tumour Profiling and Application in BTCs
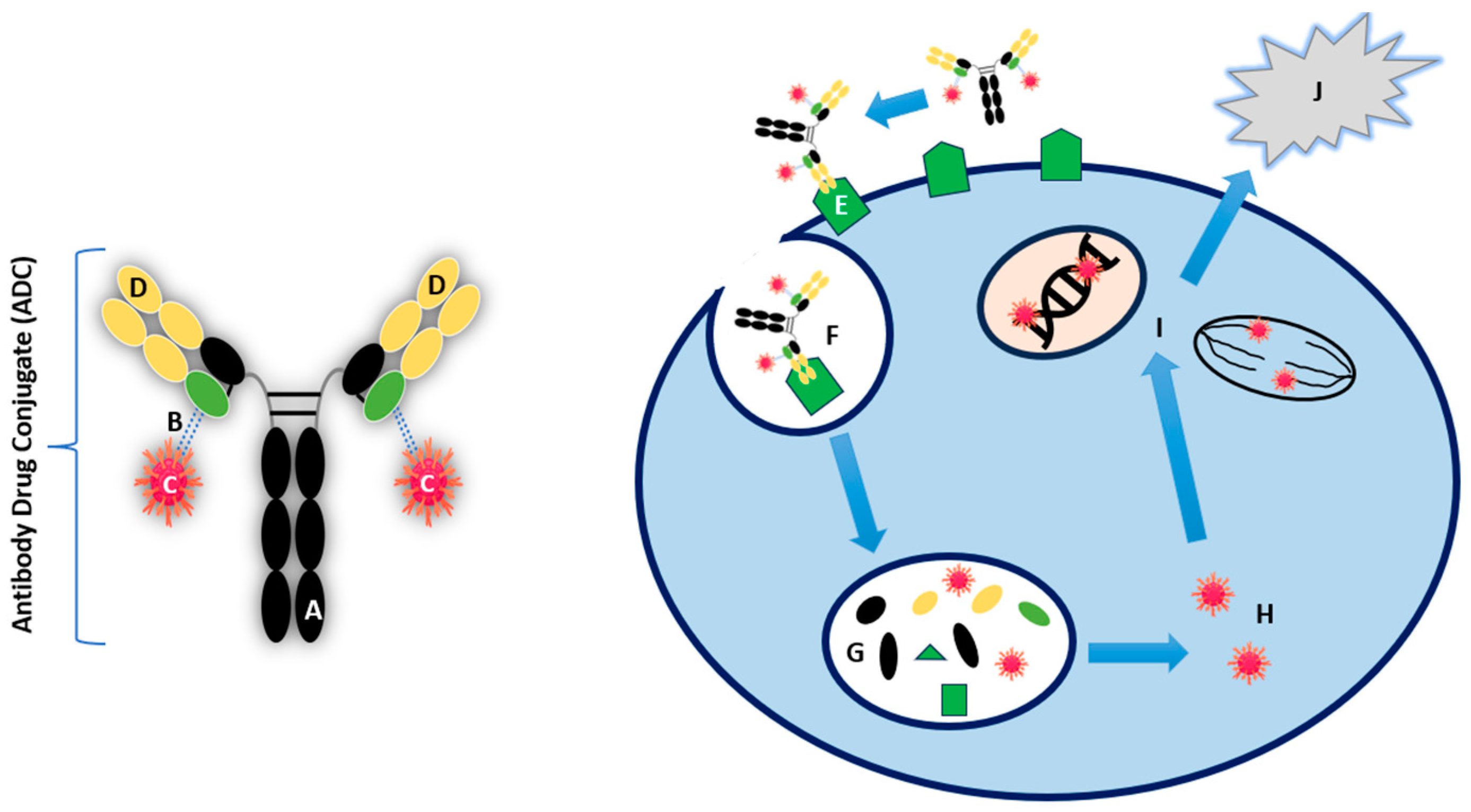
5. Key Components and Mechanism of Action of ADCs
6. Application of Biomarkers during ADC Treatment
7. Advancements in ADC Research and Development
8. ADCs in Breast Cancer
9. ADCs in Lung Cancer
10. ADCs in Urothelial Cancer
11. Use of T-Dxd in a Pan-Tumour Trial
12. Current Trials Assessing the Use of ADCs in BTCs
13. Limitations and Ongoing Challenges Associated with ADCs
14. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e194–e203. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef]
- Baria, K.; De Toni, E.N.; Yu, B.; Jiang, Z.; Kabadi, S.M.; Malvezzi, M. Worldwide Incidence and Mortality of Biliary Tract Cancer. Gastro Hep Adv. 2022, 1, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, V.; Wood, S.; Ramachandran, R.; Williams, G.; Outlaw, D.; Paluri, R.; Kim, Y.; Gbolahan, O. Short- and Long-Term Survival of Metastatic Biliary Tract Cancer in the United States from 2000 to 2018. Cancer Control 2023, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lim, J.; Han, S.-S.; Park, H.M.; Kim, S.-W.; Won, Y.-J.; Park, S.-J. Distinct Prognosis of Biliary Tract Cancer According to Stage, Treatment and Tumor Location: A Population-Based Study. HPB 2022, 24, S468–S469. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine Compared with Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Brandi, G. BILCAP Trial and Adjuvant Capecitabine in Resectable Biliary Tract Cancer: Reflections on a Standard of Care. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 483–485. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, Pemazyre. Committee for Medicinal Products for Human Use (CHMP). Available online: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-pemazyre_en.pdf (accessed on 8 June 2024).
