Simple Summary
In this study, we investigated whether the anatomical location of metastases from posterior uveal melanoma affects survival. We found that patients with newly diagnosed metastatic posterior uveal melanoma who only have extrahepatic metastases had a significantly longer survival compared to patients with liver metastases. This insight could help clinicians improve the prediction of patient outcomes and enhance the selection of patients in clinical trials.
Abstract
Background/Objectives: Metastatic posterior uveal melanoma (PUM) is one of the deadliest types of melanomas. Though the median survival is short, some patients with metastatic disease live for a long time. In this study, we investigated whether the anatomical location of the metastatic lesions is associated with differences in survival. Methods: One hundred and seventy-eight patients with metastatic PUM with baseline whole-body imaging were retrospectively included. The patients were divided into three groups based on the anatomical location of metastases: (1) exclusive liver metastases (hepatic pattern), (2) both hepatic and extrahepatic metastatic lesions (hepatic–extrahepatic pattern), and (3) exclusive extrahepatic lesions (extrahepatic pattern). Survival was investigated using Kaplan–Meier plots, log-rank test, and the Cox proportional hazard model. Results: In total, 95 patients (53%) presented with hepatic pattern, 66 patients (37%) presented with hepatic–extrahepatic pattern, and 17 patients (10%) presented with extrahepatic pattern. Overall survival was significantly longer in patients with extrahepatic pattern (median 17.0 months) compared to those with hepatic pattern (median 11.0 months) and hepatic–extrahepatic pattern (median 7.0 months) (p < 0.001, log-rank test). Multivariate Cox regression analysis showed increased hazard ratios (HR) for hepatic pattern (HR 2.37, 95% CI 1.08–5.17, p = 0.031) and hepatic–extrahepatic pattern (3.25, 95% CI 1.42–7.41, p = 0.005) compared to extrahepatic pattern. Most patients with hepatic (95%) and hepatic–extrahepatic patterns (82%) were diagnosed with metastases by liver ultrasonography screening, whereas 81% of patients with extrahepatic pattern developed symptoms that led to the diagnosis. Conclusions: Extrahepatic pattern was associated with prolonged survival in patients with metastatic PUM, despite there being a larger proportion of symptomatic patients. It is therefore important to consider the anatomical location of the metastatic lesions when stratifying patients into clinical trials.
1. Introduction
Posterior uveal melanoma (PUM) is a rare melanoma subtype that arises in the ciliary body and the choroid. It is the most common primary intraocular malignancy, with the highest incidence rates, above 8.0 cases per million person years occurring in Northern European countries and in Australia and New Zealand [1,2]. PUM has a high tendency to metastasize, primarily to the liver [3,4], and about half of the patients die due to metastatic disease [5]. The size of the eye tumor, extraocular growth, ciliary body involvement, and chromosomal and genetic alterations in the primary tumor, such as monosomy 3 and mutation in BAP1, increase the risk of metastasis [6,7,8,9]. After the development of metastatic disease, the median survival is about 12 months [10]. Though survival is short, some patients with metastatic PUM live for several years [11], indicating that there might be patient subgroups with a less aggressive phenotype. Over the last few decades, there has been an increased focus on personalized risk stratification and identification of prognostic factors among patients with metastatic PUM [12,13,14,15,16]. A better risk stratification of the metastatic patients could facilitate relevant enrollment of patients in randomized clinical trials and potentially improve prognostication for the individual patient. Several biomarkers predicting survival from the onset of metastatic disease have been proposed [13,14,15,16]. Increasing size of the largest diameter of the largest metastatic lesion (LDLM) is incorporated as a negative prognostic factor for survival in the 8th Edition of The American Joint Committee on Cancer (AJCC) staging system (M1a: LDLM ≤ 3.0 cm; M1b: LDLM 3.1–8.0 cm; M1c: LDLM ≥ 8.1 cm) [12]. Performance status, liver enzyme levels, number of liver metastases, and disease-free interval have also been associated with survival in metastatic PUM patients [13,14,15,16].
It is well known that metastases from PUM disseminate to the liver in most cases, whereas only a minority of patients present exclusively with extrahepatic metastases [3,4]. Emerging evidence indicates that the metastatic pattern at diagnosis might be associated with prognosis [3,4,11,17,18,19,20,21,22]. Several small studies [3,11,17,18,19,20,21,22] and one recent larger study [4] have suggested that exclusive extrahepatic metastases are associated with prolonged survival. In these studies, data about the anatomical location of metastases were collected from medical records, liver ultrasonography, liver magnetic resonance imaging (MRI), abdominal computed tomography (CT), and, only to a limited extent, whole-body imaging (CT or positron emission-computed tomography (PET/CT)). Hence, extrahepatic involvement might be underrepresented in these cohorts. We therefore set out to investigate whether survival depends on the anatomical location of the metastatic lesions using whole-body imaging performed at the time of metastatic diagnosis.
