Anti-MAPK Targeted Therapy for Ameloblastoma: Case Report with a Systematic Review
Abstract
Simple Summary
Abstract
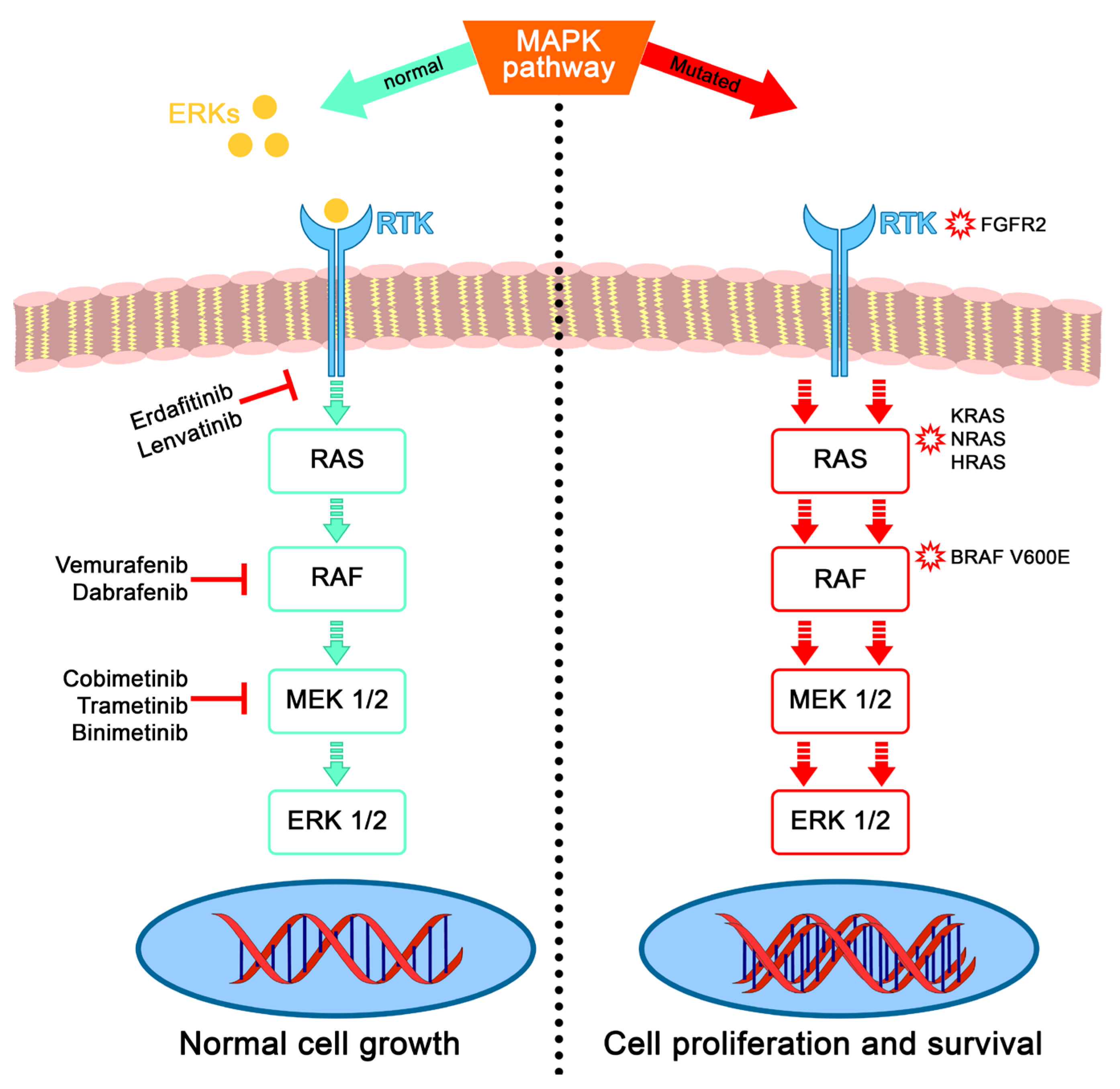
1. Introduction
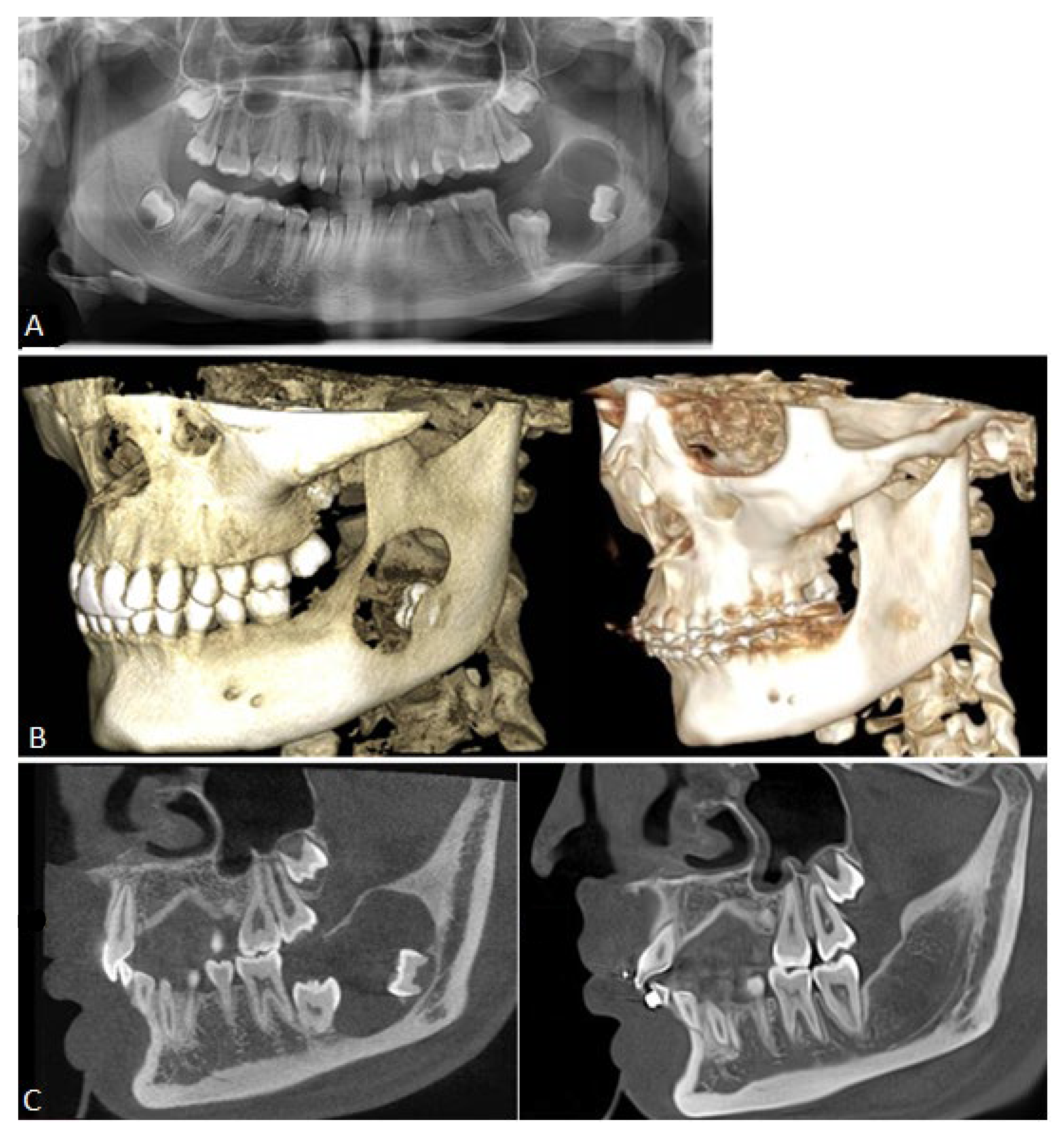
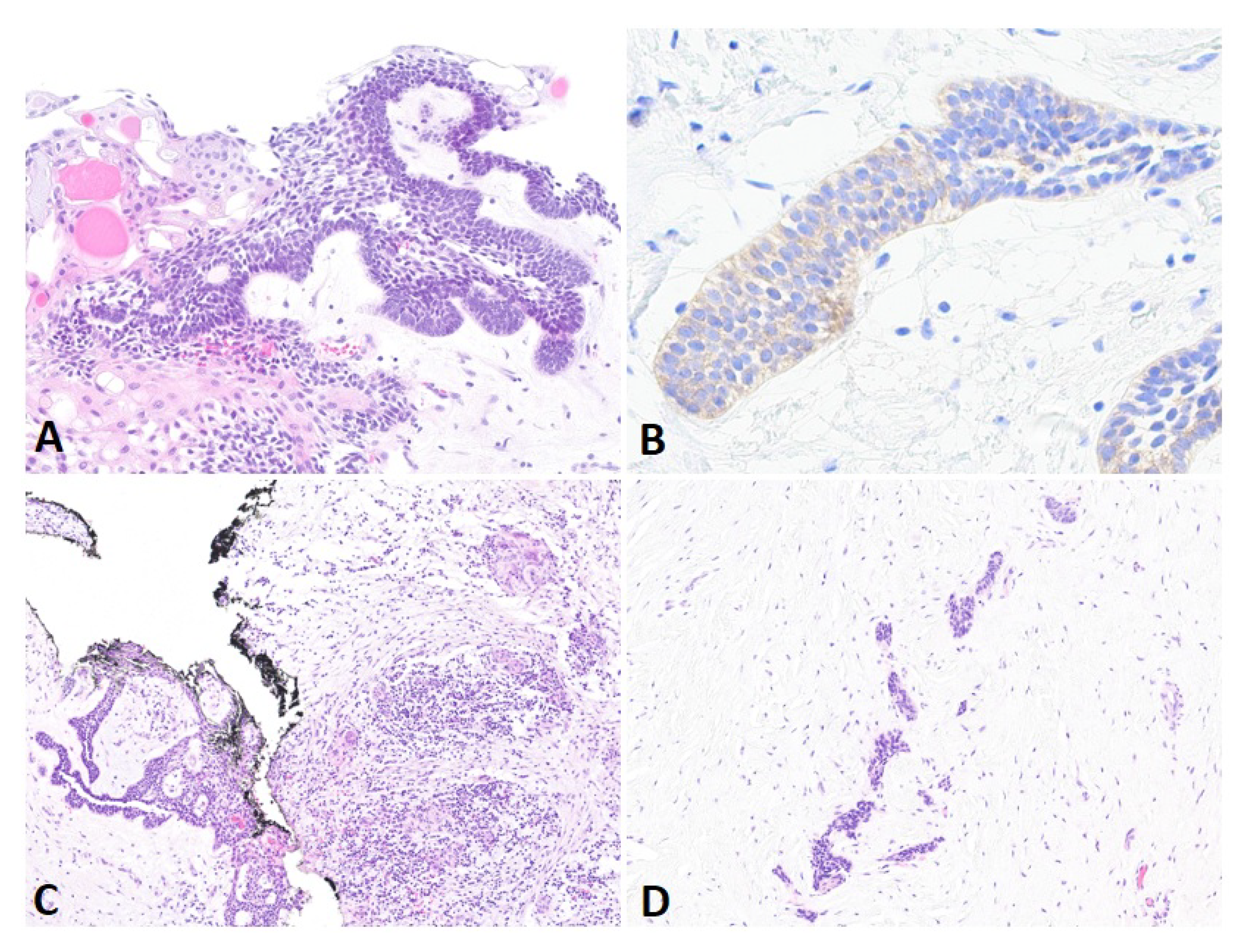
2. Case Presentation
3. Materials and Methods
3.1. Research Question, Protocol, and Eligibility Criteria
3.2. Data Sources, Search Strategy, and Study Selection
3.3. Quality Assessment and Data Synthesis
4. Results
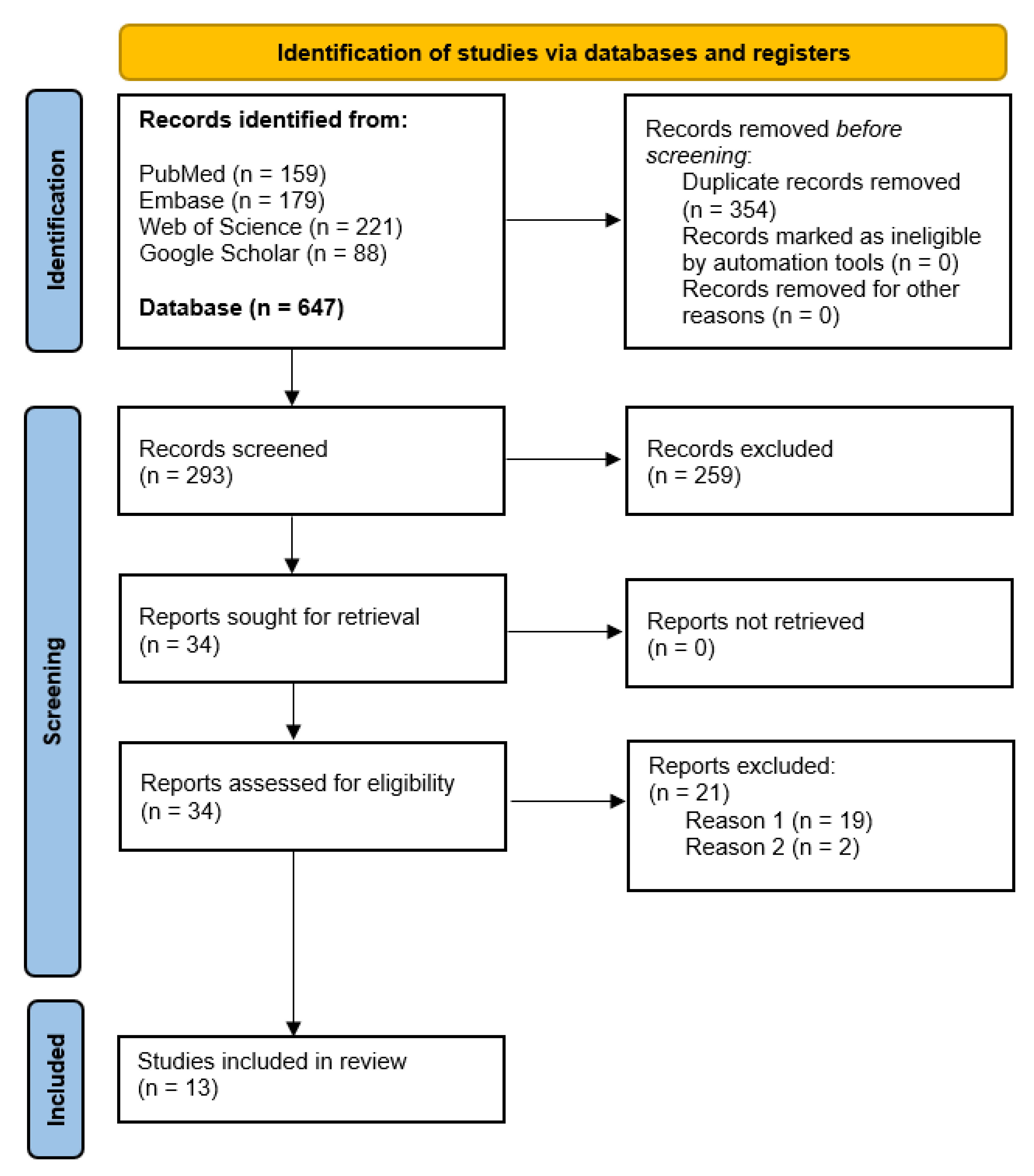
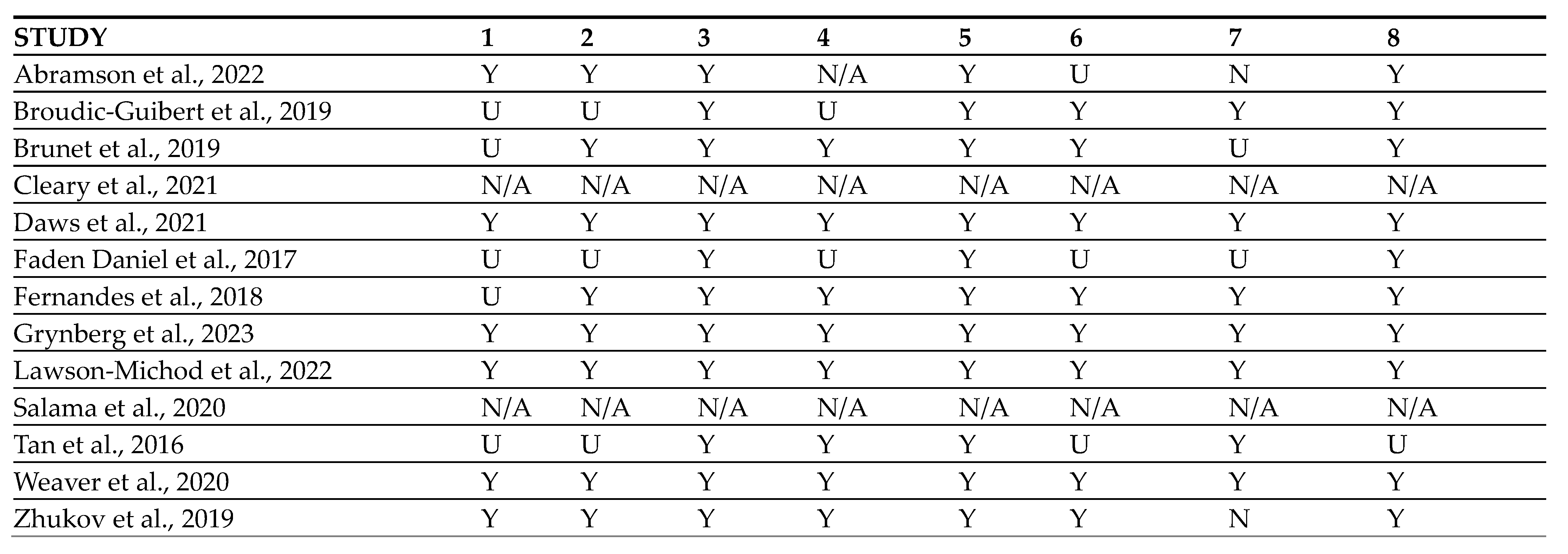
4.1. Data Search Results and Quality Assessment
4.2. Study Characteristics
4.3. Primary Outcome
4.4. Secondary Outcome
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vered, M.; Wright, J.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022, 16, 63–75. [Google Scholar] [CrossRef]
- Effiom, O.; Ogundana, O.; Akinshipo, A.; Akintoye, S. Ameloblastoma: Current etiopathological concepts and management. Oral Dis. 2018, 24, 307–316. [Google Scholar] [CrossRef]
- Hendra, F.N.; Van Cann, E.M.; Helder, M.N.; Ruslin, M.; de Visscher, J.G.; Forouzanfar, T.; De Vet, H. Global incidence and profile of ameloblastoma: A systematic review and meta-analysis. Oral Dis. 2020, 26, 12–21. [Google Scholar] [CrossRef]
- Troiano, G.; Dioguardi, M.; Cocco, A.; Laino, L.; Cervino, G.; Cicciu, M.; Ciavarella, D.; Lo Muzio, L. Conservative vs Radical Approach for the Treatment of Solid/Multicystic Ameloblastoma: A Systematic Review and Meta-analysis of the Last Decade. Oral Health Prev. Dent. 2017, 15, 421–426. [Google Scholar]
- Kalwagadda, S.; Kumar, B.; Nair, S.C.; Shah, A.K.; Shroff, S.S. Management of Ameloblastoma with Free Tissue Flap in Comparison with Other Reconstructive Options Available. J. Oral Maxillofac. Surg. 2020, 19, 283–288. [Google Scholar] [CrossRef]
- McClary, A.C.; West, R.B.; McClary, A.C.; Pollack, J.R.; Fischbein, N.J.; Holsinger, C.F.; Sunwoo, J.; Colevas, A.D.; Sirjani, D. Ameloblastoma: A clinical review and trends in management. Eur. Arch. Otorhinolaryngol. 2016, 273, 1649–1661. [Google Scholar] [CrossRef]
- Carlson, E.R.; Marx, R.E. The Ameloblastoma: Primary, Curative Surgical Management. J. Oral Maxillofac. Surg. 2006, 64, 484–494. [Google Scholar] [CrossRef]
- Bansal, S.; Desai, R.S.; Shirsat, P.; Prasad, P.; Karjodkar, F.; Andrade, N. The occurrence and pattern of ameloblastoma in children and adolescents: An Indian institutional study of 41 years and review of the literature. Int. J. Oral Maxillofac. Surg. 2015, 44, 725–731. [Google Scholar] [CrossRef]
- Fang, Q.G.; Shi, S.; Sun, C.F. Odontogenic Lesions in Pediatric Patients. J. Craniofac. Surg. 2014, 25, e248. [Google Scholar] [CrossRef]
- Ord, R.A.; Blanchaert, R.H.; Nikitakis, N.G.; Sauk, J.J. Ameloblastoma in children. J. Oral Maxillofac. Surg. 2002, 60, 762–770. [Google Scholar] [CrossRef]
- Abrahams, J.M.; McClure, S.A. Pediatric Odontogenic Tumors. Oral Maxillofac. Surg. Clin. N. Am. 2016, 28, 45–58. [Google Scholar] [CrossRef]
