Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Mesenchymal Stem Cells as Carriers of NIS Genes
2.1. Origin and Differentiation Potential
2.2. Immunomodulatory Properties
2.3. Tumor-Tropic Migration
3. Mechanism of NIS Gene Delivery
Advantages and Limitations
4. Image-Guided Approaches in Radionuclide Therapy
5. Image-Guided NIS Radionuclide Therapy in Glioblastoma
6. Challenges and Future Directions: Discussion on Image-Guided NIS Radionuclide Therapy for Glioblastoma
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, S. Novel Therapies in Glioblastoma Treatment: Review of Glioblastoma; Current Treatment Options; and Novel Oncolytic Viral Therapies. Med. Sci. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.V.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Shah, S.; Mansour, H.M.; Aguilar, T.M.; Lucke-Wold, B. Advances in Anti-Cancer Drug Development: Metformin as Anti-Angiogenic Supplemental Treatment for Glioblastoma. Int. J. Mol. Sci. 2024, 25, 5694. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Yusubalieva, G.M.; Baklaushev, V.P.; Chumakov, P.M.; Lipatova, A.V. Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef]
- Oh, J.M.; Ahn, B.C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251–6277. [Google Scholar] [CrossRef]
- Gong, Z.; Wei, M.; Vlantis, A.C.; Chan, J.Y.K.; Van Hasselt, C.A.; Li, D.; Zeng, X.; Xue, L.; Tong, M.C.F.; Chen, G.G. Sodium-iodide symporter and its related solute carriers in thyroid cancer. J. Endocrinol. 2024, 261, e230373. [Google Scholar] [CrossRef]
- Spitzweg, C.; Nelson, P.J.; Wagner, E.; Bartenstein, P.; Weber, W.A.; Schwaiger, M.; Morris, J.C. The sodium iodide symporter (NIS): Novel applications for radionuclide imaging and treatment. Endocr. Relat. Cancer. 2021, 28, T193–T213. [Google Scholar] [CrossRef]
- Darrouzet, E.; Lindenthal, S.; Marcellin, D.; Pellequer, J.-L.; Pourcher, T. The sodium/iodide symporter: State of the art of its molecular characterization. Biochim. Biophys. Acta 2014, 1838 Pt 1, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022, 80, 101684. [Google Scholar] [CrossRef]
- Herzog, E.L.; Chai, L.; Krause, D.S. Plasticity of marrow-derived stem cells. Blood 2003, 102, 3483–3493. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.; Novelli, E.; Grove, J.E.; Rinder, H.M.; Civin, C.; Cheng, L.; Krause, D.S. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp. Hematol. 2003, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Goolaerts, A.; Howard, J.P.; Lee, J.W. Mesenchymal stem cells for acute lung injury: Preclinical evidence. Crit. Care Med. 2010, 38 (Suppl. 10), S569–S573. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, L.; Zhu, L.; Zhou, B.; Yu, Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur. Neurol. 2020, 83, 235–241. [Google Scholar] [CrossRef]
- Hua, Q.; Zhang, Y.; Li, H.; Li, H.; Jin, R.; Li, L.; Xiang, Y.; Tian, M.; Wang, J.; Sun, L.; et al. Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium. Stem Cell Res. Ther. 2022, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, B.; Zhu, X.; Hu, J.; Sun, J.; Xuan, J.; Ge, Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021, 37, 51–64. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Dzobo, K. Recent Trends in Multipotent Human Mesenchymal Stem/Stromal Cells: Learning from History and Advancing Clinical Applications. OMICS 2021, 25, 342–357. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Li, T.; Xia, M.; Gao, Y.; Chen, Y.; Xu, Y. Human umbilical cord mesenchymal stem cells: An overview of their potential in cell-based therapy. Expert Opin. Biol. Ther. 2015, 15, 1293–1306. [Google Scholar] [CrossRef]