2. Materials and Methods
2.1. Patients
Uveal melanoma patients treated at The Department of Ophthalmology, Copenhagen University Hospital, Copenhagen, Denmark are registered in the Copenhagen Epidemiological Uveal Melanoma Study (COEUS) database [23]. Based on chromosomal risk profiling, high-risk primary uveal melanoma patients are offered screening with regular liver ultrasonography every 6 months for 5 years, and then once yearly for a further 5 years. Patients that develop metastatic PUM are offered imaging work-up to assess the extent of dissemination, with CT or PET/CT of at least the chest and abdomen. Data on all patients diagnosed with primary PUM at Copenhagen University Hospital, Copenhagen, Denmark between 2000 and 2020 were identified from the COEUS database and the respective medical records were retrieved. Development of metastatic disease was last checked on 31 December 2022 (corresponding to at least two years of follow-up from primary diagnosis). All patients who had an MRI, CT, and/or PET/CT of at least both the chest and the abdomen performed within five weeks from diagnosis of metastatic PUM were retrospectively included. Patients without available imaging or with imaging performed later than five weeks from metastatic diagnosis were excluded.
2.2. Clinical and Imaging Data
Images and imaging reports were assessed via the local repository at Copenhagen University Hospital, Copenhagen, Denmark the local repository at Aarhus University Hospital, Aarhus, Denmark or retrieved from the local departments of clinical physiology and nuclear medicine or radiology departments throughout Denmark. Data on the anatomical location of metastases and the LDLM were collected from imaging reports (MRI, and/or CT, and/or PET/CT). When imaging reports were not available or the LDLM was not measured, two of the authors (EB and CWB) re-examined the scans. Extrahepatic metastases verified by other diagnostic methods than imaging (e.g., a skin biopsy performed within five weeks after metastatic diagnosis) were also registered. Oncological treatment data were retrieved from the Danish Metastatic Melanoma Database (DAMMED), a research database including all Danish metastatic melanoma patients [24]. First-line treatment with the combination of ipilimumab and nivolumab (ipi+nivo) was included as a treatment variable. The patients were staged according to the AJCC 8th Edition cancer staging system for patients with metastatic PUM [12]. The vital status was last checked on 31 December 2023 (corresponding to a minimum of 12 months of follow-up from metastatic diagnosis). The cause of death was evaluated using clinical charts and data from the Danish Register of Causes of Death [25]. Approval from the Regional Ethics Committee for the Capitol Region of Denmark was obtained with dispensation for informed consent (protocol number: H-21015415, 8 July 2021), and the study adhered to the tenets of the Declaration of Helsinki [26].
2.3. Statistics
The endpoint was overall survival, defined as the time from the date of the first metastatic lesion diagnosed on imaging to the date of death or last follow-up. None of the patients included were lost to follow-up. The disease-free interval was defined as the time from primary diagnosis until the date of the first metastatic lesion diagnosed on imaging. Survival curves were visualized using Kaplan–Meier plots and compared with log-rank tests. The Cox proportional hazard model was used to estimate hazard ratios (HR). Variables included in the multivariate Cox regression were chosen based on clinical relevance. The assumption of proportional hazards was evaluated for all variables using the cumulative score process test and cumulative martingale residuals. Non-proportional variables were included as strata in the multivariate Cox model. The Kruskal–Wallis test was used to check for statistical differences among multiple groups. Statistical analysis was conducted using RStudio (version 2023.06.0) (Rstudio Team, 2023) and the packages: “survival” (version 3.5.5), “survminer” (version 0.4.9), “mets” (version 1.3.2), “timereg” (version 2.0.5), “stats” (version 4.3.0), “ggplot2” (version 3.4.4), and “dplyr” (version 1.1.2). A p-value below 0.05 was considered significant.
3. Results
3.1. Patient Characteristics
In total, 252 out of 795 patients (32%) with PUM developed metastatic disease during follow-up. Seventy-one patients (28%) with metastatic disease were excluded due to unavailability of relevant imaging. Further, three patients (2%) were excluded due to the simultaneous occurrence of histopathologically verified metastases from other primary cancers. In total, 178 patients were included in this study (Figure S1, Table 1). A total of 95 patients (53%) had metastatic disease exclusively to the liver (hepatic pattern), 66 patients (37%) had both hepatic and extrahepatic metastases (hepatic–extrahepatic pattern), and 17 patients (10%) had exclusive extrahepatic metastases (extrahepatic pattern).

Table 1.
Patient characteristics.
The median disease-free interval was 29.0 months for patients with hepatic pattern, 25.0 months for patients with hepatic–extrahepatic pattern, and 62.0 months for patients with extrahepatic pattern (Table 1). The diagnosis of PUM metastases was histopathologically verified in all 95 patients (100%) with hepatic pattern, in 61 patients with hepatic–extrahepatic pattern (92%), and in all 17 patients (100%) with extrahepatic pattern. Metastases were present at the time of primary diagnosis (AJCC stage IV) in 4 out of 95 patients (4%) with hepatic pattern, in 2 out of 66 patients (3%) with hepatic–extrahepatic pattern, and in 1 out of 17 patients (6%) with extrahepatic pattern.