- Kurppa, K.J.; Catón, J.; Morgan, P.R.; Ristimäki, A.; Ruhin, B.; Kellokoski, J.; Elenius, K.; Heikinheimo, K. High frequency of BRAF V600E mutations in ameloblastoma. J. Pathol. 2014, 232, 492–498. [Google Scholar] [CrossRef]
- Sweeney, R.T.; McClary, A.C.; Myers, B.R.; Biscocho, J.; Neahring, L.; Kwei, K.A.; Qu, K.; Gong, X.; Ng, T.; Jones, C.D.; et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat. Genet. 2014, 46, 722–725. [Google Scholar] [CrossRef]
- Brown, N.A.; Rolland, D.; McHugh, J.B.; Weigelin, H.C.; Zhao, L.; Lim, M.S.; Elenitoba-Johnson, K.S.; Betz, B.L. Activating FGFR2–RAS–BRAF Mutations in Ameloblastoma. Clin. Cancer Res. 2014, 20, 5517–5526. [Google Scholar] [CrossRef]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for Carcinogenesis and Molecular Therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.N.; Hahn, H.J.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J. Am. Acad. Dermatol. 2015, 72, 1036–1046.e2. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Xiong, J.; Li, C.; Liao, Y.; Zhu, Y.; Mao, J. Risk and Prognostic Factors for BRAFV600E Mutations in Papillary Thyroid Carcinoma. Biomed. Res. Int. 2022, 2022, 9959649. [Google Scholar]
- Levin-Sparenberg, E.; Bylsma, L.C.; Lowe, K.; Sangare, L.; Fryzek, J.P.; Alexander, D.D. A Systematic Literature Review and Meta-Analysis Describing the Prevalence of KRAS, NRAS, and BRAF Gene Mutations in Metastatic Colorectal Cancer. Gastroenterol. Res. 2020, 13, 184–198. [Google Scholar] [CrossRef]
- Bouffet, E.; Hansford, J.R.; Garrè, M.L.; Hara, J.; Plant-Fox, A.; Aerts, I.; Locatelli, F.; Van Der Lugt, J.; Papusha, L.; Sahm, F.; et al. Dabrafenib plus Trametinib in Pediatric Glioma with BRAF V600 Mutations. N. Engl. J. Med. 2023, 389, 1108–1120. [Google Scholar] [CrossRef]
- Bouffet, E.; Geoerger, B.; Moertel, C.; Whitlock, J.A.; Aerts, I.; Hargrave, D.; Osterloh, L.; Tan, E.; Choi, J.; Russo, M.; et al. Efficacy and Safety of Trametinib Monotherapy or in Combination with Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 664–674. [Google Scholar] [CrossRef]
- Wen, P.Y.; Stein, A.; van den Bent, M.; De Greve, J.; Wick, A.; de Vos, F.Y.F.L.; von Bubnoff, N.; van Linde, M.E.; Lai, A.; Prager, G.W.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022, 23, 53–64. [Google Scholar] [CrossRef]
- Mamat Yusof, M.N.; Ch’ng, E.S.; Radhiah Abdul Rahman, N. BRAF V600E Mutation in Ameloblastoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5593. [Google Scholar] [CrossRef]
- González-González, R.; López-Verdín, S.; Lavalle-Carrasco, J.; Molina-Frechero, N.; Isiordia-Espinoza, M.; Carreón-Burciaga, R.G.; Bologna-Molina, R. Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review. World J. Clin. Oncol. 2020, 11, 31–42. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Miami, FL, USA, 2020. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Kaye, F.J.; Ivey, A.M.; Drane, W.E.; Mendenhall, W.M.; Allan, R.W. Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J. Natl. Cancer Inst. 2015, 107, 378. [Google Scholar] [CrossRef]