- Zupan, J. Human Synovium-Derived Mesenchymal Stem Cells: Ex Vivo Analysis. Methods Mol. Biol. 2019, 2045, 145–154. [Google Scholar] [CrossRef]
- Okolicsanyi, R.K.; Camilleri, E.T.; Oikari, L.E.; Yu, C.; Cool, S.M.; Van Wijnen, A.J.; Griffiths, L.R.; Haupt, L.M. Human Mesenchymal Stem Cells Retain Multilineage Differentiation Capacity Including Neural Marker Expression after Extended In Vitro Expansion. PLoS ONE 2015, 10, e0137255. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna Pillai, A.; Mariappan, V.; JeanPierre, A.R.; Rao, S.R. Restoration of vascular endothelial integrity by mesenchymal stromal/stem cells in debilitating virus diseases. Hum. Cell 2022, 35, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Dapkute, D.; Steponkiene, S.; Bulotiene, D.; Saulite, L.; Riekstina, U.; Rotomskis, R. Skin-derived mesenchymal stem cells as quantum dot vehicles to tumors. Int. J. Nanomed. 2017, 12, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Y.; Xie, X.; Song, T.; Yang, G.; Su, Q.; Li, T.; Li, S.; Wu, C.; You, F.; et al. Engineered Mesenchymal Stem Cells as a Biotherapy Platform for Targeted Photodynamic Immunotherapy of Breast Cancer. Adv. Healthc. Mater. 2022, 11, e2101375. [Google Scholar] [CrossRef]
- Sadhukha, T.; O’Brien, T.D.; Prabha, S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release 2014, 196, 243–251. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, L.; Ding, S.; Chen, Y.; Zhang, X.; Bian, X.; Tian, G. Tumor-Tropic Adipose-Derived Mesenchymal Stromal Cell Mediated Bi2 Se3 Nano-Radiosensitizers Delivery for Targeted Radiotherapy of Non-Small Cell Lung Cancer. Adv. Healthc. Mater. 2022, 11, e2200143. [Google Scholar] [CrossRef]
- Senst, C.; Nazari-Shafti, T.; Kruger, S.; Zu Bentrup, K.H.; Dupin, C.L.; Chaffin, A.E.; Srivastav, S.K.; Wörner, P.M.; Abdel-Mageed, A.B.; Alt, E.U.; et al. Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res. Treat 2013, 137, 69–79. [Google Scholar] [CrossRef]
- Golinelli, G.; Talami, R.; Frabetti, S.; Candini, O.; Grisendi, G.; Spano, C.; Chiavelli, C.; Arnaud, G.F.; Mari, G.; Dominici, M. A 3D Platform to Investigate Dynamic Cell-to-Cell Interactions Between Tumor Cells and Mesenchymal Progenitors. Front. Cell Dev. Biol. 2022, 9, 767253. [Google Scholar] [CrossRef]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: Prevention and treatment of graft-versus-host disease. Stem Cell Res. Ther. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. Biomed. Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef]
- Munir, H.; Ward, L.S.C.; McGettrick, H.M. Mesenchymal Stem Cells as Endogenous Regulators of Inflammation. Adv. Exp. Med. Biol. 2018, 1060, 73–98. [Google Scholar] [CrossRef]
- Ji, K.; Ding, L.; Chen, X.; Dai, Y.; Sun, F.; Wu, G.; Lu, W. Mesenchymal Stem Cells Differentiation: Mitochondria Matter in Osteogenesis or Adipogenesis Direction. Curr. Stem Cell Res. Ther. 2020, 15, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ren, Z.; Zhong, Z.; Yan, L.; Xia, X.; Wang, J.; Yang, J. Mesenchymal Stem Cells: Potential Therapeutic Prospect of Paracrine Pathways in Neonatal Infection. J. Interferon. Cytokine Res. 2021, 41, 365–374. [Google Scholar] [CrossRef]
- Selmi-Ruby, S.; Watrin, C.; Trouttet-Masson, S.; Bernier-Valentin, F.; Flachon, V.; Munari-Silem, Y.; Rousset, B. The porcine sodium/iodide symporter gene exhibits an uncommon expression pattern related to the use of alternative splice sites not present in the human or murine species. Endocrinology 2003, 144, 1074–1085. [Google Scholar] [CrossRef]
