A Probabilistic Approach to Estimate the Temporal Order of Pathway Mutations Accounting for Intra-Tumor Heterogeneity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Method
2.2. Probability Model
2.3. Entropy Constraint
2.4. Computational Efficiency Optimization
3. Results
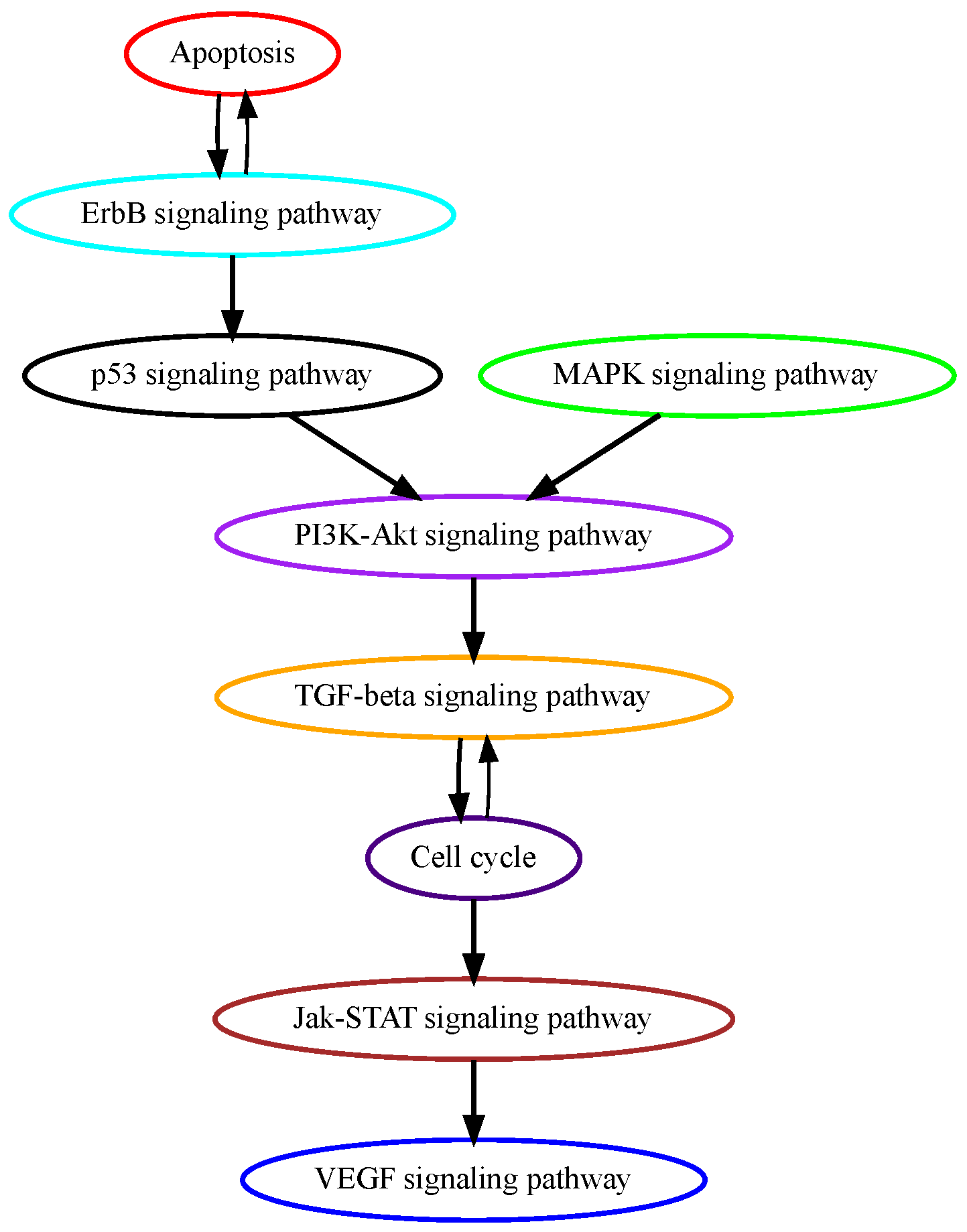
3.1. Analysis of TCGA Colon Adenocarcinoma Data
3.2. Analysis of TCGA Hepatocellular Carcinoma Data
3.3. Analysis of TCGA Glioblastoma Multiforme Data
3.4. Analysis of TCGA Pancreatic Adenocarcinoma Data
3.5. Comparison to Other Methods
Comparison of Computational Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ITH | intra-tumor heterogeneity |
| PATOPAI | a probabilistic approach for estimating the temporal order of pathway mutations by incorporating ITH information |
| TCGA | The Cancer Genome Atlas |
| HCC | hepatocellular carcinoma |
| GBM | glioblastoma multiforme |
| PAAD | pancreatic adenocarcinoma |
References
- Kuipers, E.; M Grady, W.; Lieberman, D.; Seufferlein, T.; J Sung, J.; Boelens, P.; H Cornelis, J.; De Velde, V.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Desper, R.; Jiang, F.; Kallioniemi, O.P.; Moch, H.; Papadimitriou, C.H.; Schäffer, A.A. Inferring tree models for oncogenesis from comparative genome hybridization data. J. Comput. Biol. 1999, 6, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Boucher, K. Estimating an oncogenetic tree when false negatives and positives are present. Math. Biosci. 2002, 176, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Beerenwinkel, N.; Rahnenführer, J.; Däumer, M.; Hoffmann, D.; Kaiser, R.; Selbig, J.; Lengauer, T. Learning multiple evolutionary pathways from cross-sectional data. J. Comput. Biol. 2005, 12, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Beerenwinkel, N.; Eriksson, N.; Sturmfels, B. Conjunctive bayesian networks. Bernoulli 2007, 13, 893–909. [Google Scholar] [CrossRef]
- Beerenwinkel, N.; Sullivant, S. Markov models for accumulating mutations. Biometrika 2009, 96, 645–661. [Google Scholar] [CrossRef]
- Gerstung, M.; Baudis, M.; Moch, H.; Beerenwinkel, N. Quantifying cancer progression with conjunctive Bayesian networks. Bioinformatics 2009, 25, 2809–2815. [Google Scholar] [CrossRef]
- Youn, A.; Simon, R. Estimating the order of mutations during tumorigenesis from tumor genome sequencing data. Bioinformatics 2012, 28, 1555–1561. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Liu, J.; Chen, L.; Stromberg, A.J.; Villano, J.L.; Arnold, S.M.; Liu, C.; Wang, C. A probabilistic method for leveraging functional annotations to enhance estimation of the temporal order of pathway mutations during carcinogenesis. BMC Bioinform. 2019, 20, 620. [Google Scholar] [CrossRef]
- Beerenwinkel, N.; Schwarz, R.F.; Gerstung, M.; Markowetz, F. Cancer evolution: Mathematical models and computational inference. Syst. Biol. 2015, 64, e1–e25. [Google Scholar] [CrossRef]