- Hirschhorn, A.; Campino, G.A.; Vered, M.; Greenberg, G.; Yacobi, R.; Yahalom, R.; Barshack, I.; Toren, A.; Amariglio, N.; Rechavi, G. Upfront rational therapy in BRAF V600E mutated pediatric ameloblastoma promotes ad integrum mandibular regeneration. J. Tissue Eng. Regen. Med. 2021, 15, 1155–1161. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors with BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef]
- Cleary, J.M.; Wang, V.; Heist, R.S.; Kopetz, E.S.; Mitchell, E.P.; Zwiebel, J.A.; Kapner, K.S.; Chen, H.X.; Li, S.; Gray, R.J.; et al. Differential Outcomes in Codon 12/13 and Codon 61 NRAS-Mutated Cancers in the Phase II NCI-MATCH Trial of Binimetinib in Patients with NRAS-Mutated Tumors. Clin. Cancer Res. 2021, 27, 2996–3004. [Google Scholar] [CrossRef]
- Abramson, Z.; Dayton, O.L.; Drane, W.E.; Mendenhall, W.M.; Kaye, F.J. Managing stage 4 ameloblastoma with dual BRAF/MEK inhibition: A case report with 8-year clinical follow-up. Oral Oncol. 2022, 128, 105854. [Google Scholar] [CrossRef]
- Lawson-Michod, K.A.; Le, C.H.; Tranesh, G.; Thomas, P.C.; Bauman, J.E. Precision medicine: Sustained response to erdafitinib in FGFR2-mutant, multiply recurrent ameloblastoma. Cancer Rep. 2022, 5, e1656. [Google Scholar] [CrossRef]
- Weaver, A.N.; Francisque, F.; Bowles, D.W. Tumor Regression after Treatment with Lenvatinib in FGFR2-Mutated Ameloblastoma. JCO Precis. Oncol. 2020, 4, 1403–1406. [Google Scholar] [CrossRef]
- Broudic-Guibert, M.; Blay, J.Y.; Vazquez, L.; Evrard, A.; Karanian, M.; Taïeb, S.; Hoog-Labouret, N.; Oukhatar, C.M.A.; Boustany-Grenier, R.; Arnaud, A. Persistent response to vemurafenib in metastatic ameloblastoma with BRAF mutation: A case report. J. Med. Case Rep. 2019, 13, 245. [Google Scholar] [CrossRef]
- Fernandes, G.S.; Girardi, D.M.; Bernardes, J.P.G.; Fonseca, F.P.; Fregnani, E.R. Clinical benefit and radiological response with BRAF inhibitor in a patient with recurrent ameloblastoma harboring V600E mutation. BMC Cancer 2018, 18, 887. [Google Scholar] [CrossRef]
- Daws, S.; Chaiyasate, K.; Lehal, A. Treatment of a BRAF V600E positive ameloblastoma in a pediatric patient with MEK inhibitor monotherapy. Face 2021, 2, 179–182. [Google Scholar] [CrossRef]
- Zhukov, N.; Mareeva, Y.; Konovalov, D.; Druy, A.; Grachev, N.; Litvinov, D. Potentially Curative Targeted Therapy for Undifferentiated High-Grade Sarcoma Developing After Malignant Transformation of a BRAF V600E-Mutated Ameloblastic Fibroma. JCO Precis. Oncol. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Grynberg, S.; Vered, M.; Shapira-Frommer, R.; Asher, N.; Ben-Betzalel, G.; Stoff, R.; Steinberg, Y.; Amariglio, N.; Greenberg, G.; Steinberg, Y.; et al. Neoadjuvant BRAF Targeted Therapy for Ameloblastoma of the Mandible: An Organ Preservation Approach. J. Natl. Cancer Inst. 2023, 116, djad232. [Google Scholar] [CrossRef]
