Dose Contribution to the Regional Lymph-Node Metastases and Point B from Intracavity and Interstitial Hybrid Brachytherapy in Locally Advanced Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
References
- Han, K.; Colson-Fearon, D.; Liu, Z.A.; Viswanathan, A. Updated Trends in the Utilization of Brachytherapy in Cervical Cancer in the U.S.: A Surveillance, Epidemiology, and End-Results Study. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Vittrup, A.S.; Spampinato, S.; Jensen, N.B.K.; Tanderup, K.; Kirchheiner, K.; Pötter, R.; Nout, R.; Jürgenliemk-Schulz, I.M. In Reply to Murakami et al. Int. J. Radiat. Oncol Biol. Phys. 2023, 116, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Tanderup, K.; Planchamp, F.; Chiva, L.; Humphrey, P.; Sturdza, A.; Tan, L.T.; van der Steen-Banasik, E.; Zapardiel, I.; Nout, R.A.; et al. ESGO/ESTRO quality indicators for radiation therapy of cervical cancer. Radiother. Oncol. 2023, 183, 109589. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Rosaria Raspollini, M.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Radiother. Oncol. 2023, 184, 109682. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Ohno, T.; Toita, T.; Ando, K.; Ii, N.; Okamoto, H.; Kojima, T.; Tsujino, K.; Masui, K.; Yoshida, K.; et al. Japanese Society for Radiation Oncology Consensus Guidelines of combined intracavitary and interstitial brachytherapy for gynecological cancers. J. Radiat. Res. 2022, 63, 402–411. [Google Scholar] [CrossRef]
- Murakami, N.; Watanabe, M.; Uno, T.; Sekii, S.; Tsujino, K.; Kasamatsu, T.; Machitori, Y.; Aoshika, T.; Kato, S.; Hirowatari, H.; et al. Phase I/II prospective clinical trial for the hybrid of intracavitary and interstitial brachytherapy for locally advanced uterine cervical cancer. J. Gynecol. Oncol. 2023, 34, e24. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Masui, K.; Yoshida, K.; Noda, S.E.; Watanabe, M.; Takenaka, T.; Ii, N.; Atsumi, K.; Umezawa, R.; Inaba, K.; et al. Hands-on seminar for image-guided adaptive brachytherapy and intracavitary/interstitial brachytherapy for uterine cervical cancer. Jpn. J. Clin. Oncol. 2023, 53, 508–513. [Google Scholar] [CrossRef]