- Schwartz, R.; Schäffer, A.A. The evolution of tumour phylogenetics: Principles and practice. Nat. Rev. Genet. 2017, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Goyette, M.A.; Lipsyc-Sharf, M.; Polyak, K. Clinical and translational relevance of intratumor heterogeneity. Trends Cancer 2023, 9, 726–737. [Google Scholar] [CrossRef]
- Deshwar, A.G.; Vembu, S.; Yung, C.K.; Jang, G.H.; Stein, L.; Morris, Q. PhyloWGS: Reconstructing subclonal composition and evolution from whole-genome sequencing of tumors. Genome Biol. 2015, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qiu, Y.; Minn, A.J.; Zhang, N.R. Assessing intratumor heterogeneity and tracking longitudinal and spatial clonal evolutionary history by next-generation sequencing. Proc. Natl. Acad. Sci. USA 2016, 113, E5528–E5537. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; White, B.; Foltz, S.; Miller, C.; Luo, J.; Fields, R.; Maher, C. ClonEvol: Clonal ordering and visualization in cancer sequencing. Ann. Oncol. 2017, 28, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Rampášek, L.; Safikhani, Z.; Smirnov, P.; Haibe-Kains, B.; Goldenberg, A. Machine learning approaches to drug response prediction: Challenges and recent progress. NPJ Precis. Oncol. 2020, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Sashittal, P.; Zaccaria, S.; El-Kebir, M. Parsimonious clone tree integration in cancer. Algorithms Mol. Biol. 2022, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Vembu, S.; Deshwar, A.G.; Stein, L.; Morris, Q. Inferring clonal evolution of tumors from single nucleotide somatic mutations. BMC Bioinform. 2014, 15, 35. [Google Scholar] [CrossRef]
- Ellson, J. Graphviz-Graph Visualization Software. 2008. Available online: http://www.graphviz.org/ (accessed on 1 July 2018).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Rychkov, G.Y.; Barritt, G.J. Targeting Ca2+ signaling in the initiation, promotion and progression of hepatocellular carcinoma. Cancers 2020, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Ro, S.W. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S. The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Lim, D.; Kattan, M.W.; Chakravarti, A.; Gilbert, M.R.; Lassman, A.B.; Lo, S.S.; Machtay, M.; Sloan, A.E.; Sulman, E.P.; et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro-Oncology 2017, 19, 669–677. [Google Scholar] [PubMed]
- Pei, Z.; Lee, K.C.; Khan, A.; Erisnor, G.; Wang, H.Y. Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression. Biochem. Pharmacol. 2020, 176, 113814. [Google Scholar] [CrossRef]
- Chen, W.L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321. [Google Scholar] [CrossRef]
- Tilak, M.; Holborn, J.; New, L.A.; Lalonde, J.; Jones, N. Receptor tyrosine kinase signaling and targeting in glioblastoma multiforme. Int. J. Mol. Sci. 2021, 22, 1831. [Google Scholar] [CrossRef]