- Tan, S.; Pollack, J.R.; Kaplan, M.J.; Colevas, A.D.; West, R.B. BRAF inhibitor treatment of primary BRAF-mutant ameloblastoma with pathologic assessment of response. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e5–e7. [Google Scholar] [CrossRef]
- Brunet, M.; Khalifa, E.; Italiano, A. Enabling Precision Medicine for Rare Head and Neck Tumors: The Example of BRAF/MEK Targeting in Patients with Metastatic Ameloblastoma. Front. Oncol. 2019, 9, 1204. [Google Scholar] [CrossRef]
- Faden, D.L.; Algazi, A. Durable treatment of ameloblastoma with single agent BRAFi Re: Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J. Natl. Cancer Inst. 2017, 109, djw190. [Google Scholar] [CrossRef]
- Hendra, F.N.; Natsir Kalla, D.S.; Van Cann, E.M.; de Vet, H.C.W.; Helder, M.N.; Forouzanfar, T. Radical vs conservative treatment of intraosseous ameloblastoma: Systematic review and meta-analysis. Oral Dis. 2019, 25, 1683–1696. [Google Scholar] [CrossRef]
- Amzerin, M.; Fadoukhair, Z.; Belbaraka, R.; Iraqui, M.; Boutayeb, S.; M’rabti, H.; Kebdani, T.; Hassouni, K.; Benjaafar, N.; El Gueddari, B.K.; et al. Metastatic ameloblastoma responding to combination chemotherapy: Case report and review of the literature. J. Med. Case Rep. 2011, 5, 491. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Werning, J.W.; Kaye, F.J.; Mendenhall, W.M. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur. Arch. Otorhinolaryngol. 2016, 273, 3293–3297. [Google Scholar] [CrossRef]
- Shi, H.A.; Ng, C.W.B.; Kwa, C.T.; Sim, Q.X.C. Ameloblastoma: A succinct review of the classification, genetic understanding and novel molecular targeted therapies. Surgeon 2021, 19, 238–243. [Google Scholar] [CrossRef]
- Graillon, N.; Akintoye, S.O.; Iocca, O.; Kaleem, A.; Hajjar, S.; Imanguli, M.; Shanti, R.M. Current concepts in targeted therapies for benign tumors of the jaw—A review of the literature. J. Craniomaxillofac. Surg. 2023, 51, 591–596. [Google Scholar] [CrossRef]
- Diniz, M.G.; Gomes, C.C.; Guimarães, B.V.A.; Castro, W.H.; Lacerda, J.C.T.; Cardoso, S.V.; de Faria, P.R.; Dias, F.L.; Eisenberg, A.L.A.; Loyola, A.M.; et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour. Biol. 2015, 36, 5649–5653. [Google Scholar] [CrossRef]
- Gültekin, S.E.; Aziz, R.; Heydt, C.; Sengüven, B.; Zöller, J.; Safi, A.F.; Kreppel, M.; Buettner, R. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch. 2018, 472, 807–814. [Google Scholar] [CrossRef]
- Guimarães, L.M.; Coura, B.P.; Gomez, R.S.; Gomes, C.C. The Molecular Pathology of Odontogenic Tumors: Expanding the Spectrum of MAPK Pathway Driven Tumors. Front. Oral Health 2021, 2, 740788. [Google Scholar] [CrossRef]
- Desvignes, C.; Abi Rached, H.; Templier, C.; Drumez, E.; Lepesant, P.; Desmedt, E.; Mortier, L. BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response. Melanoma Res. 2017, 27, 281–287. [Google Scholar] [CrossRef]
