Melanoma in Pregnancy—Diagnosis, Treatment, and Consequences for Fetal Development and the Maintenance of Pregnancy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria of the Study
2.2. Results
3. Prognosis
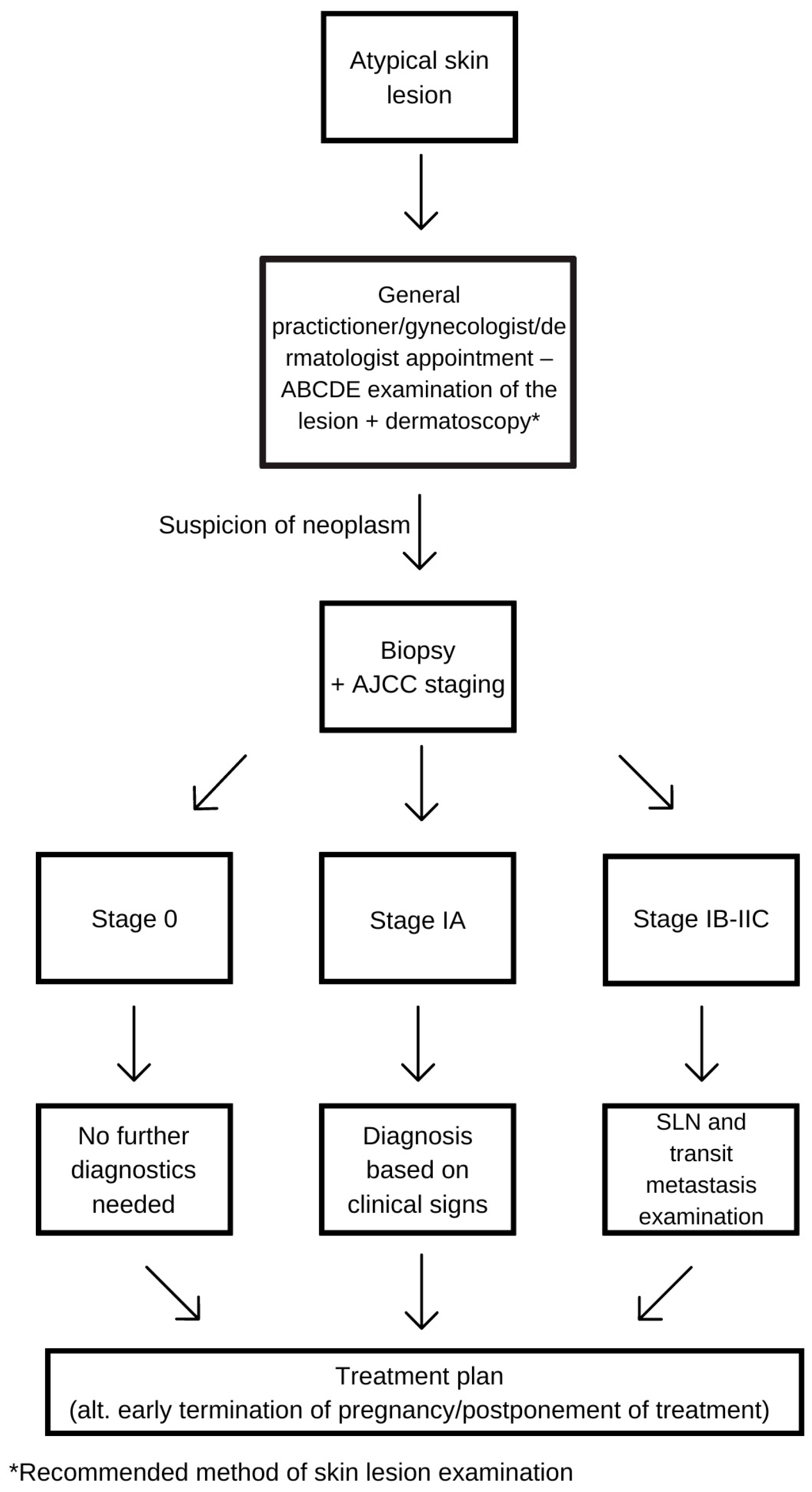
4. Diagnosis
4.1. Physiological Changes during Pregnancy and Delayed Recognition
4.2. Observation of the Lesion and Dermatoscopy
- (a)
- color of the lesion;
- (b)
- shape;
- (c)
- pigmentation (multifocal, central, eccentric, and uniform);
- (d)
- location, especially special sites—face, genital area, nails, and mucous membrane [25].