- Knoop, K.; Kolokythas, M.; Klutz, K.; Willhauck, M.J.; Wunderlich, N.; Draganovici, D.; Zach, C.; Gildehaus, F.-J.; Böning, G.; Göke, B.; et al. Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol. Ther. 2011, 19, 1704–1713. [Google Scholar] [CrossRef]
- Cho, J.Y. A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: Imaging and therapy. Curr. Gene Ther. 2002, 2, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.; Clerc, J.; Gérolami, R.; Hervé, J.; Longuet, M.; Liu, B.; Roux, J.; Moal, F.; Perricaudet, M.; Bréchot, C. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar. Cancer Res. 2004, 64, 8045–8051. [Google Scholar] [CrossRef] [PubMed]
- Belmar-López, C.; Vassaux, G.; Medel-Martinez, A.; Burnet, J.; Quintanilla, M.; Cajal, S.R.Y.; Hernandez-Losa, J.; De la Vieja, A.; Martin-Duque, P. Mesenchymal Stem Cells Delivery in Individuals with Different Pathologies: Multimodal Tracking, Safety and Future Applications. Int. J. Mol. Sci. 2022, 23, 1682. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.-Y.; Jhiang, S.M. Cell surface targeting accounts for the difference in iodide uptake activity between human Na+/I- symporter and rat Na+/I− symporter. J. Clin. Endocrinol. Metab. 2005, 90, 6131–6140. [Google Scholar] [CrossRef]
- Yao, X.; Li, J.; Liu, J.; Liu, K. An Arabidopsis mitochondria-localized RRL protein mediates abscisic acid signal transduction through mitochondrial retrograde regulation involving ABI4. J. Exp. Bot. 2015, 66, 6431–6445. [Google Scholar] [CrossRef]
- Nowakowski, A.; Walczak, P.; Lukomska, B.; Janowski, M. Genetic Engineering of Mesenchymal Stem Cells to Induce Their Migration and Survival. Stem Cells Int. 2016, 2016, 4956063. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Sakeena, Q.; Wani, M.Y.; Ismail, A.A.B.; Ahmad, S.M.; Shah, R.A. Mesenchymal stem cells: A promising antimicrobial therapy in veterinary medicine. Microb. Pathog. 2023, 182, 106234. [Google Scholar] [CrossRef]
- Siristatidis, C.; Vogiatzi, P.; Salamalekis, G.; Creatsa, M.; Vrachnis, N.; Glujovsky, D.; Iliodromiti, Z.; Chrelias, C. Granulocyte macrophage colony stimulating factor supplementation in culture media for subfertile women undergoing assisted reproduction technologies: A systematic review. Int. J. Endocrinol. 2013, 2013, 704967. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, G.; Incontro, S.; Iemmolo, R.; La Cognata, V.; Barbagallo, I.; Costanzo, E.; Barcellona, M.L.; Pellitteri, R.; Cavallaro, S. Dental mesenchymal stem cells and neuro-regeneration: A focus on spinal cord injury. Cell Tissue Res. 2020, 379, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Etchebehere, E.C.S.C.; Cendes, F.; Lopes-Cendes, I.; Pereira, J.A.; Lima, M.C.L.; Sansana, C.R.; Silva, C.A.M.; Camargo, M.F.A.G.; Santos, A.O.; Ramos, C.D.; et al. Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease. Arch. Neurol. 2001, 58, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Nose, N.; Nogami, S.; Koshino, K.; Chen, X.; Werner, R.A.; Kashima, S.; Rowe, S.P.; Lapa, C.; Fukuchi, K.; Higuchi, T. [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci. Rep. 2021, 11, 10896. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, Y.A.; Ke, C.C.; Chiu, S.J.; Chen, C.C.; Hsieh, Y.J.; Yang, B.H.; Liu, R.S. Preclinical Characterization and In Vivo Imaging of 111In-Labeled Mesenchymal Stem Cell-Derived Extracellular Vesicles. Mol. Imaging Biol. 2021, 23, 361–371. [Google Scholar] [CrossRef]