3.2. Survival Analysis
Metastatic uveal melanoma was the only cause of death. Patients with extrahepatic pattern had a significantly longer overall survival (median 17.0 months) compared to patients with hepatic pattern (median 11.0 months) and to patients with hepatic–extrahepatic pattern (median 7.0 months) (Table 1, Figure 1a, overall log-rank test p < 0.001; pair-wise log-rank test: hepatic vs. hepatic–extrahepatic p = 0.001, hepatic vs. extrahepatic p = 0.001, extrahepatic vs. hepatic–extrahepatic p < 0.001).
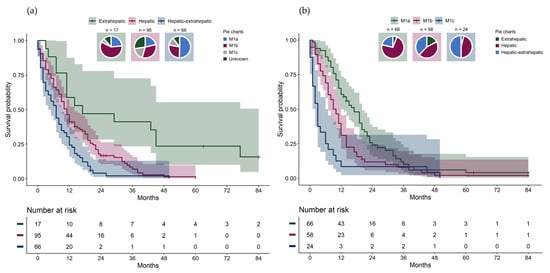
Figure 1.
(a) Overall survival from metastatic diagnosis stratified by metastatic pattern with pie charts showing the distribution of American Joint Committee on Cancer (AJCC) stage IV category in each metastatic pattern group. (b) Overall survival from metastatic diagnosis stratified by stage IV AJCC category with pie charts showing the distribution of metastatic pattern groups in each AJCC stage.
Due to a small number at risk, two patients with extrahepatic pattern were censored after 84 months (7 years) in the Kaplan–Meier plots. There were significant differences in overall survival between AJCC stage IV categories (Figure 1b, overall log-rank test p < 0.001; pair-wise log-rank test: M1a vs. M1b p = 0.005, M1a vs. M1c p < 0.001, M1b vs. M1c p = 0.006). For each metastatic pattern, survival differences between the AJCC stage IV categories were investigated (Figure 2a-c). Significant differences in overall survival were found between the AJCC stage IV categories among patients with hepatic pattern (Figure 2a, overall log-rank test p < 0.001; pair-wise log-rank test: M1a vs. M1b p = 0.01, M1a vs. M1c p < 0.001, M1b vs. M1c p = 0.005), but not among patients with hepatic–extrahepatic pattern (Figure 2b, p = 0.11, overall log-rank test) or extrahepatic pattern (Figure 2c, p = 0.31, overall log-rank test).
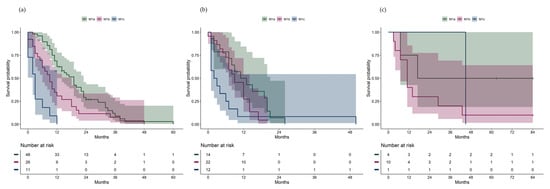
Figure 2.
(a) Overall survival from metastatic diagnosis in patients with hepatic pattern, stratified by AJCC stage IV category. (b) Overall survival from metastatic diagnosis in patients with hepatic–extrahepatic pattern, stratified by AJCC stage IV category. (c) Overall survival from metastatic diagnosis in patients with extrahepatic pattern, stratified by AJCC stage IV category.
No significant differences in overall survival from the date of metastatic diagnosis were found when stratifying patients by disease-free interval groups (0–3 years, 3–6 years, and >6 years) (Figure 3, p = 0.380, overall log-rank).
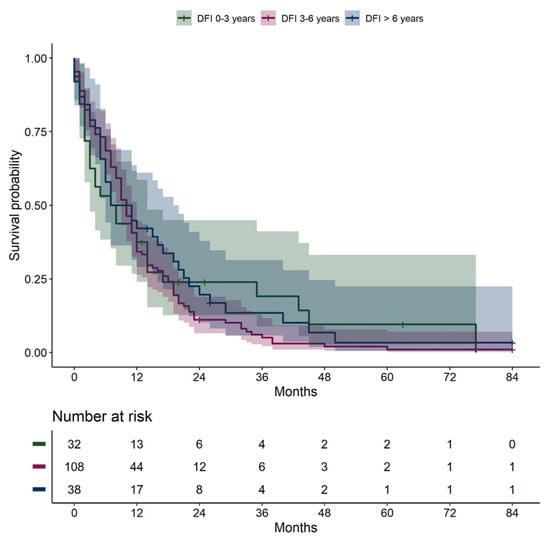
Figure 3.
Overall survival from date of metastatic diagnosis, stratified by disease-free interval: 0–3 years, 3–6 years, >6 years. DFI, disease-free interval.
The hazard ratio (HR) for death was significantly higher in patients with hepatic pattern (HR 2.57) and hepatic–extrahepatic pattern (HR 4.36) compared to those with extrahepatic pattern in the univariate Cox regression (Table 2). The metastatic pattern remained significant in the multivariate Cox regression, with an HR of 2.37 for hepatic pattern and an HR of 3.25 for hepatic–extrahepatic pattern (Table 2).