- Muramoto, Y.; Murakami, N.; Karino, T.; Sugimoto, S.; Takatsu, J.; Oshima, M.; Kosugi, Y.; Kawamoto, T.; Hirayama, T.; Fujino, K.; et al. MucoUp® as a spacer in brachytherapy for uterine cervical cancer: A first-in-human experience. Clin. Transl. Radiat. Oncol. 2023, 42, 100659. [Google Scholar] [CrossRef]
- Kobayashi, R.; Murakami, N.; Chiba, T.; Okuma, K.; Inaba, K.; Takahashi, K.; Kaneda, T.; Kashihara, T.; Takahashi, A.; Shimizu, Y.; et al. Effect of Hyaluronate Acid Injection on Dose-Volume Parameters in Brachytherapy for Cervical Cancer. Adv. Radiat. Oncol. 2022, 7, 100918. [Google Scholar] [CrossRef]
- Takatsu, J.; Murakami, N.; Muramoto, Y.; Karino, T.; Oshima, M.; Kosugi, Y.; Kawamoto, T.; Terao, Y.; Shikama, N. Safe dose escalation and reduction of the fraction number of uterine cervical brachytherapy using a gel spacer in the rectovaginal and vesicouterine septum: A planning study. Brachytherapy 2024, 23, 115–122. [Google Scholar] [CrossRef]
- Lee, L.J.; Sadow, C.A.; Russell, A.; Viswanathan, A.N. Correlation of point B and lymph node dose in 3D-planned high-dose-rate cervical cancer brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 803–809. [Google Scholar] [CrossRef][Green Version]
- van den Bos, W.; Beriwal, S.; Velema, L.; de Leeuw, A.A.; Nomden, C.N.; Jürgenliemk-Schulz, I.M. Image guided adaptive brachytherapy for cervical cancer: Dose contribution to involved pelvic nodes in two cancer centers. J. Contemp. Brachyther. 2014, 6, 21–27. [Google Scholar] [CrossRef]
- Brower, J.V.; Bradley, K.A.; Russo, A.L. Management of Radiographically Positive Pelvic and/or Para-aortic Lymph Nodes During Primary Chemoradiation Therapy for Cervix Cancer. Pract. Radiat. Oncol. 2023, 13, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Moghani, M.M.; Siavashpour, Z.; Ogorodniitchouk, O.; Moreno-Acosta, P.; Plattard, D.; Vallard, A.; Sotton, S.; Bouleftour, W.; Langrand-Escure, J.; Magné, N. Dose to pelvic lymph nodes in locally advanced cervical cancer during high-dose-rate brachytherapy with tandem-ring applicators. J. Contemp. Brachyther. 2022, 14, 183–188. [Google Scholar] [CrossRef]
- Bacorro, W.; Dumas, I.; Levy, A.; Rivin Del Campo, E.; Canova, C.H.; Felefly, T.; Huertas, A.; Marsolat, F.; Haie-Meder, C.; Chargari, C.; et al. Contribution of image-guided adaptive brachytherapy to pelvic nodes treatment in locally advanced cervical cancer. Brachytherapy 2017, 16, 366–372. [Google Scholar] [CrossRef]