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- European Medicines Agency. Tibsovo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tibsovo (accessed on 8 June 2024).
- FDA. FDA Approves Ivosidenib for Advanced or Metastatic Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma (accessed on 8 June 2024).
- Jain, A.; Javle, M. Molecular Profiling of Biliary Tract Cancer: A Target Rich Disease. J. Gastrointest. Oncol. 2016, 7, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast Growth Factor Receptor 2 Tyrosine Kinase Fusions Define a Unique Molecular Subtype of Cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and Prognostic Significance of Isocitrate Dehydrogenase 1 Mutations in Cholangiocarcinoma: A Systematic Literature Review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Lin, F.P.; Epstein, R.J. How Aging of the Global Population Is Changing Oncology. Ecancermedicalscience 2021, 15, ed119. [Google Scholar] [CrossRef]
- Li, H.; Wei, W.; Xu, H. Drug Discovery Is an Eternal Challenge for the Biomedical Sciences. Acta Mater. Medica 2022, 1, 1–3. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Li, J.; Gu, A.; Nong, X.; Zhai, S.; Yue, Z.; Li, M.; Liu, Y. Six-Membered Aromatic Nitrogen Heterocyclic Anti-Tumor Agents: Synthesis and Applications. Chem. Rec. 2023, 23, e202300293. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; Goyal, L. How I Treat Biliary Tract Cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef]
- Kim, J.W.; Suh, K.J.; Kim, J.-W.; Park, J.H.; Kim, K.H.; Kim, Y.J.; Kim, J.-S.; Kim, J.H.; Choi, I.S. A Randomized Phase II Study of Oxaliplatin/5-FU (MFOLFOX) versus Irinotecan/5-FU (MFOLFIRI) Chemotherapy in Locally Advanced or Metastatic Biliary Tract Cancer Refractory to First-Line Gemcitabine/Cisplatin Chemotherapy. J. Clin. Oncol. 2020, 38, 4603. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal Irinotecan plus Fluorouracil and Leucovorin versus Fluorouracil and Leucovorin for Metastatic Biliary Tract Cancer after Progression on Gemcitabine plus Cisplatin (NIFTY): A Multicentre, Open-Label, Randomised, Phase 2b Study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-Line FOLFOX Chemotherapy versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.; Wasan, H.S.; Ross, P.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. 54MO Quality of Life (QoL) and Value of Health (V-He) in Advanced Biliary Cancers (ABC) Treated with Second-Line Active-Symptom-Control (ASC) Alone or ASC with Oxaliplatin/5-FU Chemotherapy (ASC+FOLFOX) in the Randomised Phase III, Multi-Centre, Open-Label ABC-06 Trial. Ann. Oncol. 2022, 33, S564–S565. [Google Scholar] [CrossRef]
- Walter, T.; Horgan, A.M.; McNamara, M.; McKeever, L.; Min, T.; Hedley, D.; Serra, S.; Krzyzanowska, M.K.; Chen, E.; Mackay, H.; et al. Feasibility and Benefits of Second-Line Chemotherapy in Advanced Biliary Tract Cancer: A Large Retrospective Study. Eur. J. Cancer 2013, 49, 329–335. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Center for Drug Evaluation Research FDA Grants Accelerated Approval to Pemigatinib for Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (accessed on 8 June 2024).