- Li, J.; Goh, E.L.K.; He, J.; Li, Y.; Fan, Z.; Yu, Z.; Yuan, P.; Liu, D.X. Emerging Intrinsic Therapeutic Targets for Metastatic Breast Cancer. Biology 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Huang, T.; Wu, B.-L.; He, W.-X.; Liu, D. Stem cells in cancer therapy: Opportunities and challenges. Oncotarget 2017, 8, 75756–75766. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cruz, C.M.; Shearer, J.J.; Neto, M.F.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 4015039. [Google Scholar] [CrossRef]
- Szewc, M.; Radzikowska-Bűchner, E.; Wdowiak, P.; Kozak, J.; Kuszta, P.; Niezabitowska, E.; Matysiak, J.; Kubiński, K.; Masłyk, M. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents-A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. Int. J. Mol. Sci. 2022, 23, 2745. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Mashal, M.; Puras, G.; Pedraz, J.L. Mesenchymal Stem Cells as a Gene Delivery Tool: Promise, Problems, and Prospects. Pharmaceutics 2021, 13, 843. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Karnoub, A.E. Mesenchymal stem cells in tumor development: Emerging roles and concepts. Cell Adh. Migr. 2012, 6, 220–230. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of Polymer-Based Nanoformulations for Glioblastoma Brain Cancer Therapy and Diagnosis: An Update. Polymers 2021, 13, 4114. [Google Scholar] [CrossRef]
- Yang, C.T.; Lai, R.C.; Phua, V.J.X.; Aw, S.E.; Zhang, B.; Sim, W.K.; Lim, S.K.; Ng, D.C.E. Standard Radio-Iodine Labeling Protocols Impaired the Functional Integrity of Mesenchymal Stem/Stromal Cell Exosomes. Int. J. Mol. Sci. 2024, 25, 3742. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Zhao, G.; Zhang, L.; Zhao, Z.; Wang, Z.; Zhao, L.; Hua, L.; Naveena, K.; Lu, J.; et al. Hypoxia-Responsive Lipid-Polymer Nanoparticle-Combined Imaging-Guided Surgery and Multitherapy Strategies for Glioma. ACS Appl. Mater. Interfaces 2020, 12, 52319–52328. [Google Scholar] [CrossRef]
- Schug, C.; Gupta, A.; Urnauer, S.; Steiger, K.; Cheung, P.F.-Y.; Neander, C.; Savvatakis, K.; Schmohl, K.A.; Trajkovic-Arsic, M.; Schwenk, N.; et al. A Novel Approach for Image-Guided 131I Therapy of Pancreatic Ductal Adenocarcinoma Using Mesenchymal Stem Cell-Mediated NIS Gene Delivery. Mol. Cancer Res. 2019, 17, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Basquin, C.; Portulano, C.; Reyna-Neyra, A.; Paroder, M.; Carrasco, N. The Na+/I− symporter mediates active iodide uptake in the intestine. Am. J. Physiol. Cell Physiol. 2009, 296, C654–C662. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) and Institute of Medicine (US) Committee on the Mathematics and Physics of Emerging Dynamic Biomedical Imaging. Single Photon Emission Computed Tomography. In Mathematics and Physics of Emerging Biomedical Imaging; National Academies Press: Washington, WA, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK232492/ (accessed on 8 August 2024).
- Penheiter, A.R.; Russell, S.J.; Carlson, S.K. The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr. Gene Ther. 2012, 12, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; DeGrado, T.R. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics 2018, 8, 3918–3931. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shilyagina, N.Y.; Vodeneev, V.A.; Zvyagin, A.V. Targeted Radionuclide Therapy of Human Tumors. Int. J. Mol. Sci. 2015, 17, 33. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Guo, R.; Miao, Y.; Li, B. Bone Marrow-Derived Mesenchymal Stem Cell-Mediated Dual-Gene Therapy for Glioblastoma. Hum. Gene Ther. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Gut, P.; Borowska, M.; Dyrka, K.; Ruchała, M.; Ferlito, A. A new hypothesis in the treatment of recurrent glioblastoma multiforme (GBM). PART 1: Introduction. Pol. Merkur. Lekarski. 