4.3. Staging
4.4. Sentinel Lymph Node Biopsy and Excision of the Lesion
4.5. Radiology Diagnostic Possibilities
4.6. New Diagnostic Possibilities
5. Diagnostics and Treatment
5.1. Surgery of the Primary Melanoma
5.2. Metastases and Recurrence of Melanoma
5.3. Radiation
5.4. MRI
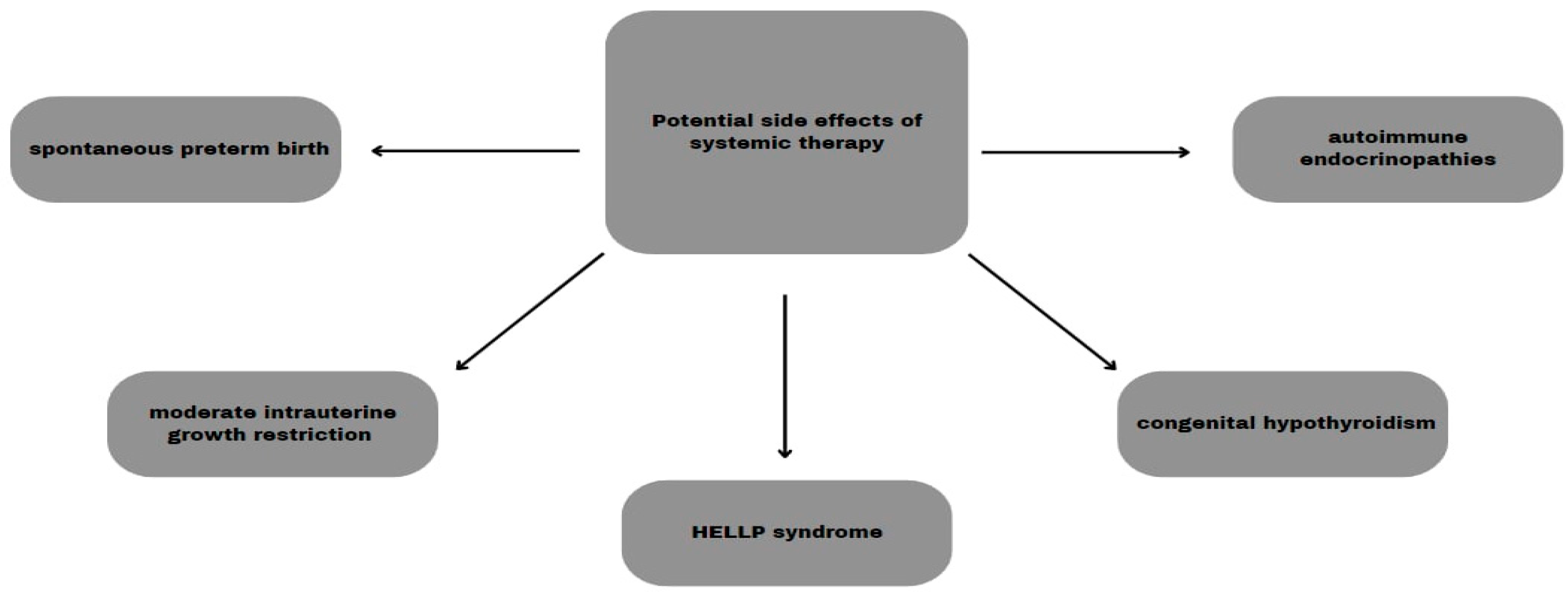
5.5. Systemic Therapy
6. Consequences for Both Mother and Child
6.1. Perinatal Complications
6.2. Placental Metastasis
6.3. Maternal Organism
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef]
- Yuan, T.A.; Lu, Y.; Edwards, K.; Jakowatz, J.; Meyskens, F.L.; Liu-Smith, F. Race-, Age-, and Anatomic Site-Specific Gender Differences in Cutaneous Melanoma Suggest Differential Mechanisms of Early- and Late-Onset Melanoma. Int. J. Environ. Res. Public Health 2019, 16, 908. [Google Scholar] [CrossRef]
- Karimkhani, C.; Green, A.C.; Nijsten, T.; Weinstock, M.A.; Dellavalle, R.P.; Naghavi, M.; Fitzmaurice, C. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 2017, 177, 134–140. [Google Scholar] [CrossRef]
- Andersson, T.M.; Johansson, A.L.; Fredriksson, I.; Lambe, M. Cancer during pregnancy and the postpartum period: A population-based study. Cancer 2015, 121, 2072–2077. [Google Scholar] [CrossRef]
- Maggen, C.; Wolters, V.E.R.A.; Cardonick, E.; Fumagalli, M.; Halaska, M.J.; Lok, C.A.R.; de Haan, J.; Van Tornout, K.; Van Calsteren, K.; Amant, F. Pregnancy and Cancer: The INCIP Project. Curr. Oncol. Rep. 2020, 22, 17. [Google Scholar] [CrossRef]
- Byrom, L.; Olsen, C.; Knight, L.; Khosrotehrani, K.; Green, A.C. Increased mortality for pregnancy-associated melanoma: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1457–1466. [Google Scholar] [CrossRef]
- Tellez, A.; Rueda, S.; Conic, R.Z.; Powers, K.; Galdyn, I.; Mesinkovska, N.A.; Gastman, B. Risk factors and outcomes of cutaneous melanoma in women less than 50 years of age. J. Am. Acad. Dermatol. 2016, 74, 731–738. [Google Scholar] [CrossRef]
- O’Meara, A.T.; Cress, R.; Xing, G.; Danielsen, B.; Smith, L.H. Malignant melanoma in pregnancy. A population-based evaluation. Cancer 2005, 103, 1217–1226. [Google Scholar] [CrossRef]
- Johansson, A.L.; Andersson, T.M.; Plym, A.; Ullenhag, G.J.; Møller, H.; Lambe, M. Mortality in women with pregnancy-associated malignant melanoma. J. Am. Acad. Dermatol. 2014, 71, 1093–1101. [Google Scholar] [CrossRef]
- Jones, M.S.; Lee, J.; Stern, S.L.; Faries, M.B. Is Pregnancy-Associated Melanoma Associated with Adverse Outcomes? J. Am. Coll. Surg. 2017, 225, 149–158. [Google Scholar] [CrossRef]