Table 2.
Univariate and multivariate Cox proportional hazard model for overall survival from date of diagnosis of metastasis until death or end of follow-up.
3.3. Extrahepatic Lesions
The extrahepatic lesions were located in various organs (Table 3 and Table 4). Among the patients with hepatic–extrahepatic pattern, bones (45%), lungs (36%), and lymph nodes (36%) were the most common extrahepatic organs. For patients with extrahepatic pattern, lymph nodes (59%) and lungs (53%) were the most common sites, whereas bone metastases were found in 18%.

Table 3.
Sites and number of involved extrahepatic lesions in patients with hepatic–extrahepatic metastatic pattern.

Table 4.
Affected extrahepatic organs in patients with extrahepatic metastatic pattern.
3.4. The Largest Diameter of the Largest Metastatic Lesion
The median LDLM was smallest in patients with hepatic pattern (27 mm [interquartile range (IQR) 18 to 50]) compared to those with hepatic–extrahepatic pattern (42 mm [IQR 30 to 73]) and extrahepatic pattern (46 mm [IQR 34–63]). The Kruskal–Wallis test showed differences between the groups (p = 0.005). In 93% of patients with hepatic–extrahepatic pattern, the metastasis with the LDLM was located in the liver (data available in 45 patients). In two patients with hepatic and extrahepatic pattern, the LDLM was measured in the bones (15 mm compared to 10 mm in the liver and 22 mm compared to 20 mm in the liver, respectively), and in one patient, the LDLM was measured in the spleen (34 mm compared to 30 mm in the liver).
3.5. Detection of Metastases: Screening vs. Symptoms
We investigated how many patients our follow-up program detected before the symptoms developed. Data were not available for all patients. A total of 61 out of 64 patients with hepatic pattern (95%) were diagnosed with metastases at a planned liver ultrasonography screening visit, whereas two patients (3%) developed symptoms that led to the diagnosis, and in one patient (2%), a metastatic liver lesion was discovered incidentally (Table 5). In 27 out of 33 patients with hepatic–extrahepatic pattern (82%), the metastatic lesions were detected at the planned liver ultrasonography screening visit, whereas six patients (18%) developed symptoms (Table 5). For the extrahepatic pattern group, 13 out of 16 patients (81%) were diagnosed with metastases due to the onset of symptoms, and for three patients (19%), uveal melanoma metastases were an incidental finding, of which one was diagnosed with metastases at the time of primary diagnosis.

Table 5.
The cause leading to the detection of the first metastatic lesion, either at a scheduled liver ultrasonography visit, due to symptoms, or as an incidental finding while investigating for other diseases.
3.6. Excluded Patients
The metastatic pattern could not be described in 71 patients, as images or imaging reports could not be retrieved (Table S1). The median overall survival was 2.3 months in the excluded group compared to 9.5 months in the included patient group. There was a male predominance (55%), and the median age was higher (median age 69 years) in the excluded patients compared to that in the included patients (43% men, median age 66 years). A total of 60 out of the 71 excluded patients (85%) were registered with liver metastases either histopathologically or through liver ultrasonography.
4. Discussion
To the best of our knowledge, this study is the first to describe and investigate the impact of the anatomical location of metastatic lesions on survival, exclusively including patients who have undergone imaging assessment covering at least the chest and the abdomen. This study shows that patients with disseminated PUM without liver metastases have a significantly longer overall survival compared to patients presenting with hepatic metastases, even though a large proportion of these patients were diagnosed with metastatic disease based on symptoms and not by screening. As the Danish surveillance program includes regular liver ultrasonography, one would expect patients without liver metastases to demonstrate an even shorter survival than patients with liver metastases, as the disease will be diagnosed at a presumed later disease stage. Paradoxically, the survival was longer in the extrahepatic pattern group. A possible explanation could be that extrahepatic lesions are located at sites that are less lethal. This is supported by a higher percentage of resection of metastatic lesions among patients with hepatic–extrahepatic pattern compared to the other two groups. Another explanation could be that lesions developing exclusively in extrahepatic organs are more indolent and less aggressive than hepatic metastases. Ten percent of the patients presented with only extrahepatic metastases, which is in line with recent large cohort studies [3,4,27]. Among all the patients with hepatic metastases, patients with hepatic pattern demonstrated a longer survival than patients with hepatic–extrahepatic pattern, possibly due to a larger total tumor burden in the latter. Further, the median LDLM was smaller in patients with hepatic pattern compared to patients with hepatic–extrahepatic pattern, indicating that the latter group has more advanced disease.