2023, 51, 430–432. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Gut, P.; Sowiński, J.; Ruchała, M.; Ferlito, A.; Dyrka, K. A new hypothesis in the treatment of recurrent glioblastoma multiforme (GBM). Part 2: Is there an alternative therapy option in recurrent GM when all standard treatments have been exhausted? Pol. Merkur. Lekarski. 2023, 51, 433–435. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Shen, D.H.; Yang, W.; Williams, B.; Buckwalter, T.L.; La Perle, K.M.; Hinkle, G.; Pozderac, R.; Kloos, R.; Nagaraja, H.N.; et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002, 9, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xi, Y.; Zhang, M.; Miao, Y.; Zhang, M.; Li, B. Human sodium iodide transporter gene-mediated imaging and therapy of mouse glioma, comparison between 188Re and 131I. Oncol. Lett. 2018, 15, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Opyrchal, M.; Allen, C.; Iankov, I.; Aderca, I.; Schroeder, M.; Sarkaria, J.; Galanis, E. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS). Hum. Gene Ther. 2012, 23, 419–427. [Google Scholar] [CrossRef]
- Kitzberger, C.; Shehzad, K.; Morath, V.; Spellerberg, R.; Ranke, J.; Steiger, K.; Kälin, R.E.; Multhoff, G.; Eiber, M.; Schilling, F.; et al. Interleukin-6-controlled, mesenchymal stem cell-based sodium/iodide symporter gene therapy improves survival of glioblastoma-bearing mice. Mol. Ther. Oncolytics 2023, 30, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, R.; Benli-Hoppe, T.; Kitzberger, C.; Hageneier, M.; Schwenk, N.; Öztürk, Ö; Steiger, K.; Multhoff, G.; Eiber, M.; Schilling, F.; et al. Dual EGFR- and TfR-targeted gene transfer for sodium iodide symporter gene therapy of glioblastoma. Mol. Ther. Oncolytics 2022, 27, 272–287. [Google Scholar] [CrossRef]
- Fu, Y.; Ong, L.-C.; Ranganath, S.H.; Zheng, L.; Kee, I.; Zhan, W.; Yu, S.; Chow, P.K.H.; Wang, C.-H. A Dual Tracer 18F-FCH/18F-FDG PET Imaging of an Orthotopic Brain Tumor Xenograft Model. PLoS ONE 2016, 11, e0148123. [Google Scholar] [CrossRef]
- Kitzberger, C.; Spellerberg, R.; Han, Y.; Schmohl, K.A.; Stauss, C.; Zach, C.; Kälin, R.E.; Multhoff, G.; Eiber, M.; Schilling, F.; et al. Mesenchymal Stem Cell-mediated Image-guided Sodium Iodide Symporter (NIS) Gene Therapy Improves Survival of Glioblastoma-bearing Mice. Clin. Cancer Res. 2023, 29, 930–942. [Google Scholar] [CrossRef]
- Abo-Aziza, F.A.M.; Zaki, A.K.A.; Abo El-Maaty, A.M. Bone marrow-derived mesenchymal stem cell (BM-MSC): A tool of cell therapy in hydatid experimentally infected rats. Cell Regen. 2019, 8, 58–71. [Google Scholar] [CrossRef]
- Kitzberger, C.; Spellerberg, R.; Morath, V.; Schwenk, N.; Schmohl, K.A.; Schug, C.; Urnauer, S.; Tutter, M.; Eiber, M.; Schilling, F.; et al. The sodium iodide symporter (NIS) as theranostic gene: Its emerging role in new imaging modalities and non-viral gene therapy. EJNMMI Res. 2022, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeong, M.; Kim, D.W.; Jeong, H.; Lim, S.T.; Sohn, M. Iodine 125-labeled mesenchymal-epithelial transition factor binding peptide-click-cRGDyk heterodimer for glioma imaging. Cancer Sci. 2011, 102, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bullard, D.E.; Zalutsky, M.R.; Coleman, R.E.; Wikstrand, C.J.; Friedman, H.S.; Colapinto, E.V.; Bigner, D.D. Therapeutic efficacy of antiglioma mesenchymal extracellular matrix 131I-radiolabeled murine monoclonal antibody in a human glioma xenograft model. Cancer Res. 1988, 48, 559–566. [Google Scholar] [PubMed]