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. FIGHT-202: A Phase II Study of Pemigatinib in Patients (Pts) with Previously Treated Locally Advanced or Metastatic Cholangiocarcinoma (CCA). Ann. Oncol. 2019, 30, v876. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Results from ClarIDHy, a Global, Phase III, Randomized, Double-Blind Study of Ivosidenib (IVO) versus Placebo (PBO) in Patients (Pts) with Previously Treated Cholangiocarcinoma (CCA) and an Isocitrate Dehydrogenase 1 (IDH1) Mutation. J. Clin. Oncol. 2021, 39, 266. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kamgar, M.; Mahipal, A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers 2020, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Valery, M.; Vasseur, D.; Fachinetti, F.; Boilève, A.; Smolenschi, C.; Tarabay, A.; Antoun, L.; Perret, A.; Fuerea, A.; Pudlarz, T.; et al. Targetable Molecular Alterations in the Treatment of Biliary Tract Cancers: An Overview of the Available Treatments. Cancers 2023, 15, 4446. [Google Scholar] [CrossRef] [PubMed]
- Farha, N.; Dima, D.; Ullah, F.; Kamath, S. Precision Oncology Targets in Biliary Tract Cancer. Cancers 2023, 15, 2105. [Google Scholar] [CrossRef]
- DiPeri, T.P.; Javle, M.M.; Meric-Bernstam, F. Next Generation Sequencing for Biliary Tract Cancers. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 471–474. [Google Scholar] [CrossRef]
- Wu, C.-E.; Pan, Y.-R.; Yeh, C.-N.; Lunec, J. Targeting P53 as a Future Strategy to Overcome Gemcitabine Resistance in Biliary Tract Cancers. Biomolecules 2020, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Jeong, M.; In, H.; Kim, J.H.; Lin, C.-W.; Han, K.H. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies 2023, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; McShane, L.M.; Dowsett, M. HER2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J. Oncol. Pract. 2018, 14, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of Resistance to Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Trastuzumab Emtansine for Treating HER2-Positive Advanced Breast Cancer after Trastuzumab and a Taxane. Available online: https://www.nice.org.uk/guidance/ta458 (accessed on 22 July 2024).
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of Antibody–Drug Conjugates (ADCs) for Cancer Therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Lucas, A.; Price, L.; Schorzman, A.; Storrie, M.; Piscitelli, J.; Razo, J.; Zamboni, W. Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies 2018, 7, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Gou, L.; Li, W.; Wang, Y. Antibody–Drug Conjugates: Recent Advances in Payloads. Acta Pharm. Sin. B 2023, 13, 4025–4059. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting Cancer with Antibody-Drug Conjugates: Promises and Challenges. MAbs 2021, 13, 1951427. [Google Scholar] [CrossRef]
- Widdison, W.C.; Chari, R.V.J. Factors Involved in the Design of Cytotoxic Payloads for Antibody–Drug Conjugates. In Antibody-Drug Conjugates and Immunotoxins; Springer: New York, NY, USA, 2013; pp. 93–115. [Google Scholar]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload Diversification: A Key Step in the Development of Antibody–Drug Conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L. A Brief Introduction to Antibody–Drug Conjugates for Toxicologic Pathologists. Toxicol. Pathol. 2018, 46, 746–752. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-Drug Conjugates: Recent Advances in Conjugation and Linker Chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Hayes, D.F. Cancer Biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Eberly, H.W.; Sciscent, B.Y.; Lorenz, F.J.; Rettig, E.M.; Goyal, N. Current and Emerging Diagnostic, Prognostic, and Predictive Biomarkers in Head and Neck Cancer. Biomedicines 2024, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Y.; Park, K.S.; Nam, S.E.; Yoo, Y.B.; Park, W.S.; Yun, I.J. BRCA1/2 Serves as a Biomarker for Poor Prognosis in Breast Carcinoma. Int. J. Mol. Sci. 2022, 23, 3754. [Google Scholar] [CrossRef] [PubMed]