It has previously been described that patients without liver involvement exhibit longer survival. Four decades ago, Bedikian et al. [17] found that 31 patients with metastatic lesions exclusively in the liver had a poorer prognosis compared to 21 patients with single-site extrahepatic metastases. Rajpal et al. [18] published a study in 1983, where they analyzed a series of 35 patients and found that the survival varied, in favor of patients with lung metastases compared to patients with liver metastases. Hepatic metastases have been associated with a poor prognosis in other previously published small cohort studies and case series [11,19,20,21,22], as well as in a more recent, larger cohort study [4]. The assessment of metastatic sites varied across the studies and did not always include whole-body imaging. In the present study, the univariate and multivariate Cox analysis both showed that the metastatic pattern was an independent prognostic factor for survival, even when adjusting for the AJCC stage, which confirms the previous findings.
In the present study, only patients who had undergone imaging of at least the chest and the abdomen were included. We used this approach to reduce the risk of missing any potential extrahepatic metastases. Consequently, due to a lack of imaging data, we had to exclude 71 out of 252 patients diagnosed with metastases. Among the excluded patients, 85% had verified liver metastases, indicating that the distribution of metastatic liver involvement is comparable between the included (90%) and excluded patients. Nonetheless, the median survival in the excluded patients was considerably shorter than in the included patients. A possible explanation could be that the excluded patients may have had a more advanced disease stage when the metastases were detected and, consequently, may have been unable or unwilling to undergo further investigation. That only 1 out of the 71 excluded patients (1%) was treated with ipi+nivo, and that there was a larger proportion of men and older patients in the extrahepatic group, supports a possible selection bias.
LDLM as a prognostic factor for survival was proposed by Eskelin et al. [16] However, it is unclear whether the LDLM applies to liver metastases only, or if it could also be applied to extrahepatic metastases. In the present study, we staged patients with extrahepatic pattern according to the AJCC 8th Edition [12], using the largest diameter of the largest metastatic lesion in the whole body (Table 1). In the total cohort, the survival varied significantly between the AJCC stages M1a, M1b, and M1c. This was also the case when only looking at patients with hepatic pattern, but there were no differences in survival between the M1a, M1b, or M1c stages within the hepatic–extrahepatic pattern or the extrahepatic pattern groups, though it should be noted that the sub-cohorts were small.
It is noteworthy that 41% of patients with hepatic metastases also had extrahepatic metastases. In patients with hepatic–extrahepatic pattern, bones were the most common extrahepatic organ (45%), which was only the case for 18% of patients with extrahepatic pattern. Lymph nodes (59%) and lungs (53%) were the most common metastatic sites in patients with extrahepatic pattern, which was also the second most commonly involved organs for hepatic–extrahepatic pattern. We were not able to identify any metastatic sub-patterns among patients with extrahepatic pattern, and larger, multicenter studies are needed to explore the frequency and distribution of metastatic sites related to survival among this small patient group. The question is whether to expand the follow-up program to include monitoring for the development of extrahepatic lesions. As the extrahepatic lesions develop in various organs, including lymph nodes and bones, surveillance for extrahepatic lesions would require whole-body imaging. We find that it would be unethical to regularly expose all patients to the radiation dose of a whole-body CT or PET/CT to find the 10% without hepatic lesions. In total, 18% of the patients with hepatic–extrahepatic pattern and 81% of the patients with extrahepatic pattern developed symptoms before being diagnosed with metastatic PUM. This emphasizes that it is important to inform all patients to be aware of symptoms from other organs than the liver and to seek medical help when experiencing any unexplained symptoms. Of the 66 patients that presented with hepatic–extrahepatic pattern, whether the first metastasis was in the liver or an extrahepatic organ is not known. However, in 93% of patients with hepatic–extrahepatic pattern, the largest metastatic lesion was found in the liver, indicating that the liver was the primary site (given that the tumor doubling time is the same in all metastatic lesions). This supports maintaining surveillance that focuses on the liver.
The disease-free interval has been suggested as a prognostic factor for survival in previous studies [13,14,16,28], where a short disease-free interval has been associated with a shorter survival from the date of metastatic diagnosis. We were not able to identify any differences in survival between patients with a short, medium, or long disease-free interval (Figure 3), and our results align with other studies that likewise have not been able to demonstrate this association [29,30]. The median disease-free survival was longer in patients with extrahepatic pattern (62.0 months, IQR 53.0–116.0) compared to those with hepatic (29.0 months, IQR 17.5–41.5) and hepatic–extrahepatic patterns (25.0 months, 12.0–66.0). As we only include patients who have developed metastases, the disease-free survival should be interpreted with caution due to selection bias. With this in mind, we still find it noteworthy that the disease-free interval was longer for the extrahepatic pattern group. A lead-time bias is likely introduced, as patients are not routinely screened for extrahepatic metastases, and patients with only extrahepatic metastases are therefore diagnosed at a later disease stage. An explanation could also be that the extrahepatic pattern represents a less aggressive tumor subtype that develops more slowly than hepatic metastases. This is highly speculative, and we do not know whether the longer disease-free interval is caused only by the lead-time bias or by a slower development and growth of extrahepatic lesions.