- Stege, H.; Haist, M.; Schultheis, M.; Fleischer, M.I.; Mohr, P.; Meier, F.; Schadendorf, D.; Ugurel, S.; Livingstone, E.; Zimmer, L.; et al. Discontinuation of BRAF/MEK-Directed Targeted Therapy after Complete Remission of Metastatic Melanoma—A Retrospective Multicenter ADOReg Study. Cancers 2021, 13, 2312. [Google Scholar] [CrossRef]
- Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; Li, J.R.; Kim, G.; Rezaee, M.; Ally, M.S.; Kim, J.; Yao, C.; Chang, A.L.S.; et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 2015, 27, 342–353. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Gray, R.J.; Flaherty, K.T.; Chen, A.P.; Li, S.; Wang, V.; McShane, L.M.; Patton, D.R.; Tricoli, J.V.; Williams, P.M.; et al. The NCI-MATCH trial: Lessons for precision oncology. Nat. Med. 2023, 29, 1349–1357. [Google Scholar] [CrossRef]
- Knispel, S.; Zimmer, L.; Kanaki, T.; Ugurel, S.; Schadendorf, D.; Livingstone, E. The safety and efficacy of dabrafenib and trametinib for the treatment of melanoma. Expert Opin. Drug Saf. 2018, 17, 73–87. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Yu, Q.; Xie, J.; Li, J.; Lu, Y.; Liao, L. Clinical outcomes of BRAF plus MEK inhibition in melanoma: A meta-analysis and systematic review. Cancer Med. 2019, 8, 5414–5424. [Google Scholar] [CrossRef]
- Blankenstein, S.A.; Rohaan, M.W.; Klop, W.M.C.; van der Hiel, B.; van de Wiel, B.A.; Lahaye, M.J.; Adriaansz, S.; Sikorska, K.; van Tinteren, H.; Sari, A.; et al. Neoadjuvant Cytoreductive Treatment with BRAF/MEK Inhibition of Prior Unresectable Regionally Advanced Melanoma to Allow Complete Surgical Resection, REDUCTOR: A Prospective, Single-arm, Open-label Phase II Trial. Ann. Surg. 2021, 274, 383–389. [Google Scholar] [CrossRef]
| Study | Age | Sex | Diagnosis | Location | Mutational Status |
|---|---|---|---|---|---|
| Abramson et al. [33] 2022 | 47 | M | Ameloblastoma, recurrent and metastatic (2 times) | Left mandible, bilateral lung metastasis | BRAF V600E, IHC + NGS |
| Broudic-Guibert et al. [36] 2019 | 33 | F | Ameloblastoma plexiform, lung metastases | Left mandible, bilateral lung metastasis | BRAF V600E |
| Brunet et al. [42] 2019 | 26 | F | Ameloblastoma, lung metastases | Right mandible, bilateral lung metastasis | BRAF V600E, NGS |
| Cleary et al. [32] 2021 | NS | NS | Ameloblastoma, lung metastases | NS, bilateral lung metastasis | NRAS Q61R, NGS |
| Daws et al. [38] 2021 | 13 | F | Ameloblastoma, primary | Right mandible | BRAF V600E, PCR |
| Fernandes et al. [37] 2018 | 29 | F | Ameloblastoma, recurrent | Left mandibular ascending branch, extension to cavernous sinus and orbital fissure | BRAF V600E, PCR |
| Faden Daniel et al. [43] 2017 | 83 | F | Ameloblastoma, recurrent | Right mandibular body | BRAF V600E |
| Grynberg et al. [40] 2023 | 15 | M | Ameloblastoma unicystic mural type, primary | Right mandibular angle | BRAF V600E, IHC + NGS |
| 13 | M | Ameloblastoma unicystic mural type, primary | Right mandibular angle | BRAF V600E, IHC + NGS | |
| 11 | M | Ameloblastoma unicystic mural type, primary | Left mandibular angle | BRAF V600E, IHC + NGS | |