- Varn, F.S.; Johnson, K.C.; Martinek, J.; Huse, J.T.; Nasrallah, M.P.; Wesseling, P.; Cooper, L.A.; Malta, T.M.; Wade, T.E.; Sabedot, T.S.; et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 2022, 185, 2184–2199.e16. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar]
- Waters, A.M.; Der, C.J. KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733. [Google Scholar] [CrossRef]
- Moskaluk, C.A.; Hruban, R.H.; Kern, S.E. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997, 57, 2140–2143. [Google Scholar] [PubMed]
- Iacobuzio-Donahue, C.A. Genetic evolution of pancreatic cancer: Lessons learnt from the pancreatic cancer genome sequencing project. Gut 2012, 61, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Kuipers, J.; Beerenwinkel, N. Tree inference for single-cell data. Genome Biol. 2016, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Tzen, A.; Navin, N.; Chen, K.; Nakhleh, L. SiFit: Inferring tumor trees from single-cell sequencing data under finite-sites models. Genome Biol. 2017, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Navin, N.; Chen, K.; Nakhleh, L. SiCloneFit: Bayesian inference of population structure, genotype, and phylogeny of tumor clones from single-cell genome sequencing data. Genome Res. 2019, 29, 1847–1859. [Google Scholar] [CrossRef]
- Perez-Moreno, P.; Brambilla, E.; Thomas, R.; Soria, J.C. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin. Cancer Res. 2012, 18, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2014, 157, 753. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular subtypes of breast cancer: A review for breast radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Xie, Y.; Liu, J.; Li, A.; Chen, L.; Stromberg, A.; Arnold, S.M.; Liu, C.; Wang, C. A Probabilistic Approach to Estimate the Temporal Order of Pathway Mutations Accounting for Intra-Tumor Heterogeneity. Cancers 2024, 16, 2488. https://doi.org/10.3390/cancers16132488
Wang M, Xie Y, Liu J, Li A, Chen L, Stromberg A, Arnold SM, Liu C, Wang C. A Probabilistic Approach to Estimate the Temporal Order of Pathway Mutations Accounting for Intra-Tumor Heterogeneity. Cancers. 2024; 16(13):2488. https://doi.org/10.3390/cancers16132488
Chicago/Turabian StyleWang, Menghan, Yanqi Xie, Jinpeng Liu, Austin Li, Li Chen, Arnold Stromberg, Susanne M. Arnold, Chunming Liu, and Chi Wang. 2024. "A Probabilistic Approach to Estimate the Temporal Order of Pathway Mutations Accounting for Intra-Tumor Heterogeneity" Cancers 16, no. 13: 2488. https://doi.org/10.3390/cancers16132488
APA StyleWang, M., Xie, Y., Liu, J., Li, A., Chen, L., Stromberg, A., Arnold, S. M., Liu, C., & Wang, C. (2024). A Probabilistic Approach to Estimate the Temporal Order of Pathway Mutations Accounting for Intra-Tumor Heterogeneity. Cancers, 16(13), 2488. https://doi.org/10.3390/cancers16132488