- Jóhannsson, O.T.; Ranstam, J.; Borg, A.; Olsson, H. Survival of BRCA1 Breast and Ovarian Cancer Patients: A Population-Based Study from Southern Sweden. J. Clin. Oncol. 1998, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chetrit, A.; Hirsh-Yechezkel, G.; Ben-David, Y.; Lubin, F.; Friedman, E.; Sadetzki, S. Effect of BRCA1/2 Mutations on Long-Term Survival of Patients with Invasive Ovarian Cancer: The National Israeli Study of Ovarian Cancer. J. Clin. Oncol. 2008, 26, 20–25. [Google Scholar] [CrossRef]
- Patani, N.; Martin, L.-A.; Dowsett, M. Biomarkers for the Clinical Management of Breast Cancer: International Perspective. Int. J. Cancer 2013, 133, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Blanquisett, A.; Touya, D.; Strasser-Weippl, K.; Ruiz, R.; St. Louis, J.; Goss, P. Current and Emerging Therapies of HER2-Positive Metastatic Breast Cancer. Breast 2016, 29, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Bussing, D.; Sharma, S.; Li, Z.; Meyer, L.F.; Shah, D.K. Quantitative Evaluation of the Effect of Antigen Expression Level on Antibody–Drug Conjugate Exposure in Solid Tumor. AAPS J. 2021, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef] [PubMed]
- De Matos, L.L.; Trufelli, D.C.; De Matos, M.G.L.; Da Silva Pinhal, M.A. Immunohistochemistry as an Important Tool in Biomarkers Detection and Clinical Practice. Biomark. Insights 2010, 5, BMI-S2185. [Google Scholar] [CrossRef]
- Colomer, R.; Mondejar, R.; Romero-Laorden, N.; Alfranca, A.; Sanchez-Madrid, F.; Quintela-Fandino, M. When Should We Order a next Generation Sequencing Test in a Patient with Cancer? EClinicalMedicine 2020, 25, 100487. [Google Scholar] [CrossRef] [PubMed]
- Bosi, C.; Bartha, Á.; Galbardi, B.; Notini, G.; Naldini, M.M.; Licata, L.; Viale, G.; Mariani, M.; Pistilli, B.; Ali, H.R.; et al. Pan-Cancer Analysis of Antibody-Drug Conjugate Targets and Putative Predictors of Treatment Response. Eur. J. Cancer 2023, 195, 113379. [Google Scholar] [CrossRef]
- FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for HER2-Low Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (accessed on 20 July 2024).
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s Magic Bullet Concept: 100 Years of Progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Trail, P.A.; Willner, D.; Lasch, S.J.; Henderson, A.J.; Hofstead, S.; Casazza, A.M.; Firestone, R.A.; Hellström, I.; Hellström, K.E. Cure of Xenografted Human Carcinomas by BR96-Doxorubicin Immunoconjugates. Science 1993, 261, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.H.; DeHerdt, S.V.; Schneck, D.W.; Bumol, T.F. The Human Immune Response to KS1/4-Desacetylvinblastine (LY256787) and KS1/4-Desacetylvinblastine Hydrazide (LY203728) in Single and Multiple Dose Clinical Studies. Cancer Res. 1991, 51, 2286–2290. [Google Scholar] [PubMed]
- Norsworthy, K.J.; Ko, C.-W.; Lee, J.E.; Liu, J.; John, C.S.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist 2018, 23, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Tenchov, R.; Bird, R.; Iyer, K.A.; Ralhan, K.; Rodriguez, Y.; Zhou, Q.A. The Evolving Landscape of Antibody–Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjug. Chem. 2023, 34, 1951–2000. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Center for Drug Evaluation Research FDA Grants Accelerated Approval to Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HER2-Positive Solid Tumours. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed on 20 July 2024).