| 15 | M | Ameloblastoma unicystic mural type, primary | Right mandibular angle | BRAF V600E, NGS | |
| 21 | M | Ameloblastoma unicystic mural type, primary | Left mandibular angle | BRAF V600E, NGS | |
| 83 | M | Ameloblastoma conventional, primary | Mandibular symphysis | BRAF V600E, NGS | |
| 32 | M | Ameloblastoma unicystic mural type, primary | Right mandibular body | BRAF V600E, NGS | |
| 15 | F | Ameloblastoma unicystic mural type (borderline conventional, primary | Right mandible | BRAF V600E, NGS | |
| 63 | M | Ameloblastoma conventional, primary | Right mandible | BRAF V600E, NGS | |
| 19 | M | Ameloblastoma unicystic mural type, primary | Left mandibular angle | BRAF V600E, NGS | |
| 25 | M | Ameloblastoma conventional, primary | Right mandibular angle | BRAF V600E, NGS | |
| Lawson-Michod et al. [34] 2022 | 40 | M | Conventional ameloblastoma, recurrent | Right maxillary sinus, extension to lateral orbit, middle cranial fossa, temporal lobe | PD-L1 CPS 2%, FGFR2 V395D, SMO W535L |
| Salama et al. [31] 2020 | NS | NS | Ameloblastoma, NS | Mandible | BRAF V600E, NGS |
| Tan et al. [41] 2016 | 85 | M | Ameloblastoma follicular and plexiform, recurrent | Left mandibular angle | BRAF V600E, PCR + IHC |
| Weaver et al. [35] 2020 | 62 | M | Ameloblastoma plexiform, primary | right maxillary sinus | FGFR2 Y375C, SMO L412F, PALB2 H786Y |
| Zhukov et al. [39] 2019 | 10 | M | Ameloblastic fibroma, recurrent | Right mandible, extension to base skull | BRAF V600E |
| Study | N° | Medication | Dosis | Duration | Treatment Modality |
|---|---|---|---|---|---|
| Abramson et al. [33] 2022 | 1 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 4 y, stop 3 y, rechallenge 16 m (ongoing) | Rechallenge, exclusive |
| Broudic-Guibert et al. [36] 2019 | 1 | Vemurafenib | 960 mg BID, reduce to 720 mg BID, 480 mg BID | 26 m (ongoing) | exclusive |
| Brunet et al. [42] 2019 | 1 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 30 w (ongoing) | exclusive |
| Cleary et al. [32] 2021 | 1 | Binimetinib | 45 mg BID | 26 m | exclusive |
| Daws et al. [38] 2021 | 1 | Trametinib | 1.5 mg QD, reduced to 1 mg QD | 2 w, 6 w | exclusive |
| Fernandes et al. [37] 2018 | 1 | Vemurafenib | 960 mg BID | 12 m (ongoing) | exclusive |
| Faden Daniel et al. [43] 2017 | 1 | dabrafenib | 75 mg BID, reduced to 32.5 mg BID | 12 m (ongoing) | exclusive |
| Grynberg et al. [40] 2023 | 1 | Dabrafenib | 4.5 mg/kg/d | 20 m | Neo-adjuvant |
| 2 | Dabrafenib | 4.5 mg/kg/d | 18 m | Neo-adjuvant | |
| 3 | Dabrafenib | 4.5 mg/kg/d | 12 m | Neo-adjuvant | |
| 4 | Dabrafenib | 4.5 mg/kg/d | 6 m | Neo-adjuvant | |
| 5 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 13 m | Neo-adjuvant and adjuvant | |
| 6 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 4 m | Neo-adjuvant | |
| 7 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 16 m | Neo-adjuvant | |
| 8 | Dabrafenib | 4.5 mg/kg/d | 12 m | Neo-adjuvant | |