- Carter, T.J.; George, C.; Harwood, C.; Nathan, P. Melanoma in pregnancy: Diagnosis and management in early-stage and advanced disease. Eur. J. Cancer 2022, 166, 240–253. [Google Scholar] [CrossRef]
- Vora, R.V.; Gupta, R.; Mehta, M.J.; Chaudhari, A.H.; Pilani, A.P.; Patel, N. Pregnancy and skin. J. Fam. Med. Prim. Care 2014, 3, 318–324. [Google Scholar] [CrossRef]
- Bannister-Tyrrell, M.; Roberts, C.L.; Hasovits, C.; Nippita, T.; Ford, J.B. Incidence and outcomes of pregnancy-associated melanoma in New South Wales 1994–2008. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 116–122. [Google Scholar] [CrossRef]
- Khosrotehrani, K.; Nguyen Huu, S.; Prignon, A.; Avril, M.-F.; Boitier, F.; Oster, M.; Mortier, L.; Richard, M.-A.; Maubec, E.; Kerob, D.; et al. Pregnancy promotes melanoma metastasis through enhanced lymphangiogenesis. Am. J. Pathol. 2011, 178, 1870–1880. [Google Scholar] [CrossRef]
- Dadras, S.S.; Lange-Asschenfeldt, B.; Velasco, P.; Nguyen, L.; Vora, A.; Muzikansky, A.; Jahnke, K.; Hauschild, A.; Hirakawa, S.; Mihm, M.C.; et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod. Pathol. 2005, 18, 1232–1242. [Google Scholar] [CrossRef]
- Wolters, V.; Heimovaara, J.; Maggen, C.; Cardonick, E.; Boere, I.; Lenaerts, L.; Amant, F. Management of pregnancy in women with cancer. Int. J. Gynecol. Cancer 2021, 31, 314–322. [Google Scholar] [CrossRef]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575. [Google Scholar] [CrossRef]
- Costanzo, V.; Bardelli, A.; Siena, S.; Abrignani, S. Exploring the links between cancer and placenta development. Open Biol. 2018, 8, 180081. [Google Scholar] [CrossRef]
- Still, R.; Brennecke, S. Melanoma in pregnancy. Obstet. Med. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Prithviraj, P.; Anaka, M.; McKeown, S.J.; Permezel, M.; Walkiewicz, M.; Cebon, J.; Behren, A.; Jayachandran, A. Pregnancy associated plasma protein—A links pregnancy and melanoma progression by promoting cellular migration and invasion. Oncotarget 2015, 6, 15953–15965. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Liebman, T.N.; Stein, J.A. Pregnancy and Melanoma: Recommendations for Clinical Scenarios. Int. J. Womens Dermatol. 2018, 4, 113–115. [Google Scholar] [CrossRef]
- Friedman, E.B.; Scolyer, R.A.; Thompson, J.F. Management of pigmented skin lesions during pregnancy. Aust. J. Gen. Pract. 2019, 48, 621–624. [Google Scholar] [CrossRef]