- Nakada, T. Discovery Research and Translation Science of Trastuzumab Deruxtecan, from Non-Clinical Study to Clinical Trial. Transl. Regul. Sci. 2021, 3, 65–71. [Google Scholar] [CrossRef]
- Martín, M.; Pandiella, A.; Vargas-Castrillón, E.; Díaz-Rodríguez, E.; Iglesias-Hernangómez, T.; Martínez Cano, C.; Fernández-Cuesta, I.; Winkow, E.; Perelló, M.F. Trastuzumab Deruxtecan in Breast Cancer. Crit. Rev. Oncol. Hematol. 2024, 198, 104355. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, N.L.; Brown, M.P.; Liapis, V.; Staudacher, A.H. Antibody Drug Conjugates: Hitting the Mark in Pancreatic Cancer? J. Exp. Clin. Cancer Res. 2023, 42, 280. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Bragaia, V.P.H.; Yeo, W.; Kim, S.-B.; Bianchini, G.; Yamashita, T.; Yonemori, K.; Inoue, K.; Curigliano, G.; Hurvitz, S.A.; et al. Trastuzumab Deruxtecan (T-DXd) versus Trastuzumab Emtansine (T-DM1) in Patients (Pts) with HER2-Positive (HER2+) Unresectable and/or Metastatic Breast Cancer (MBC): Safety Follow-up of the Randomized, Phase 3 Study DESTINY-Breast03. J. Clin. Oncol. 2022, 40, 1000. [Google Scholar] [CrossRef]
- FDA Grants Regular Approval to Fam-Trastuzumab Deruxtecan-Nxki for Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-fam-trastuzumab-deruxtecan-nxki-breast-cancer (accessed on 20 July 2024).
- Smit, E.F.; Felip, E.; Uprety, D.; Nakagawa, K.; Paz-Ares, L.; Pacheco, J.; Li, B.T.; Planchard, D.; Baik, C.; Goto, Y.; et al. 975P Trastuzumab Deruxtecan in Patients (Pts) with HER2-Overexpressing (HER2-OE) Metastatic Non-Small Cell Lung Cancer (NSCLC): Results from the DESTINY-Lung01 Trial. Ann. Oncol. 2022, 33, S994–S995. [Google Scholar] [CrossRef]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; de Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients with HER2-Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results from the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Interstitial Lung Disease/Pneumonitis: ENHERTU® (FAM-Trastuzumab Deruxtecan-Nxki), n.d. ENHERTU®. Available online: https://www.enhertuhcp.com/en/managing-adverse-reactions/interstitial-lung-disease-pneumonitis (accessed on 20 July 2024).
- Powell, C.A.; Modi, S.; Iwata, H.; Takahashi, S.; Smit, E.F.; Siena, S.; Chang, D.-Y.; Macpherson, E.; Qin, A.; Singh, J.; et al. Pooled Analysis of Drug-Related Interstitial Lung Disease and/or Pneumonitis in Nine Trastuzumab Deruxtecan Monotherapy Studies. ESMO Open 2022, 7, 100554. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Crossno, C.L.; Gesthalter, Y.B.; Kelley, K.; Moore, H.N.; Rimawi, M.F.; Westbrook, K.E.; Buys, S.S. Real-World Perspectives and Practices for Pneumonitis/Interstitial Lung Disease Associated with Trastuzumab Deruxtecan Use in Human Epidermal Growth Factor Receptor 2–Expressing Metastatic Breast Cancer. JCO Oncol. Pract. 2023, 19, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Shen, G.; Li, J.; Qiu, T.; Fang, Q.; Zheng, Y.; Xin, Y.; Liu, Z.; Zhao, F.; Ren, D.; et al. Incidence of Antibody–Drug Conjugates-Related Pneumonitis in Patients with Solid Tumors: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2023, 184, 103960. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, G.; Deng, X.; Zhang, Y.; Jia, Z.; Huang, Z. Antibody-Drug Conjugates in Urinary Tumors: Clinical Application, Challenge, and Perspectives. Front. Oncol. 2023, 13, 1259784. [Google Scholar] [CrossRef]