Ipi+nivo is the only available treatment regimen in Denmark proven to have any efficacy in patients with metastatic PUM [31,32,33]. Interestingly, in a phase II trial investigating the effect of ipi+nivo in metastatic uveal melanoma [31], 6 out of 35 patients (17%) had extrahepatic lesions only, and one of these patients (17%) experienced (as the only patient) complete remission. Three of the patients (50%) reached stable disease of at least 6 months, which was only the case for 11% of the patients with liver metastases. The authors also found a longer progression-free survival for patients with extrahepatic lesions only, though it was not significant. Another phase II study including 52 patients also investigated the efficacy of ipi+nivo and found a longer overall survival among patients with extrahepatic lesions only, though it was also not significant [32]. Whether this is caused by ipi+nivo being more effective on extrahepatic lesions or whether it is caused by extrahepatic lesions representing a less aggressive metastatic subtype is not known, but it emphasizes the need to consider the anatomical location of metastatic lesions when stratifying patients into clinical trials. It has been hypothesized that the limited effect of ipi+nivo and other immunotherapy in metastatic PUM could be caused by the high immune tolerance of the liver [34]. It has been shown that tryptophan 2,3-dioxygenase (TDO), which is predominantly expressed in the liver, is also expressed by metastatic uveal melanoma cells [34]. TDO has a suppressive effect on the immunological activity of the liver and could therefore impair the efficacy of immunotherapy.
It is still not evident why the metastases bypass the liver in some cases, but it is apparent that PUM can exhibit heterogeneous clinical courses. With genetic profiling of the primary tumor, we can quite precisely predict if the patients are at risk of developing metastases [9,35,36,37], but the genetic signatures driving the different dissemination patterns remain to be explored. The molecular profile of the primary tumor could potentially hold the key to differentiating between patients who will develop liver metastases and those who will develop metastases in other organs. This could pave the way for a more personalized follow-up program, with surveillance focusing on specific organs depending on metastatic pattern risk profiles.
5. Conclusions
Metastatic PUM patients with only extrahepatic lesions represented 10% of the total cohort. Despite diagnosis at the onset of symptoms, they still had significantly better overall survival compared to patients with liver metastases. Of the 90% with hepatic metastases, 41% also had extrahepatic lesions. The patients with both hepatic and extrahepatic lesions had larger liver metastases and poorer overall survival than patients with only liver metastases, indicating a more advanced stage of disease. The high percentage of liver metastases and the low incidence of symptoms among patients with only liver metastases support maintaining surveillance that focuses on the liver. The heterogenous anatomical distribution of extrahepatic lesions suggests that whole-body imaging is mandated if surveillance for extrahepatic lesions should be included; however, due to radiation exposure, we do not recommend this as a screening tool.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16193346/s1, Figure S1: Flow diagram showing the inclusion and exclusion of patients; Table S1: Patient characteristics of the excluded patients.
Author Contributions
Conceptualization, T.G.H., M.B.S. and J.F.K.; Methodology, T.G.H., M.B.S., M.D., I.M.S., E.E., S.H., K.M. and J.F.K.; Software, T.G.H. and J.F.K.; Validation, T.G.H., P.S.J., M.B.S., K.N., C.W.B., E.v.B., C.F., S.F.U., M.D., I.M.S., E.E., S.H. and J.F.K.; Formal Analysis, T.G.H., M.B.S. and J.F.K.; Investigation, T.G.H., P.S.J., M.B.S., K.N., C.W.B., E.v.B., C.F., M.D., I.M.S., E.E., S.H. and J.F.K.; Resources, P.S.J., M.B.S., S.F.U., M.D., I.M.S., E.E., S.H., K.M. and J.F.K.; Data Curation, T.G.H., M.B.S., K.N., C.F., M.D., I.M.S., E.E. and J.F.K.; Writing—Original Draft, T.G.H. and J.F.K.; Writing—Review and Editing, T.G.H., P.S.J., M.B.S., K.N., C.W.B., E.v.B., C.F., S.F.U., M.D., I.M.S., E.E., S.H., K.M. and J.F.K.; Visualization, T.G.H.; Supervision, M.B.S., S.H., K.M. and J.F.K.; Project Administration, T.G.H. and J.F.K.; Funding Acquisition, T.G.H. and J.F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Danish Cancer Society (Kræftens Bekæmpelse), Copenhagen University Hospital-Rigshospitalet Research Grant, The Synoptik Foundation (Synoptik-Fonden), Fight for Sight Denmark (Øjenforeningen), and Minister Erna Hamilton’s Grant for Science and Art (Minister Erna Hamiltons Legat for Videnskab og Kunst).
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee of The Capital Region of Denmark (protocol code (protocol no. H-21015415, 8 July 2021). The Ethics Committee waived approval of this study with dispensation for informed consent.
Informed Consent Statement
Patient consent was waived due to the majority of patients being deceased at the time of this study’s conduction.
Data Availability Statement
Data cannot be fully anonymized and therefore cannot be made publicly available as per the European General Data Protection Regulation (GDPR). Inquiries regarding data can be directed to J.F.K. (jens.folke.kiilgaard@regionh.dk).