- Zalaudek, I.; Docimo, G.; Argenziano, G. Using dermoscopic criteria and patient-related factors for the management of pigmented melanocytic nevi. Arch. Dermatol. 2009, 145, 816–826. [Google Scholar] [CrossRef]
- Cosgarea, I.; Trevisan-Herraz, M.; Ungureanu, L.; Zalaudek, I. Dermatoscopic Features of Naevi during Pregnancy—A Mini Review. Front. Med. 2021, 8, 727319. [Google Scholar] [CrossRef]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert. Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef]
- Mestnik, N.C.; Afonso, J.P.; Enokihara, M.M.; Enokihara, M.Y.; Porro, A.M.; Hirata, S.H. Melanoma developed during pregnancy—A case report. Bras. Dermatol. 2014, 89, 157–159. [Google Scholar] [CrossRef]
- Curti, A.; Piana, S.; Banzi, M.; Castagnetti, F.; Lai, M.; Longo, C. Diving Into the Blue: A Case of Melanoma Arising in a Giant Congenital Blue Nevus during Pregnancy. Dermatol. Pract. Concept. 2023, 13, e2023059. [Google Scholar] [CrossRef]
- Todd, S.P.; Driscoll, M.S. Prognosis for women diagnosed with melanoma during, before, or after pregnancy: Weighing the evidence. Int. J. Womens Dermatol. 2017, 3, 26–29. [Google Scholar] [CrossRef]
- Pabuccu, R.; Kiseli, M.; Kahyaoğlu, I.; Cağlar, G.S.; Yılmaz, M.B. Malignant melanoma arising in an in vitro fertilisation pregnancy: A case report. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 186–187. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi160–vi170. [Google Scholar] [CrossRef]
- Li, J.N.; Nijhawan, R.I.; Srivastava, D. Cutaneous Surgery in Patients Who Are Pregnant or Breastfeeding. Dermatol. Clin. 2019, 37, 307–317. [Google Scholar] [CrossRef]
- Zelin, E.; Conforti, C.; Giuffrida, R.; Deinlein, T.; di Meo, N.; Zalaudek, I. Melanoma in pregnancy: Certainties unborn. Melanoma Manag. 2020, 7, MMT48. [Google Scholar] [CrossRef]
- El-Shourbagy, K.H.; Mashaly, E.M.; Khodair, S.A.; Houseni, M.M.; Khadrah, R.S.A. PET/CT in restaging, prognosis, and recurrence in patients with malignant melanoma. Egypt. J. Radiol. Nucl. Med. 2020, 51, 167. [Google Scholar] [CrossRef]
- Korenaga, T.K.; Tewari, K.S. Gynecologic cancer in pregnancy. Gynecol. Oncol. 2020, 157, 799–809. [Google Scholar] [CrossRef]
- Mitrou, S.; Zarkavelis, G.; Fotopoulos, G.; Petrakis, D.; Pavlidis, N. A mini review on pregnant mothers with cancer: A paradoxical coexistence. J. Adv. Res. 2016, 7, 559–563. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association between MRI Exposure during Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Davis, J.R.; Trocha, S.D.; Hale, A.L.; Bartz, M.J. Videoscopic inguinal lymphadenectomy in malignant melanoma: Safe in pregnancy? J. Surg. Case Rep. 2014, 2014, rju103. [Google Scholar] [CrossRef][Green Version]