| 9 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 8 m | Neo-adjuvant | |
| 10 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 3 m | Neo-adjuvant | |
| 11 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 4 m | Exclusive | |
| Lawson-Michod et al. [34] 2022 | 1 | Pembrolizumab Erdafitinib | NS 8 mg QD | 12 w 12 m, stop 14 m, rechallenge 12 w (ongoing) | Rechallenge, exclusive |
| Salama et al. [31] 2020 | 1 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | 35 m (ongoing) | Exclusive |
| Tan et al. [41] 2016 | 1 | Dabrafenib | 150 mg BID | 73 d | Neo-adjuvant |
| Weaver et al. [35] 2020 | 1 | Lenvatinib | 24 mg QD, reduced to 20 mg, reduced to 20–10 mg, reduced to 14 mg and again 20 mg | 1 m, 1 m, 13 m in total (ongoing) | Exclusive |
| Zhukov et al. [39] 2019 | 1 | Vemurafenib Cobimetinib | 960 mg BID 60 mg QD | 18 m | Neo-adjuvant and adjuvant |
| Study | N° | Radiological Response | Pathological Response | Effect on Symptoms | Follow-Up | Recurrence |
|---|---|---|---|---|---|---|
| Abramson et al. [33] 2022 | 1 | CR for lung metastasis, PR locally | CR after 2 w | 7 y | Yes (treated with rechallenge) | |
| Broudic-Guibert et al. [36] 2019 | 1 | PR, 30% decrease in diameter of lung meta after 3.5 m (RECIST) | CR after 4 m | 26 m | N | |
| Brunet et al. [42] 2019 | 1 | CR (RECIST + PERCIST) | CR after 30 w | 30 w | N | |
| Cleary et al. [32] 2021 | 1 | PR | NS | 26 m | N | |
| Daws et al. [38] 2021 | 1 | SD | No necrosis, no squamous differentiation, no architectural change | SD | 3 m | / |
| Fernandes et al. [37] 2018 | 1 | PR | CR after 2 w | 12 m | N | |
| Faden Daniel et al. [43] 2017 | 1 | PR, 75% volume reduction after 8 months | CR at 8 m | 12 m | N | |
| Grynberg et al. [40] 2023 | 1 | PR | Near Complete Response | NS | 58 m | N |
| 2 | PR | PR | NS | 51 m | N | |
| 3 | PR | PR | NS | 42 m | N | |
| 4 | PR | NCR | NS | 17 m | N | |
| 5 | PR | PR | NS | 33 m | Yes (treated with re-operation followed by adjuvant rechallenge) | |
| 6 | PR | PR | NS | 23 m | N | |
| 7 | PR | NCR | NS | 26 m | N | |
| 8 | PR | NCR | NS | 30 m | N | |
| 9 | PR | PR | NS | 19 m | N | |
| 10 | PR | PR | NS | 15 m | N | |
| 11 | CR | NS | 15 m | N | ||
| Lawson-Michod et al. [34] 2022 | 1 | PR, NCR at rechallenge | CR after 4w | 29 m | Yes (treated with rechallenge) | |
| Salama et al. [31] 2020 | 1 | SD | NS | 35 m | NS | |
| Tan et al. [41] 2016 | 1 | SD | Squamous cell differentiation. Less than 10% of ameloblastic cells | NS | 1 y | N |
| Weaver et al. [35] 2020 | 1 | PR, 40% volume reduction after 6 m (RECIST) | CR et 6m | 13 m | N | |
| Zhukov et al. [39] 2019 | 1 | PR, 79% volume reduction after 1 m | No BRAF sensitive allele-specific PCR, CR | CR after 2d | 18 m | N |
| Study | N° | Medication | Dose | CTCAE (Grade, Symptoms) | TTT Modification | |
|---|---|---|---|---|---|---|
| Abramson et al. [33] 2022 | 1 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | NS | ||