- Ungaro, A.; Tucci, M.; Audisio, A.; Di Prima, L.; Pisano, C.; Turco, F.; Delcuratolo, M.D.; Di Maio, M.; Scagliotti, G.V.; Buttigliero, C. Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022, 11, 803. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing after Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results from the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Rubin, E.; Shan, K.; Dalal, S.; Vu, D.; Milillo-Naraine, A.; Guaqueta, D.; Ergle, A. Molecular Targeting of the Human Epidermal Growth Factor Receptor-2 (HER2) Genes across Various Cancers. Int. J. Mol. Sci. 2024, 25, 1064. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Noske, A.; Ramach, C.; Padberg, B.; Moch, H. Assessment of HER2 Status in Breast Cancer: Overall Positivity Rate and Accuracy by Fluorescence in Situ Hybridization and Immunohistochemistry in a Single Institution over 12 Years: A Quality Control Study. BMC Cancer 2013, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Ayasun, R.; Ozer, M.; Sahin, I. The Role of HER2 Status in the Biliary Tract Cancers. Cancers 2023, 15, 2628. [Google Scholar] [CrossRef] [PubMed]
- Gagan, J.; Van Allen, E.M. Next-Generation Sequencing to Guide Cancer Therapy. Genome Med. 2015, 7, 80. [Google Scholar] [CrossRef]
- ten Haaft, B.H.E.A.; Pedregal, M.; Prato, J.; Klümpen, H.-J.; Moreno, V.; Lamarca, A. Revolutionizing Anti-HER2 Therapies for Extrahepatic Cholangiocarcinoma and Gallbladder Cancer: Current Advancements and Future Perspectives. Eur. J. Cancer 2024, 199, 113564. [Google Scholar] [CrossRef] [PubMed]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab Deruxtecan (T-DXd; DS-8201) in Patients (Pts) with HER2-Expressing Unresectable or Recurrent Biliary Tract Cancer (BTC): An Investigator-Initiated Multicenter Phase 2 Study (HERB Trial). J. Clin. Oncol. 2022, 40, 4006. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Tazbirkova, A.; Yang, J.; Yue, J.; Sun, Y.; Pan, Y.; Sun, M.; Qin, Y.; Shen, L.; et al. Safety and Efficacy of IBI343 (Anti-Claudin18.2 Antibody-Drug Conjugate) in Patients with Advanced Pancreatic Ductal Adenocarcinoma or Biliary Tract Cancer: Preliminary Results from a Phase 1 Study. J. Clin. Oncol. 2024, 42, 3037. [Google Scholar] [CrossRef]
- D’Arienzo, A.; Verrazzo, A.; Pagliuca, M.; Napolitano, F.; Parola, S.; Viggiani, M.; Caputo, R.; Puglisi, F.; Giuliano, M.; Del Mastro, L.; et al. Toxicity Profile of Antibody-Drug Conjugates in Breast Cancer: Practical Considerations. EClinicalMedicine 2023, 62, 102113. [Google Scholar] [CrossRef]
- Colombo, R.; Rich, J.R. The Therapeutic Window of Antibody Drug Conjugates: A Dogma in Need of Revision. Cancer Cell 2022, 40, 1255–1263. [Google Scholar] [CrossRef]
- Bogenberger, J.M.; DeLeon, T.T.; Arora, M.; Ahn, D.H.; Borad, M.J. Emerging Role of Precision Medicine in Biliary Tract Cancers. NPJ Precis. Oncol. 2018, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.-Z.; Zhang, Y.; Guo, Y.; Guo, W.; Nian, W.; Liao, W.; Xu, Z.; Zhang, W.; Zhao, H.-Y.; Wei, X.; et al. Evaluation of Safety of Treatment with Anti–Epidermal Growth Factor Receptor Antibody Drug Conjugate MRG003 in Patients with Advanced Solid Tumors. JAMA Oncol. 2022, 8, 1042. [Google Scholar] [CrossRef]
- Xie, N.; Cai, J.-B.; Zhang, L.; Zhang, P.-F.; Shen, Y.-H.; Yang, X.; Lu, J.-C.; Gao, D.-M.; Kang, Q.; Liu, L.-X.; et al. Upregulation of B7-H4 Promotes Tumor Progression of Intrahepatic Cholangiocarcinoma. Cell Death Dis. 2017, 8, 3205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, F.; Li, Z.; Jiang, P.; Deng, X.; Tian, F.; Li, X.; Wang, S. Aberrant Expression of B7-H4 Correlates with Poor Prognosis and Suppresses Tumor-Infiltration of CD8+ T Lymphocytes in Human Cholangiocarcinoma. Oncol. Rep. 2016, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Teng, S.; Zhang, Y.; Zhang, W.; Zhang, X.; Xu, K.; Yao, H.; Yao, J.; Wang, H.; Liang, X.; et al. TROP2 Promotes Proliferation, Migration and Metastasis of Gallbladder Cancer Cells by Regulating PI3K/AKT Pathway and Inducing EMT. Oncotarget 2017, 8, 47052–47063. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.; Filetti, M.; Falcone, R.; Altamura, V.; Paroni Sterbini, F.; Bria, E.; Fabi, A.; Giannarelli, D.; Scambia, G.; Daniele, G. Overview of Trop-2 in Cancer: From Pre-Clinical Studies to Future Directions in Clinical Settings. Cancers 2023, 15, 1744. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Lisberg, A.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Vicente Baz, D.; Sugawara, S.; Cobo Dols, M.; Pérol, M.; et al. LBA12 Datopotamab Deruxtecan (Dato-DXd) vs Docetaxel in Previously Treated Advanced/Metastatic (Adv/Met) Non-Small Cell Lung Cancer (NSCLC): Results of the Randomized Phase III Study TROPION-Lung01. Ann. Oncol. 2023, 34, S1305–S1306. [Google Scholar] [CrossRef]
- Lee, J.K.; Sivakumar, S.; Schrock, A.B.; Madison, R.; Fabrizio, D.; Gjoerup, O.; Ross, J.S.; Frampton, G.M.; Napalkov, P.; Montesion, M.; et al. Comprehensive Pan-Cancer Genomic Landscape of KRAS Altered Cancers and Real-World Outcomes in Solid Tumors. NPJ Precis. Oncol. 2022, 6, 91. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Döppler, H.; Necela, B.; Edenfield, B.; Zhang, L.; Dawson, D.W.; Storz, P. Mutant KRAS–Induced Expression of ICAM-1 in Pancreatic Acinar Cells Causes Attraction of Macrophages to Expedite the Formation of Precancerous Lesions. Cancer Discov. 2015, 5, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, A.; Romayor, I.; Arteta, B. Role of Liver ICAM-1 in Metastasis. Oncol. Lett. 2017, 14, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Agoston, A.T.; Guo, P.; Moses, M.A. A Rationally Designed ICAM1 Antibody Drug Conjugate for Pancreatic Cancer. Adv. Sci. 2020, 7, 2002852. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.S.; Frantz, C.; Schutten, M.M.; Zhong, F.; del Rosario, G.; Go, M.A.T.; Yu, S.-F.; Leipold, D.D.; Kamath, A.V.; Ng, C.; et al. Calicheamicin Antibody–Drug Conjugates with Improved Properties. Mol. Cancer Ther. 2021, 20, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, A.; Sapkota, S.; Sharma, A.; Riano, I.; Kurzrock, R.; Adashek, J.J. Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload. Pharmaceutics 2023, 15, 2160. [Google Scholar] [CrossRef]
- Loganzo, F.; Sung, M.; Gerber, H.-P. Mechanisms of Resistance to Antibody-Drug Conjugates. Mol. Cancer Ther. 2016, 15, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor Cells Chronically Treated with a Trastuzumab–Maytansinoid Antibody–Drug Conjugate Develop Varied Resistance Mechanisms but Respond to Alternate Treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef]
- Owen, S.C.; Patel, N.; Logie, J.; Pan, G.; Persson, H.; Moffat, J.; Sidhu, S.S.; Shoichet, M.S. Targeting HER2 + Breast Cancer Cells: Lysosomal Accumulation of Anti-HER2 Antibodies Is Influenced by Antibody Binding Site and Conjugation to Polymeric Nanoparticles. J. Control. Release 2013, 172, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Shao, Z.; Yu, K. Resistance to Antibody-drug Conjugates in Breast Cancer: Mechanisms and Solutions. Cancer Commun. 2023, 43, 297–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of Hypoxia in the Tumor Microenvironment and Targeted Therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef]
- Hanker, A.B.; Garrett, J.T.; Estrada, M.V.; Moore, P.D.; Ericsson, P.G.; Koch, J.P.; Langley, E.; Singh, S.; Kim, P.S.; Frampton, G.M.; et al. HER2-Overexpressing Breast Cancers Amplify FGFR Signaling upon Acquisition of Resistance to Dual Therapeutic Blockade of HER2. Clin. Cancer Res. 2017, 23, 4323–4334. [Google Scholar] [CrossRef]
- European Medicines Agency. Enhertu, 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu (accessed on 22 July 2024).