- Wyon, Y.; Synnerstad, I.; Fredrikson, M.; Rosdahl, I. Spectrophotometric analysis of melanocytic naevi during pregnancy. Acta Derm. Venereol. 2007, 87, 231–237. [Google Scholar] [CrossRef]
- De Haan, J.; Vandecaveye, V.; Han, S.N.; Van de Vijver, K.K.; Amant, F. Difficulties with diagnosis of malignancies in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 33, 19–32. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502, Erratum in Lancet 2023, 402, 450. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Spatz, A.; Grob, J.J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.; et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2010, 46, 270–283. [Google Scholar] [CrossRef]
- Czeyda-Pommersheim, F.; Kluger, H.; Langdon, J.; Menias, C.; VanBuren, W.; Leventhal, J.; Baumann, R., Jr.; Revzin, M. Melanoma in pregnancy. Abdom. Radiol. 2023, 48, 1740–1751. [Google Scholar] [CrossRef]
- Hasan, B.; Asif, T.; Hasan, M. Lidocaine-Induced Systemic Toxicity: A Case Report and Review of Literature. Cureus 2017, 9, e1275, Erratum in Cureus 2019, 11, c24. [Google Scholar] [CrossRef]
- Murase, J.E.; Heller, M.M.; Butler, D.C. Safety of dermatologic medications in pregnancy and lactation: Part, I. Pregnancy. J. Am. Acad. Dermatol. 2014, 70, 401.e1–401.e14. [Google Scholar] [CrossRef]
- Rashid, S.; Shaughnessy, M.; Tsao, H. Melanoma classification and management in the era of molecular medicine. Dermatol. Clin. 2023, 41, 49–63. [Google Scholar] [CrossRef]
- Ravindra, G.L.; Madamangalam, A.S.; Seetharamaiah, S. Anaesthesia for non-obstetric surgery in obstetric patients. Indian J. Anaesth. 2018, 62, 710–716. [Google Scholar] [CrossRef]
- Eskandari, A.; Alipour, S. Aspects of Anesthesia for Breast Surgery during Pregnancy. Adv. Exp. Med. Biol. 2020, 1252, 107–114. [Google Scholar] [CrossRef]
- Reitman, E.; Flood, P. Anaesthetic considerations for non-obstetric surgery during pregnancy. Br. J. Anaesth. 2011, 107 (Suppl. S1), i72–i78. [Google Scholar] [CrossRef]
- Mainprize, J.G.; Yaffe, M.J.; Chawla, T.; Glanc, P. Effects of ionizing radiation exposure during pregnancy. Abdom. Radiol. 2023, 48, 1564–1578. [Google Scholar] [CrossRef]
- American College of Radiology ACR-SPR Practice Parameterfor Imaging Pregnant or Potentially Pregnant Patients with Ionizing Radiation, Revised 2023 (Resolution 31). Available online: https://www.acr.org (accessed on 2 May 2024).