- Nowak, B.; Rogujski, P.; Janowski, M.; Lukomska, B.; Andrzejewska, A. Mesenchymal stem cells in glioblastoma therapy and progression: How one cell does it all. Biochim. Biophys. Acta Rev Cancer. 2021, 1876, 188582. [Google Scholar] [CrossRef]
- Spellerberg, R.; Benli-Hoppe, T.; Kitzberger, C.; Berger, S.; Schmohl, K.A.; Schwenk, N.; Yen, H.-Y.; Zach, C.; Schilling, F.; Weber, W.A.; et al. Selective sodium iodide symporter (NIS) genetherapy of glioblastoma mediatedby EGFR-targeted lipopolyplexes. Mol. Ther. Oncolytics 2021, 23, 432–446. [Google Scholar] [CrossRef]
- Jansen, J.A.; Omuro, A.; Lucca, L.E. T cell dysfunction in glioblastoma: A barrier and an opportunity for the development of successful immunotherapies. Curr. Opin. Neurol. 2021, 34, 827–833. [Google Scholar] [CrossRef]

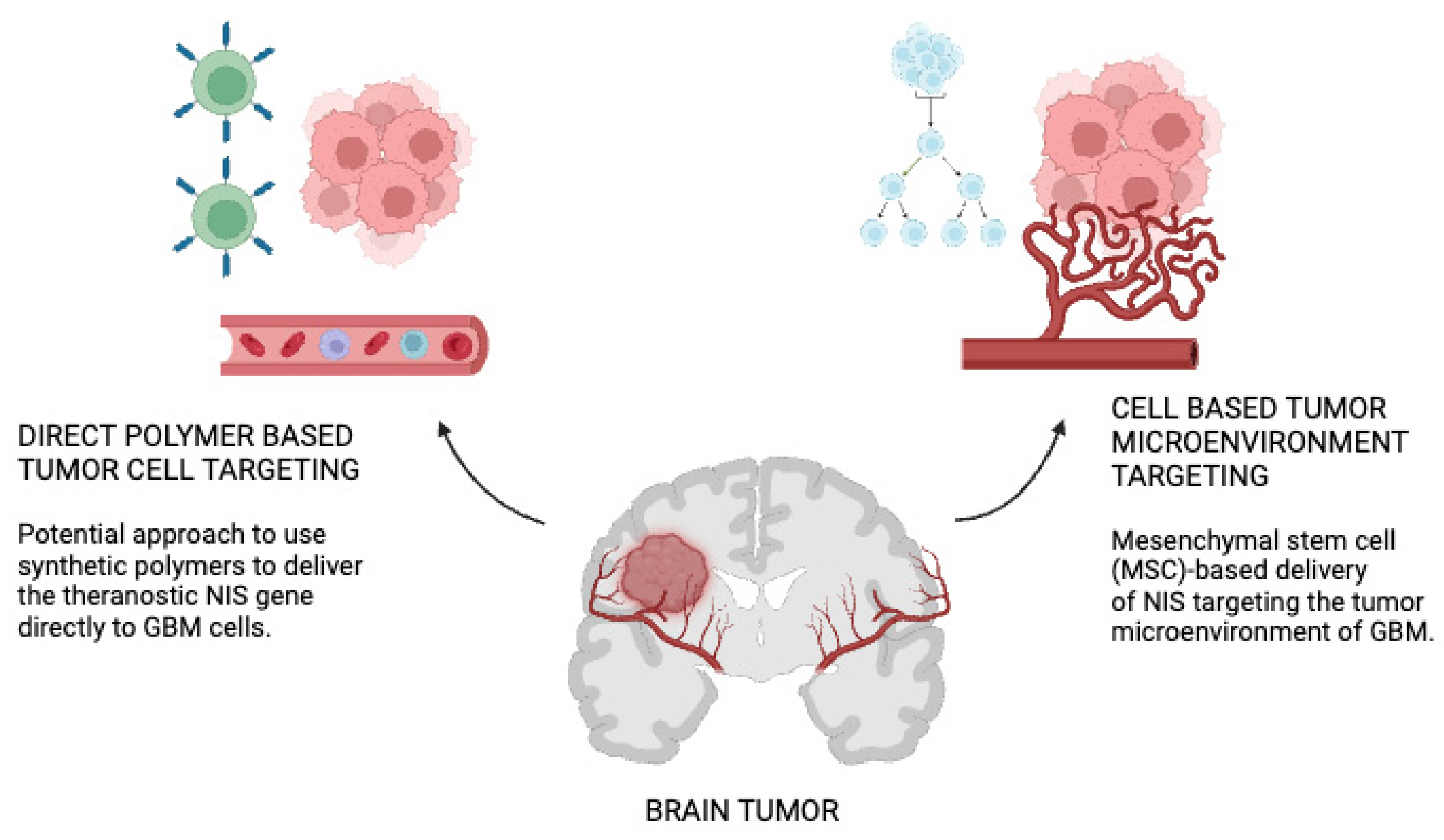
| NIS Gene Delivery Strategies | Polymer-Based | MSC-Based NIS Delivery |
|---|---|---|
| Advantages [71,72] |
|
|
| Disadvantages [71,72] |
|
|
| Studies | Abstract | Conclusion |
|---|---|---|
| Mesenchymal stem cell-mediated image-guided sodium iodide symporter (NIS) gene therapy improves survival of glioblastoma-bearing mice [91] | Due in part to their innate capacity to locate tumors, mesenchymal stem cells (MSC) have become viable cellular carriers for the delivery of therapeutic genes in cancer treatment. The sodium iodide symporter (NIS), a theranostic gene, is a promising target for non-invasive radionuclide-based imaging and therapeutics. In this work, we used genetically modified MSCs to target the NIS gene for tumor-targeted transfer in experimental glioblastoma (GBM), a tumor with a very bad prognosis. | By using NIS-mediated in vivo imaging, a strong tumoral NIS-specific radionuclide accumulation was seen following the administration of NIS-MSC and radioiodide. Tumor-selective MSC homing was seen in conjunction with NIS expression in GBM and non-target tissues stained with NIS immunofluorescence. When compared to controls, the application of therapeutically effective 131I resulted in a considerable delay in tumor development and an extended median survival following NIS-MSC therapy. |
| Bone marrow-derived mesenchymal stem cell-mediated dual-gene therapy for glioblastoma [92] | A highly effective BMSC-based therapy approach has been created that permits the concurrent elimination of implanted BMSCs following glioblastoma treatment, evaluation, and suppression of tumor angiogenesis. The human sodium iodide symporter (NIS), which is involved in the uptake of radioisotopes and is controlled by the early growth response factor 1 (Egr1), a radiation-activated promoter, and the angiogenesis inhibitor kringle 5 (K5) of human plasminogen were engineered to co-express in BMSCs. | Mesenchymal stem cells (MSCs) have been used as a delivery vector for anticancer drugs in many tumor models due to their capacity to move precisely to malignancies. Tumor necrosis factor apoptosis-inducing ligand, or NIS, has been engineered to express and deliver immunomodulatory cytokines such as interleukin-12, interferons (IFN) like IFN-α and IFN-β, prodrug converting enzymes like thymidine kinase from the herpes simplex virus, and many other tumor types. The administration of 131I after systemic MSC-mediated NIS gene transfer caused a notable postponement in the formation of tumors. K5 and NIS were selected as the treatment agents in this investigation because of their strong synergistic anticancer impact. |