Conflicts of Interest
J.F.K. is a consultant at Aura Biosciences. I.M.S. has received consulting fees from MSD, IO Biotech, Novartis, Pierre Fabre, and TILT Biotherapeutics, honoraria for presentations/lectures from MSD, Novartis, Sanofi Aventis, Pierre Fabre, BMS, Novo Nordisk, and Takeda, travel/conference expenses from MSD, and owns stocks in IO Biotech. E.E. has received honoraria from BMS, Pierre Fabre, and Novartis for consultancies, lectures, and travel/conference expenses from Pierre Fabre and MSD. M.D. is an advisor at Achilles Therapeutics and has received proprietary data access from Genentech and Bristol-Myers Squibb. All remaining authors have declared no conflicts of interest. The funding organizations had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wu, M.; Yavuzyiğitoğlu, S.; Brosens, E.; Ramdas, W.D.; Kiliç, E. Worldwide Incidence of Ocular Melanoma and Correlation with Pigmentation-Related Risk Factors. Investig. Ophthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef] [PubMed]
- Smidt-Nielsen, I.; Bagger, M.; Heegaard, S.; Andersen, K.K.; Kiilgaard, J.F. Posterior Uveal Melanoma Incidence and Survival by AJCC Tumour Size in a 70-Year Nationwide Cohort. Acta Ophthalmol. 2021, 99, e1474–e1482. [Google Scholar] [CrossRef]
- Jochems, A.; van der Kooij, M.K.; Fiocco, M.; Schouwenburg, M.G.; Aarts, M.J.; van Akkooi, A.C.; van den Berkmortel, F.W.P.J.; Blank, C.U.; van den Eertwegh, A.J.M.; Franken, M.G.; et al. Metastatic Uveal Melanoma: Treatment Strategies and Survival-Results from the Dutch Melanoma Treatment Registry. Cancers 2019, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Kujala, E.; Damato, B.; Coupland, S.E.; Desjardins, L.; Bechrakis, N.E.; Grange, J.D.; Kivelä, T. Staging of Ciliary Body and Choroidal Melanomas Based on Anatomic Extent. J. Clin. Oncol. 2013, 31, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of Uveal Melanoma Millimeter-by-Millimeter in 8033 Consecutive Eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sisley, K.; Rennie, I.G.; Parsons, M.A.; Jacques, R.; Hammond, D.W.; Bell, S.M.; Potter, A.M.; Rees, R.C. Abnormalities of Chromosomes 3 and 8 in Posterior Uveal Melanoma Correlate with Prognosis. Genes Chromosom. Cancer 1997, 19, 22–28. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivelä, T.T. Overall Survival after Treatment for Metastatic Uveal Melanoma: A Systematic Review and Meta-Analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, P.; Panageas, K.S.; Hanlon, C.; Patel, A.; Abramson, D.H.; Chapman, P.B. Variates of Survival in Metastatic Uveal Melanoma. J. Clin. Oncol. 2005, 23, 8076–8080. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Edge, S.; Green, F.; Byrd, D.; Brookland, R.; Washington, M.; Gershenwald, J.; Compton, C.; Hess, K.; Sullivan, D.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Valpione, S.; Moser, J.C.; Parrozzani, R.; Bazzi, M.; Mansfield, A.S.; Mocellin, S.; Pigozzo, J.; Midena, E.; Markovic, S.N.; Aliberti, C.; et al. Development and External Validation of a Prognostic Nomogram for Metastatic Uveal Melanoma. PLoS ONE 2015, 10, e0120181. [Google Scholar] [CrossRef]
- Mariani, P.; Dureau, S.; Savignoni, A.; Rouic, L.L.L.; Levy-Gabriel, C.; Piperno-Neumann, S.; Rodrigues, M.J.; Desjardins, L.; Cassoux, N.; Servois, V. Development of a Prognostic Nomogram for Liver Metastasis of Uveal Melanoma Patients Selected by Liver MRI. Cancers 2019, 11, 863. [Google Scholar] [CrossRef]
- Kivelä, T.T.; Piperno-Neumann, S.; Desjardins, L.; Schmittel, A.; Bechrakis, N.; Midena, E.; Leyvraz, S.; Zografos, L.; Grange, J.D.; Ract-Madoux, G.; et al. Validation of a Prognostic Staging for Metastatic Uveal Melanoma: A Collaborative Study of the European Ophthalmic Oncology Group. Am. J. Ophthalmol. 2016, 168, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, S.; Pyrhönen, S.; Hahka-Kemppinen, M.; Tuomaala, S.; Kivelä, T. A Prognostic Model and Staging for Metastatic Uveal Melanoma. Cancer 2003, 97, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Kantarjian, H.; Young, S.E.; Bodey, G.P. Prognosis in Metastatic Choroidal Melanoma. South. Med. J. 1981, 74, 574–577. [Google Scholar] [CrossRef]