- Applegate, K.E.; Findlay, Ú.; Fraser, L.; Kinsella, Y.; Ainsbury, L.; Bouffler, S. Radiation exposures in pregnancy, health effects and risks to the embryo/foetus—Information to inform the medical management of the pregnant patient. J. Radiol. Prot. 2021, 41, S522. [Google Scholar] [CrossRef]
- Michalet, M.; Dejean, C.; Schick, U.; Durdux, C.; Fourquet, A.; Kirova, Y. Radiotherapy and pregnancy. Cancer Radiother. 2022, 26, 417–423. [Google Scholar] [CrossRef]
- Jha, P.; Pōder, L.; Glanc, P.; Patel-Lippmann, K.; McGettigan, M.; Moshiri, M.; Nougaret, S.; Revzin, M.V.; Javitt, M.C. Imaging Cancer in Pregnancy. Radiographics 2022, 42, 1494–1513. [Google Scholar] [CrossRef]
- Nomeda, R.V.; Varyte, G.; Zakarevičienė, J.; Kontrimavičiūtė, E.; Ramašauskaitė, D.; Rutkauskaitė-Valančienė, D. Use of Magnetic Resonance Imaging in Evaluating Fetal Brain and Abdomen Malformations during Pregnancy. Medicina 2019, 55, 55. [Google Scholar] [CrossRef]
- Pejman, J.M.; Pai, V.; Ertl-Wagner, B.B. Safety of Magnetic Resonance Imaging in Pregnancy. Radiologie 2023, 63, 34–40. [Google Scholar]
- Zhang, Y.H.; Sun, H.X. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef]
- Xu, W.; Moor, R.J.; Walpole, E.T.; Atkinson, V.G. Pregnancy with successful foetal and maternal outcome in a melanoma patient treated with nivolumab in the first trimester: Case report and review of the literature. Melanoma Res. 2019, 29, 333–337. [Google Scholar] [CrossRef]
- Bucheit, A.D.; Hardy, J.T.; Szender, J.B.; Oliva, I.C.G. Conception and viable twin pregnancy in a patient with metastatic melanoma while treated with CTLA-4 and PD-1 checkpoint inhibition. Melanoma Res. 2020, 30, 423–425. [Google Scholar] [CrossRef]
- Hassel, J.C.; Livingstone, E.; Allam, J.; Behre, H.; Bojunga, J.; Klein, H.; Landsberg, J.; Nawroth, F.; Schüring, A.; Susok, L.; et al. Fertility preservation and management of pregnancy in melanoma patients requiring systemic therapy. ESMO Open 2021, 6, 100248. [Google Scholar] [CrossRef]
- Pagan, M.; Jinks, H.; Sewell, M. Treatment of metastatic malignant melanoma during pregnancy with a BRAF kinase inhibitor: A case report. Case Rep. Women’s Health 2019, 24, e00142. [Google Scholar] [CrossRef]
- Morton, S.K.; Morton, A.P. Melanoma and pregnancy. Australas. J. Dermatol. 2017, 58, 259–267. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Ward, K.C.; Kramer, M.R.; Lash, T.L.; Mertens, A.C.; Spencer, J.B.; Fothergill, A.; Howards, P.P. The risk of preterm birth and growth restriction in pregnancy after cancer. Int. J. Cancer 2017, 141, 2187–2196. [Google Scholar] [CrossRef]
- Cottreau, C.M.; Dashevsky, I.; Andrade, S.E.; Li, D.-K.; Nekhlyudov, L.; Raebel, M.A.; Ritzwoller, D.P.; Partridge, A.H.; Pawloski, P.A.; Toh, S. Pregnancy-Associated Cancer: A U.S. Population-Based Study. J. Women’s Health 2002, 28, 250–257. [Google Scholar] [CrossRef]