| The sodium iodide symporter (NIS) as a theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy [93] | The sodium iodide symporter (NIS) was cloned in 1996, opening the door to its potential application as a potent theranostic transgene. Innovative gene therapy approaches use therapeutic radionuclides after image-guided selective NIS gene transfer in non-thyroidal cancers. The development of the NIS gene therapy approach, which uses genetically modified mesenchymal stem cells and synthetic polyplexes as selective non-viral gene delivery vehicles, has advanced significantly over the previous 20 years, as this overview demonstrates. | The tumor micromilieu may potentially be involved in the control of NIS function and/or NIS membrane targeting, which might impact the effectiveness of NIS gene therapy techniques. Extensive evidence from advanced cancer models, including our own data, suggest that the NIS gene therapy idea may be extended to low volume, disseminated illnesses like glioblastoma. NIS transgene expression can be comparatively low in low volume diseases. In this case, the great sensitivity and resolution of emerging imaging techniques should be quite helpful in tailoring treatment plans. |
| Iodine 125-labeled mesenchymal–epithelial transition factor binding peptide-click-cRGDyk heterodimer for glioma imaging [94] | Using mini polyethylene glycol-conjugated cMBP-3 glycine (GGG), a single name of amino acids (SC) (Ser-Cys), and cRGDyk through a click (1° + 3° cycloaddition), a cMBP-click-cRGDyk (cyclic Arg-Gly-Asp-Tyr-Lys) heterodimer was created. It was then labeled with iodine 125 (I-125) via histidine in the cMBP and tyrosine in the cRGDyk. Both in vitro and in vivo tests were performed to evaluate the tumor-targeting effectiveness and receptor-binding properties of cMBP-click-cRGDyk. | A biodistribution research study found that at 4 h, 125I-cMBP-GGG-SC had the greatest T/B. On the other hand, at 1 and 4 h, static pin-hole pictures of 125I-cMBP-GGG-SC revealed a comparatively poor tumor uptake and high body background activity, with considerably greater pancreatic and renal activities throughout. To increase the targetability for an in vivo cancer model, cMBP-GGG-SC had to be modified by the heterodimerization of two ligands, one of which targeted c-Met and the other integrin. |
| Therapeutic efficacy of antiglioma mesenchymal extracellular matrix 131I-radiolabeled murine monoclonal antibody(mab) in a human glioma xenograft model [95] | The discovery of Mabs—especially those reacting with primary brain tumors but not with the normal brain—offers a possible way to target human malignant gliomas specifically with therapeutic medicines. It has been demonstrated that Mab 81C6, an IgG2b immunoglobulin, binds to human glioma cell lines, glioma xenografts in nude mice, and primary human gliomas, but not to the normal adult or fetal brain. Mab 81C6 specifies an epitope of the glioma-associated extracellular matrix protein tenascin. | In several animal models, tumor-associated Mabs have demonstrated therapeutic effects. However, Mabs alone have often only proven effective against tiny or freshly infected tumors, with the exception of p 185, an IgG2a Mab directed against a neuoncogene-associated transmembrane glycoprotein. Drug–antibody conjugates have also only demonstrated activity against freshly infected tumor cells or in vitro. In contrast, a number of models have demonstrated the effectiveness of radiolabeled Mabs against well-established malignancies. |