- Rajpal, S.; Moore, R.; Karakousis, C.P. Survival in Metastatic Ocular Melanoma. Cancer 1983, 52, 334–336. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Egan, K.M.; Seddon, J.M.; Glynn, R.J.; Walsh, S.M.; Finn, S.M.; Munzenrider, J.E.; Spar, M.D. Survival of Patients with Metastases from Uveal Melanoma. Ophthalmology 1991, 98, 383–390. [Google Scholar] [CrossRef]
- Kath, R.; Hayungs, J.; Bornfeld, N.; Sauerwein, W.; Höffken, K.; Seeber, S. Prognosis and Treatment of Disseminated Uveal Melanoma. Cancer 1993, 72, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, E.C.; Essner, R.; Foshag, L.J.; Ye, X.; Wang, H.J.; Morton, D.L. Prolonged Survival after Complete Resection of Metastases from Intraocular Melanoma. Cancer 2004, 100, 122–129. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, S.J.; Heo, S.J.; Choe, E.A.; Kim, C.G.; Jung, M.; Keum, K.C.; Yoon, J.S.; Lee, S.C.; Shin, S.J. Prognoses and Clinical Outcomes of Primary and Recurrent Uveal Melanoma. Cancer Res. Treat. 2018, 50, 1238–1251. [Google Scholar] [CrossRef]
- Bagger, M.; Smidt-Nielsen, I.; Andersen, M.K.; Jensen, P.K.; Heegaard, S.; Andersen, K.K.; Friis, S.; Kiilgaard, J.F. Long-Term Metastatic Risk after Biopsy of Posterior Uveal Melanoma. Ophthalmology 2018, 125, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Ellebaek, E.; Svane, I.M.; Schmidt, H.; Haslund, C.A.; Donia, M.; Hoejberg, L.; Ruhlmann, C.; Guldbrandt, L.M.; Køhler, U.H.; Bastholt, L. The Danish Metastatic Melanoma Database (DAMMED): A Nation-Wide Platform for Quality Assurance and Research in Real-World Data on Medical Therapy in Danish Melanoma Patients. Cancer Epidemiol. 2021, 73, 101943. [Google Scholar] [CrossRef]
- Helweg-Larsen, K. The Danish Register of Causes of Death. Scand. J. Public Health 2011, 39, 26–29. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA-J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar]
- Nicholas, M.N.; Khoja, L.; Atenafu, E.G.; Hogg, D.; Quirt, I.; Butler, M.; Joshua, A.M. Prognostic Factors for First-Line Therapy and Overall Survival of Metastatic Uveal Melanoma: The Princess Margaret Cancer Centre Experience. Melanoma Res. 2018, 28, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, D.; Ochoa, M.; Piulats, J.M.; Gutiérrez, C.; Arias, L.; Català, J.; Grau, M.; Peñafiel, J.; Cobos, E.; Garcia-Bru, P.; et al. Prognostic Factors and Decision Tree for Long-Term Survival in Metastatic Uveal Melanoma. Cancer Res. Treat. 2018, 50, 1130–1139. [Google Scholar] [CrossRef]
- Kodjikian, L.; Grange, J.D.; Baldo, S.; Baillif, S.; Garweg, J.G.; Rivoire, M. Prognostic Factors of Liver Metastases from Uveal Melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 985–993. [Google Scholar] [CrossRef]
- Xu, L.T.; Funchain, P.F.; Bena, J.F.; Li, M.; Tarhini, A.; Berber, E.; Singh, A.D. Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul. Oncol. Pathol. 2019, 5, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results From a Single-Arm Phase II Study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef]
- Piulats, J.M.; Espinosa, E.; de la Cruz Merino, L.; Varela, M.; Alonso Carrión, L.; Martín-Algarra, S.; López Castro, R.; Curiel, T.; Rodríguez-Abreu, D.; Redrado, M.; et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 2021, 39, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Navrazhina, K.; Ding, F.; Bhatia, R.; Tsai, K.; Abbate, K.; Durden, B.; Eroglu, Z.; Bhatia, S.; Park, S.; et al. Ipilimumab plus Nivolumab for Patients with Metastatic Uveal Melanoma: A Multicenter, Retrospective Study. J. Immunother. Cancer 2020, 8, e000331. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Dogrusöz, M.; Bagger, M.; Van Duinen, S.G.; Kroes, W.G.; Ruivenkamp, C.A.L.; Böhringer, S.; Andersen, K.K.; Luyten, G.P.M.; Kiilgaard, J.F.; Jager, M.J. The Prognostic Value of AJCC Staging in Uveal Melanoma Is Enhanced by Adding Chromosome 3 and 8q Status. Investig. Ophthalmol. Vis. Sci. 2017, 58, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220e15. [Google Scholar] [CrossRef]
- Dogrusöz, M.; Jager, M.J. Genetic Prognostication in Uveal Melanoma. Acta Ophthalmol. 2018, 96, 331–347. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).