- Wiedemann, S.V.; Müller, V.; Toth, B.; Erdmann, M.; Bühler, B.; Dugas-Breit, S.; Schatton, K.; Reinhardt, L.; Meissner, M.; Mickler, M.; et al. CLAUDIUS Study: Risk of materno-fetal transmission of melanoma cells in pregnant women with high grade melanoma—A retrospective multicenter study and literature review. EJC Ski. Cancer 2023, 1, 100005. [Google Scholar] [CrossRef]
- Alexander, A.; Samlowski, W.E.; Grossman, D.; Bruggers, C.S.; Harris, R.M.; Zone, J.J.; Noyes, R.D.; Bowen, G.M.; Leachman, S.A. Metastatic melanoma in pregnancy: Risk of transplacental metastases in the infant. J. Clin. Oncol. 2003, 21, 2179–2186, Erratum in J. Clin. Oncol. 2010, 28, 3670. [Google Scholar] [CrossRef]
| Cancer Type | % |
|---|---|
| Breast Cancer | 41% |
| Lymphoma | 12% |
| Cervical Cancer | 10% |
| Leukemia | 8% |
| Ovarian Cancer | 7% |
| Gastrointestinal Cancer | 5% |
| Melanoma | 5% |
| Other | 12% |
| Melanoma Staging | Recommended Therapy |
|---|---|
| Stage 0 (Melanoma in situ) | Excision of the lesion a |
| Stage I | Excision of the lesion a + SLNB |
| Stage II | Excision of the lesion a + SLNB |
| Stage III (lymph nodes involved) | Excision of the lesion and sentinel/involved lymph nodes b; Consider preponed labor; Adjuvant immunotherapy c |
| Stage IV (presence of distant metastases) | Treatment based on clinical presentation and advancement of the pregnancy d; Excision of the lesion and sentinel/involved lymph nodes b; Consider preponed labor; Adjuvant immunotherapy c Radiotherapy e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelczar, P.; Kosteczko, P.; Wieczorek, E.; Kwieciński, M.; Kozłowska, A.; Gil-Kulik, P. Melanoma in Pregnancy—Diagnosis, Treatment, and Consequences for Fetal Development and the Maintenance of Pregnancy. Cancers 2024, 16, 2173. https://doi.org/10.3390/cancers16122173
Pelczar P, Kosteczko P, Wieczorek E, Kwieciński M, Kozłowska A, Gil-Kulik P. Melanoma in Pregnancy—Diagnosis, Treatment, and Consequences for Fetal Development and the Maintenance of Pregnancy. Cancers. 2024; 16(12):2173. https://doi.org/10.3390/cancers16122173
Chicago/Turabian StylePelczar, Patrycja, Pola Kosteczko, Ewelina Wieczorek, Maciej Kwieciński, Aleksandra Kozłowska, and Paulina Gil-Kulik. 2024. "Melanoma in Pregnancy—Diagnosis, Treatment, and Consequences for Fetal Development and the Maintenance of Pregnancy" Cancers 16, no. 12: 2173. https://doi.org/10.3390/cancers16122173
APA StylePelczar, P., Kosteczko, P., Wieczorek, E., Kwieciński, M., Kozłowska, A., & Gil-Kulik, P. (2024). Melanoma in Pregnancy—Diagnosis, Treatment, and Consequences for Fetal Development and the Maintenance of Pregnancy. Cancers, 16(12), 2173. https://doi.org/10.3390/cancers16122173