- Chua, G.W.Y.; Foo, Y.W.; Tay, G.H.; Tan, D.B.H. Assessing dose contribution to pelvic lymph nodes in intracavitary brachytherapy for cervical cancer. J. Contemp. Brachyther. 2017, 9, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Erickson, B.; Gaffney, D.K.; Beriwal, S.; Bhatia, S.K.; Lee Burnett, O., 3rd; D’Souza, D.P.; Patil, N.; Haddock, M.G.; Jhingran, A.; et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 320–328. [Google Scholar] [CrossRef]
- Tod, M.; Meredith, W.J. Treatment of cancer of the cervix uteri, a revised Manchester method. Br. J. Radiol. 1953, 26, 252–257. [Google Scholar] [CrossRef]
- Matsukawa, H.; Sasaki, T.; Hirayama, R.; Hirose, T.A.; Fukunaga, J.I. Assessment of the anatomical position of point B and the relationship between point B dose and the dose delivered to pelvic lymph nodes in CT-based high-dose-rate brachytherapy for uterine cervical cancer. J. Contemp. Brachyther. 2019, 11, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, R.; Subramanian, S.; Gopalakrishnan, K.; Jothi, V.; Krishnamurthy, K. Dose to pelvic lymph nodes in image based high dose rate brachytherapy of carcinoma cervix. Rep. Pract. Oncol. Radiother. 2019, 24, 80–85. [Google Scholar] [CrossRef]
- Vargo, J.A.; Kim, H.; Choi, S.; Sukumvanich, P.; Olawaiye, A.B.; Kelley, J.L.; Edwards, R.P.; Comerci, J.T.; Beriwal, S. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Ohno, T.; Kato, S.; Ando, K.; Noda, S.E.; Kiyohara, H.; Shibuya, K.; Karasawa, K.; Kamada, T.; Nakano, T. Impact of boost irradiation on pelvic lymph node control in patients with cervical cancer. J. Radiat. Res. 2014, 55, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Miyagi, E.; Numazaki, R.; Asai-Sato, M.; Kasuya, T.; Kaizu, H.; Matsui, T.; Hirahara, F.; Inoue, T. Radiation therapy for pelvic lymph node metastasis from uterine cervical cancer. Gynecol. Oncol. 2013, 131, 99–102. [Google Scholar] [CrossRef]
- Nomden, C.N.; Pötter, R.; de Leeuw, A.A.C.; Tanderup, K.; Lindegaard, J.C.; Schmid, M.P.; Fortin, I.; Haie-Meder, C.; Mahantshetty, U.; Hoskin, P.; et al. Nodal failure after chemo-radiation and MRI guided brachytherapy in cervical cancer: Patterns of failure in the EMBRACE study cohort. Radiother. Oncol. 2019, 134, 185–190. [Google Scholar] [CrossRef]