| Broudic-Guibert et al. [36] 2019 | 1 | Vemurafenib | 960 mg BID, reduce to 720 mg BID, 480 mg BID | Grade 1–2 | Arthralgia, nausea, rash | Dose reduction (50%) |
| Brunet et al. [42] 2019 | 1 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | NS | ||
| Cleary et al. [32] 2021 | 1 | binimetinib | 45 mg BID | Grade 2 | Myalgia | Discontinuation |
| Daws et al. [38] 2021 | 1 | trametinib | 1.5 mg QD, reduced to 1 mg QD | (Grade 1–2) | Modeste pustular facial acne | TTT held 1w, dose reduction |
| Fernandes et al. [37] 2018 | 1 | vemurafenib | 960 mg BID | Grade 1 | Anorexia, nausea, fatigue | none |
| Faden Daniel et al. [43] 2017 | 1 | dabrafenib | 75 mg BID, reduced to 32.5 mg BID | NS | ||
| Grynberg et al. [40] 2023 | 1 | Dabrafenib | 4.5 mg/kg/d | Grade 2 Grade 1 | Fever Curly hair | TTT held |
| 2 | Dabrafenib | 4.5 mg/kg/d | Grade 1 | Fever, rash acneiform | none | |
| 3 | Dabrafenib | 4.5 mg/kg/d | Grade 2 Grade 1 | Erythema nodosum Folliculitis | TTT held | |
| 7 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | Grade 1–2 | Fever, weakness, arthralgia | TTT held and dose reduction | |
| 9 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | Grade 1–2 | Fever, myalgia, aphthous ulcers | TTT held and dose reduction | |
| 10 | Dabrafenib Trametinib | 150 mg BID 2 mg QD | Grade 3 | Hepatitis | discontinuation | |
| unspecified | unspecified | / | Grade 1–2 (3 patients) | unspecified | none | |
| Lawson-Michod et al. [34] 2022 | 1 | pembrolizumab erdafitinib | NS 8 mg QD | Grade 2 | Myalgia, fatigue | TTT held |
| Salama et al. [31] 2020 | 1 | dabrafenib trametinib | 150 mg BID 2 mg QD | NS | ||
| Tan et al. [41] 2016 | 1 | dabrafenib | 150 mg BID | Fatigue, actinic keratoses, voice change | discontinuation | |
| Weaver et al. [35] 2020 | 1 | lenvatinib | 24 mg QD, reduced to 20 mg, reduced to 20–10 mg, reduced to 14 mg and again 20 mg | Grade 2 Grade 1 | HTA, hypothyroidism, weight loss diarrhea | none |
| Zhukov et al. [39] 2019 | 1 | vemurafenib cobimetinib | 960 mg BID 60 mg QD | NS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raemy, A.; May, L.; Sala, N.; Diezi, M.; Beck-Popovic, M.; Broome, M. Anti-MAPK Targeted Therapy for Ameloblastoma: Case Report with a Systematic Review. Cancers 2024, 16, 2174. https://doi.org/10.3390/cancers16122174
Raemy A, May L, Sala N, Diezi M, Beck-Popovic M, Broome M. Anti-MAPK Targeted Therapy for Ameloblastoma: Case Report with a Systematic Review. Cancers. 2024; 16(12):2174. https://doi.org/10.3390/cancers16122174
Chicago/Turabian StyleRaemy, Anton, Laurence May, Nathalie Sala, Manuel Diezi, Maja Beck-Popovic, and Martin Broome. 2024. "Anti-MAPK Targeted Therapy for Ameloblastoma: Case Report with a Systematic Review" Cancers 16, no. 12: 2174. https://doi.org/10.3390/cancers16122174
APA StyleRaemy, A., May, L., Sala, N., Diezi, M., Beck-Popovic, M., & Broome, M. (2024). Anti-MAPK Targeted Therapy for Ameloblastoma: Case Report with a Systematic Review. Cancers, 16(12), 2174. https://doi.org/10.3390/cancers16122174