| ID | Title of Study | Antibody Drug Conjugate | Primary Site | Primary Outcome | Target Recruitment Number |
|---|---|---|---|---|---|
| NCT04482309 | A Phase 2, Multicentre, Open-label Study to Evaluate the Efficacy and Safety of Trastuzumab Deruxtecan (T-DXd, DS-8201a) for the Treatment of Selected HER2 Expressing Tumours (DESTINY-PanTumor02) | Trastuzumab Deruxtecan | Biliary tract, bladder, cervical, colorectal, endometrial, epithelial, gastric, non-small cell lung, ovarian, pancreatic. | Evaluate the efficacy and safety of Trastuzumab Deruxtecan (T-DXd) for the treatment of selected HER2-expressing tumours | 468 |
| NCT04644068 | A Modular Phase I/IIa, Open-label, Multicentre Study to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Preliminary Efficacy of Ascending Doses of AZD5305 as Monotherapy and in Combination With Anti-cancer Agents in Patients With Advanced Solid Malignancies | Trastuzumab Deruxtecan | Biliary tract, ovarian, breast, pancreatic, prostate, small cell and non-small cell lung, colorectal, bladder, gastric, cervical, endometrial | Determine if experimental treatment with PARP inhibitor, AZD5305, alone, or in combination with anti-cancer agents is safe, tolerable, and has anti-cancer activity in patients with advanced solid tumours. | 804 |
| NCT04838964 | An Open-label, Single-arm, Multi-center, Phase II Clinical Study of MRG003 in the Treatment of Patients With EGFR-positive Unresectable, Locally Advanced or Metastatic Biliary Tract Cancer | MRG003 | Advanced or metastatic biliary cancer | Assess the safety, efficacy, pharmacokinetics, and immunogenicity of MRG003 as single agent in EGFR-positive unresectable locally advanced or metastatic biliary tract cancer patients who have progressed during or relapsed after at least one prior standard therapy. | 80 |
| NCT05123482 | A Phase I/IIa Multi-center, Open-label Master Protocol Dose Escalation and Expansion Study of AZD8205 as Monotherapy and in Combination With Anticancer Agents in Participants With Advanced Solid Tumours (BLUESTAR) | AZD8205 | Biliary tract, breast, endometrial, ovarian | Study a possible treatment for advanced or metastatic solid tumours alone or in combination with anti-cancer agents | 340 |
| NCT05489211 | A Phase II, Multicentre, Open-label, Master Protocol to Evaluate the Efficacy and Safety of Datopotamab Deruxtecan (Dato-DXd) as Monotherapy and in Combination With Anticancer Agents in Patients With Advanced/Metastatic Solid Tumours | Datopotamab Deruxtecan | Biliary tract, urothelial, colorectal, ovarian, endometrial, gastric, prostate | Investigate the safety, tolerability, and anti-tumour activity of Datopotamab Deruxtecan (Dato-DXd) as monotherapy and in combination with anti-cancer agents in patients with advanced/metastatic solid tumours. | 582 |
| NCT04329429 | An Open-label, Single-arm, Multi-center, Phase II Study of RC48-ADC in Subjects With HER2 Overexpressed Locally Advanced or Metastatic Biliary Tract Cancer (BTC) Who Have Failed First-line Chemotherapy | RC48-ADC | Biliary tract | Evaluate the efficacy and safety of intravenous RC48-ADC in patients with locally advanced or metastatic HER2 overexpressed biliary tract cancer who have failed first-line chemotherapy. | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, S.; Aleem, U.; Jacobs, T.; Frizziero, M.; Foy, V.; Hubner, R.A.; McNamara, M.G. Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer. Cancers 2024, 16, 3345. https://doi.org/10.3390/cancers16193345
Alexander S, Aleem U, Jacobs T, Frizziero M, Foy V, Hubner RA, McNamara MG. Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer. Cancers. 2024; 16(19):3345. https://doi.org/10.3390/cancers16193345
Chicago/Turabian StyleAlexander, Shaun, Umair Aleem, Timothy Jacobs, Melissa Frizziero, Victoria Foy, Richard A. Hubner, and Mairéad G. McNamara. 2024. "Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer" Cancers 16, no. 19: 3345. https://doi.org/10.3390/cancers16193345
APA StyleAlexander, S., Aleem, U., Jacobs, T., Frizziero, M., Foy, V., Hubner, R. A., & McNamara, M. G. (2024). Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer. Cancers, 16(19), 3345. https://doi.org/10.3390/cancers16193345