| Mesenchymal stem cells in glioblastoma therapy and progression: how one cell does it all [96] | One of the somatic stem cells that is extensively studied and used in experimental treatments for the regeneration of damaged tissues is the mesenchymal stem cell (MSC). Furthermore, MSCs could have anti-tumor qualities, as was recently suggested. Glioblastoma (GBM) is a grade IV tumor of the central nervous system that has an unfavorable prognosis with no effective treatment currently available. Many debates have arisen from experimental trials that used MSCs to treat GBM. It has been demonstrated that native MSCs have anti-GBM action through apoptosis induction, cell cycle regulation, and angiogenesis control. | The actual nature of the connections between GBM cells and endogenous MSCs remains unknown; nonetheless, it appears that both cell types undergo functional alterations as a result of reciprocal signaling processes. MSCs grown in vitro appear to have GBM inhibitory properties. Notwithstanding these observations, a number of preclinical investigations showed that MSCs might effectively limit the development of GBM. Moreover, a number of strategies have demonstrated effective MSC-based drug delivery for anticancer purposes, which is extremely promising for potential therapeutic uses. However, as animal research provides the majority of experimental data, caution must be used when extrapolating this information to human treatment. |
| Selective sodium iodide symporter (NIS) gene therapy of glioblastoma mediated by EGFR-targeted lipopolyplexes [97] | When post-functionalized with ligands to improve targeting, lipo-oligomers offer a potential new vehicle for delivering therapeutic genes, like the sodium iodide symporter (NIS), to particular tumors. NIS is an effective theranostic technique for therapeutic radionuclide application and diagnostic imaging because of its iodide-trapping action. Applications of 131I allow for cytoreduction, whereas 124I PET imaging permits non-invasive monitoring of the in vivo biodistribution of functional NIS expression. We employed EGFR-targeted polyplexes (GE11) in our experimental design, which were first described in vitro using 125I uptake experiments. | Based on dosimetric calculations, NIS imaging enables an accurate assessment of radiation dosage for radioablation of the specific tumor. When 131I is applied, radionuclide entrapment occurs inside NIS-positive cells, and beta decay causes cell death. The impact of 131I is further enhanced by the cross-fire effect, which causes nearby cells to also undergo cytotoxic death. Because of their natural NIS expression, the thyroid and salivary glands are primarily affected by off-target damage. The TSH dependence of NIS expression results in a downregulation of thyroidal iodide absorption following pretreatment with LT4. Thyroid hormone replacement therapy can be used to treat hypothyroidism if it still develops following treatment. Even in cases of advanced metastatic illness, radioiodide therapy has a proven track record of success in treating thyroid cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.; Lucke-Wold, B. Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma. Cancers 2024, 16, 2892. https://doi.org/10.3390/cancers16162892
Shah S, Lucke-Wold B. Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma. Cancers. 2024; 16(16):2892. https://doi.org/10.3390/cancers16162892
Chicago/Turabian StyleShah, Siddharth, and Brandon Lucke-Wold. 2024. "Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma" Cancers 16, no. 16: 2892. https://doi.org/10.3390/cancers16162892
APA StyleShah, S., & Lucke-Wold, B. (2024). Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma. Cancers, 16(16), 2892. https://doi.org/10.3390/cancers16162892