| Variable | No. of Patients | |
|---|---|---|
| Median age (years, range) | 56 (30–78) | 11 |
| Median Body Mass Index (range) | 23.2 (17.4–30.4) | 11 |
| ECOG PS (patients) | 0 | 9 |
| 1 | 2 | |
| T classification | T2b | 5 |
| T3b | 6 | |
| N classification | N0 | 0 |
| N1 | 11 | |
| FIGO stage (2018) | IIIC1 | 9 |
| IIIC2 | 2 | |
| Histology | Squamous cell carcinoma | 9 |
| Adenocarcinoma | 2 |
| Lymph Node Locations | Total Number (Left, Right Side) | Mean Total D100 (Range) | Mean Total D90 (Range) | Mean Total D50 (Range) | Mean Total D0.1cm3 (Range) | p-Value |
|---|---|---|---|---|---|---|
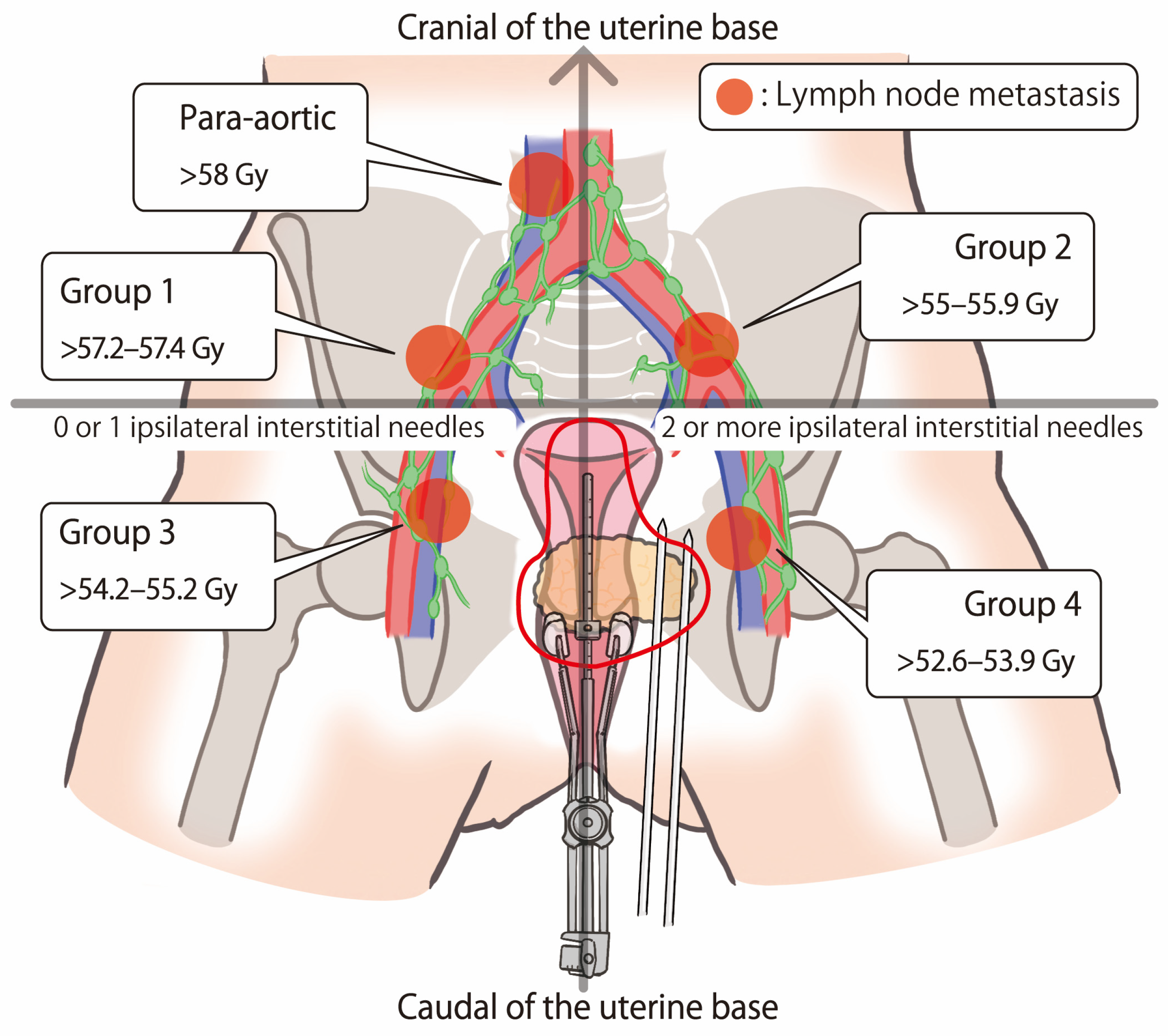
| Para-aortic lymph nodes | 4 (2, 2) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | - |
| Common iliac lymph nodes | 6 (4, 2) | 42.2 (0.0–119.7) | 48.5 (0.0–139.1) | 83.2 (0–219.1) | 182.5 (75.8–410.0) | - |
| External iliac lymph nodes | 14 (7, 7) | 264.8 (72.1–516.5) | 313.8 (95.8–578.9) | 382.9 (109.8–690.9) | 569.2 (137.3–1153.7) | - |
| Internal iliac lymph nodes | 11 (6, 5) | 264.4 (0.0–700.9) | 304.3 (0.0–793.3) | 379.0 (41.4–953.8) | 578.6 (87.6–1412.5) | - |
| Obturator lymph nodes | 2 (1, 1) | 408.2 (351.3–465.1) | 449.5 (389.6–509.4) | 540.1 (494.1–586.1) | 705.2 (660.7–749.6) | - |
| Pre-sacral lymph nodes | 1 (0, 1) | 451.2 | 480.3 | 526.9 | 633.6 | - |
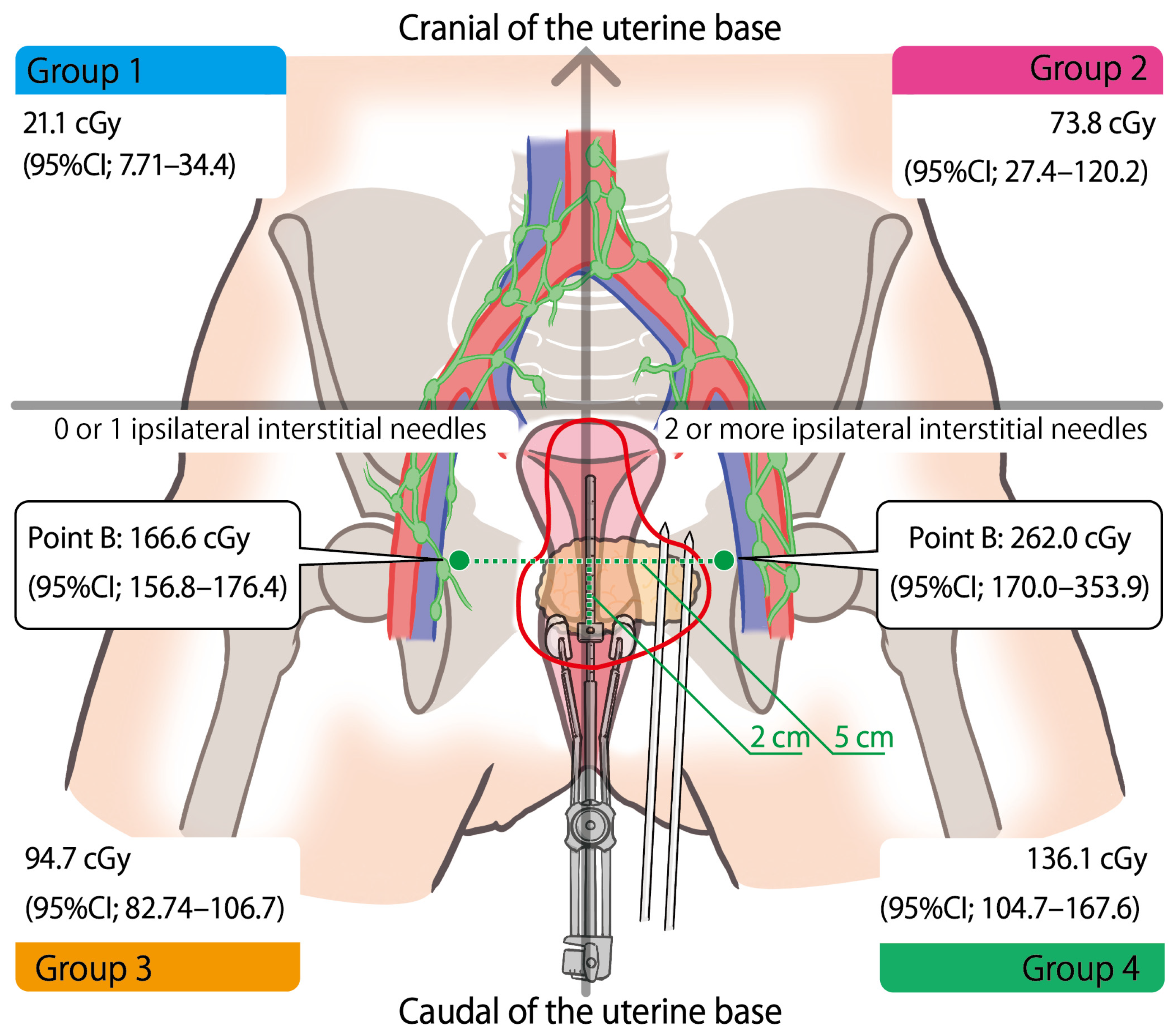
| Cranial of the uterine base | 17 | 72.3 (0–386.3) | 85.5 (0–473.0) | 117.8 (0–602.8) | 197.6 (0–870.8) | <0.0001 |
| Caudal of the uterine base | 21 | 328.9 (72.1–700.9) | 378.9 (95.8–793.3) | 460.0 (109.8–953.8) | 672.1 (137.3–1412.5) |
| Number of Ipsilateral Interstitial Needles | Mean D90 at Each Session (Range) | p-Value |
|---|---|---|
| 0 (n = 4) | 48.6 (0–98.7) | - |
| 1 (n = 91) | 69.2 (0–285.8) | - |
| 2 (n = 23) | 106.2 (0–254.8) | - |
| 4 (n = 1) | 101.8 | - |
| 5 (n = 2) | 185.6 (175.9–195.3) | - |
| 0–1 ipsilateral interstitial needle (n = 95) | 68.4 (95%CI; 56.4–80.3) | 0.006 |
| 2 or more ipsilateral interstitial needles (n = 26) | 112.2 (95%CI; 89.3–135.0) |
| Point B (11 Patients, 38 IC/IS Sessions) | Mean Dose of Each Session (Range) | Mean Total Dose (Range) | p-Value |
|---|---|---|---|
| Bilateral (mean of both side) | 185.4 (106.9–484.5) | 640.6 (455.9–1190.0) | - |
| Left side | 203.0 (103.8–736.9) | 703.0 (409.9–1587.8) | - |
| Right side | 167.4 (109.9–280.6) | 578.4 (377.2–792.1) | - |
| Ipsilateral 0 needle (n = 15) | 144.8 (112.0–186.0) | - | - |
| Ipsilateral 1 needle (n = 46) | 173.7 (103.8–280.6) | - | - |
| Ipsilateral 2 needles (n = 12) | 195.1 (141.6–251.7) | - | - |
| Ipsilateral 4 needles (n = 1) | 283.2 | - | - |
| Ipsilateral 5 needles (n = 2) | 652.3 (567.7–736.9) | - | - |
| 0–1 ipsilateral interstitial needle (n = 61) | 166.6 (95%CI; 156.8–176.4) | - | <0.001 |
| 2 or more ipsilateral interstitial needles (n = 15) | 262.0 (95%CI; 170.0–353.9) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muramoto, Y.; Murakami, N.; Okonogi, N.; Takatsu, J.; Iijima, K.; Inoue, T.; Kato, K.; Karino, T.; Kojima, K.; Oshima, M.; et al. Dose Contribution to the Regional Lymph-Node Metastases and Point B from Intracavity and Interstitial Hybrid Brachytherapy in Locally Advanced Cervical Cancer. Cancers 2024, 16, 2384. https://doi.org/10.3390/cancers16132384
Muramoto Y, Murakami N, Okonogi N, Takatsu J, Iijima K, Inoue T, Kato K, Karino T, Kojima K, Oshima M, et al. Dose Contribution to the Regional Lymph-Node Metastases and Point B from Intracavity and Interstitial Hybrid Brachytherapy in Locally Advanced Cervical Cancer. Cancers. 2024; 16(13):2384. https://doi.org/10.3390/cancers16132384
Chicago/Turabian StyleMuramoto, Yoichi, Naoya Murakami, Noriyuki Okonogi, Jun Takatsu, Kotaro Iijima, Tatsuya Inoue, Kanade Kato, Tatsuki Karino, Kanako Kojima, Masaki Oshima, and et al. 2024. "Dose Contribution to the Regional Lymph-Node Metastases and Point B from Intracavity and Interstitial Hybrid Brachytherapy in Locally Advanced Cervical Cancer" Cancers 16, no. 13: 2384. https://doi.org/10.3390/cancers16132384
APA StyleMuramoto, Y., Murakami, N., Okonogi, N., Takatsu, J., Iijima, K., Inoue, T., Kato, K., Karino, T., Kojima, K., Oshima, M., Kosugi, Y., Kawamoto, T., Hirayama, T., Fujino, K., Terao, Y., & Shikama, N. (2024). Dose Contribution to the Regional Lymph-Node Metastases and Point B from Intracavity and Interstitial Hybrid Brachytherapy in Locally Advanced Cervical Cancer. Cancers, 16(13), 2384. https://doi.org/10.3390/cancers16132384






