Influence of Previous Therapy for Neutropenia Caused by Combination Therapy of Ramucirumab and Docetaxel
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
2.2. Data Collection
2.3. Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Adverse Events
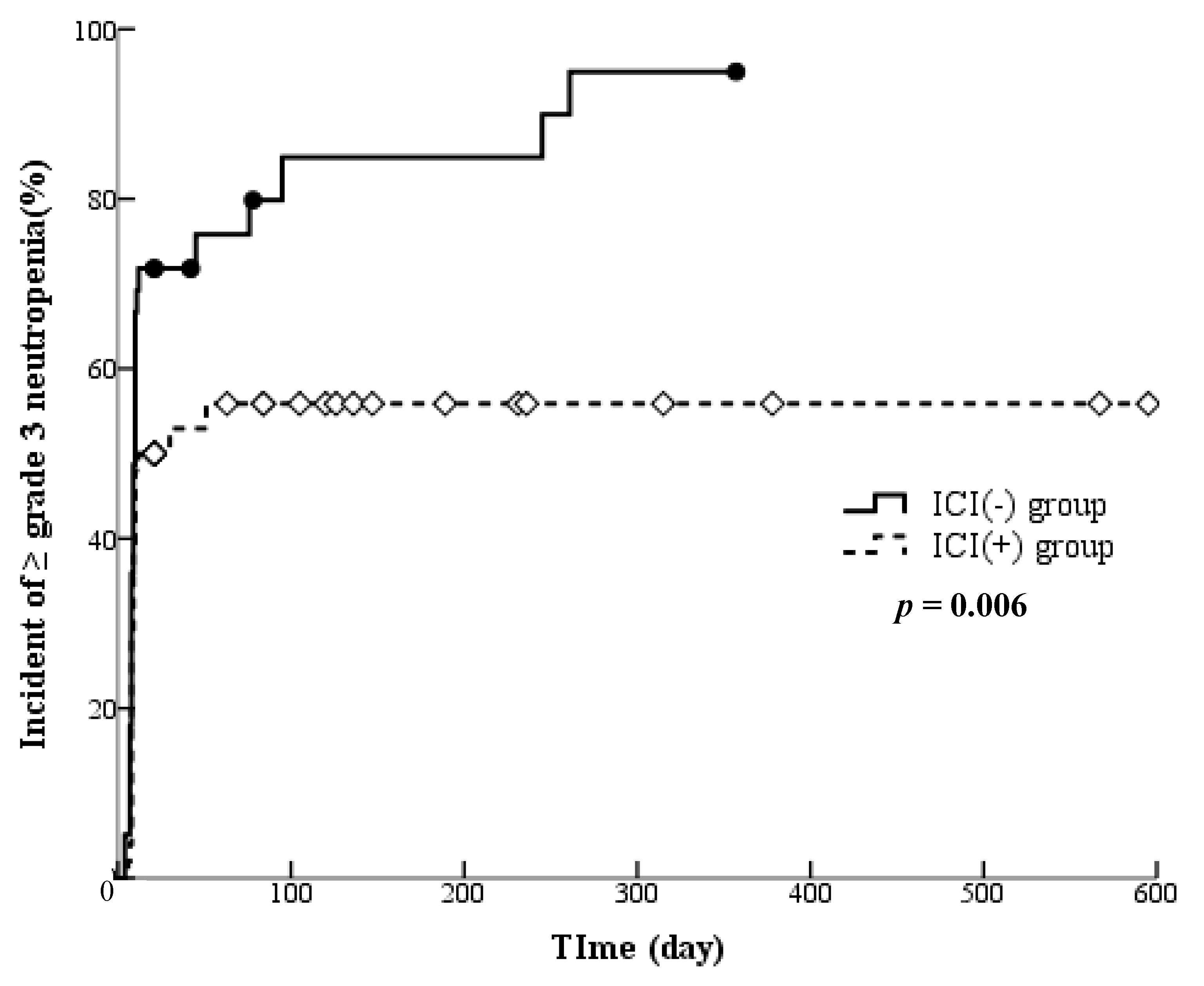
3.3. Grouping Based on ICI Treatment History
3.4. Influence of ICI Treatment History on the Onset of Adverse Events
3.5. Factors Associated with the Occurrence of Grade ≥ 3 Neutropenia
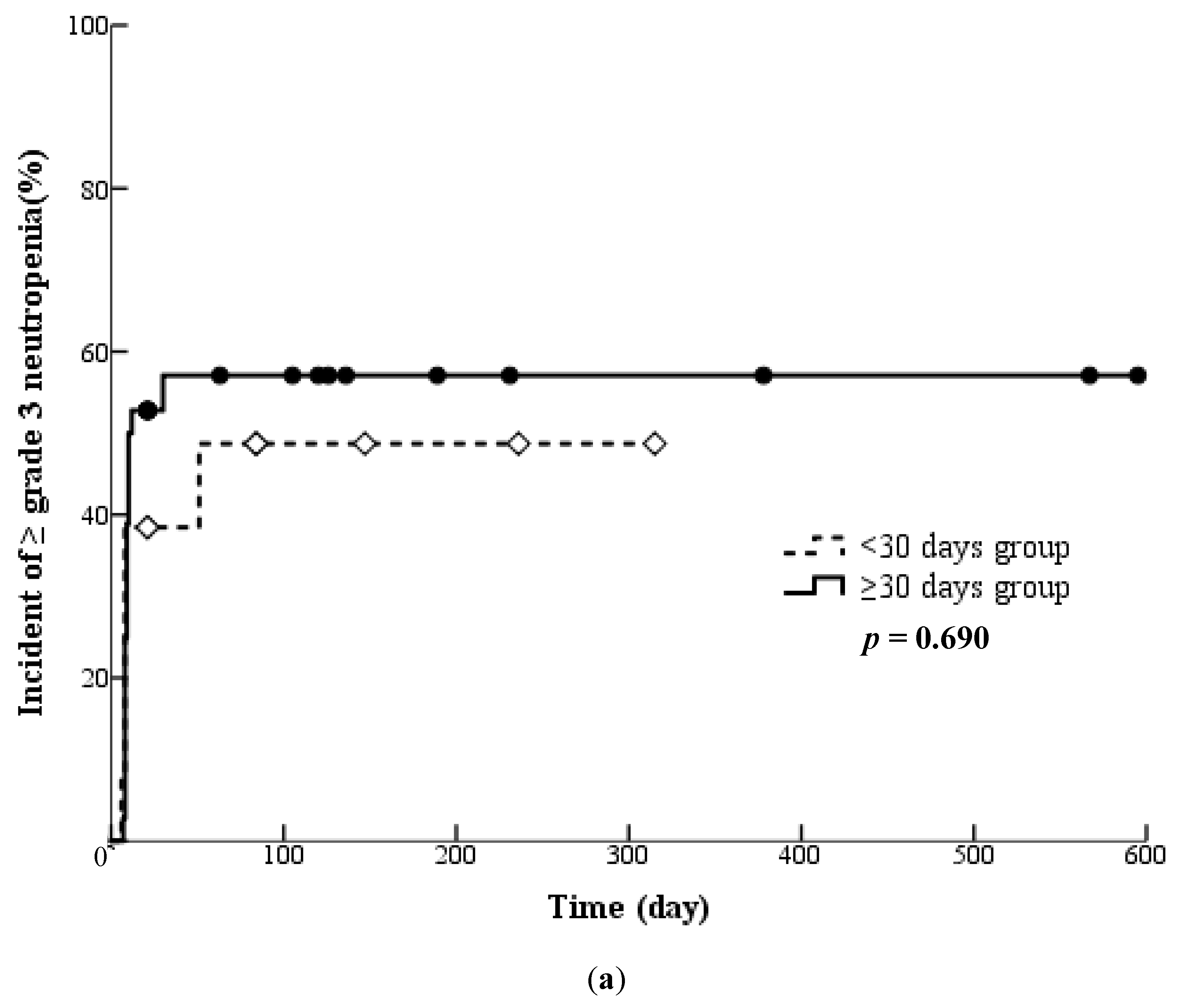
3.6. Influences of Pretreatment with Cytotoxic Chemotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiro, S.G.; Rudd, R.M.; Souhami, R.L.; Brown, J.; Fairlamb, D.J.; Gower, N.H.; Maslove, L.; Milroy, R.; Napp, V.; Parmar, M.K.; et al. Big Lung Trial participants. Chemotherapy versus supportive care in advanced non-small cell lung cancer: Improved survival without detriment to quality of life. Thorax 2004, 59, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Pérol, M.; Reck, M.; Kowalyszyn, R.D.; Gautschi, O.; Kimmich, M.; Cho, E.K.; Czyzewicz, G.; Grigorescu, A.; Karaseva, N.; et al. Efficacy and safety of ramucirumab with docetaxel versus placebo with docetaxel as second-line treatment of advanced non-small-cell lung cancer: A subgroup analysis according to patient age in the REVEL trial. Clin. Lung Cancer 2018, 19, 270–279.e3. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Scagliotti, G.V.; Gautschi, O.; Reck, M.; Thomas, M.; Iglesias Docampo, L.; Kalofonos, H.; Kim, J.H.; Gans, S.; Brustugun, O.T.; et al. Exploratory analysis of front-line therapies in REVEL: A randomised phase 3 study of ramucirumab plus docetaxel versus docetaxel for the treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy. ESMO Open 2020, 5, e000567. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology—Myeloid Growth Factors, Version 1.2018. 2018. Available online: https://oncolife.com.ua/doc/nccn/Myeloid_Growth_Factors.pdf (accessed on 14 March 2024).
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Harada, D.; Takata, K.; Mori, S.; Kozuki, T.; Takechi, Y.; Moriki, S.; Asakura, Y.; Ohno, T.; Nogami, N. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res. 2019, 39, 4987–4993. [Google Scholar] [CrossRef] [PubMed]
- Molife, C.; Hess, L.M.; Cui, Z.L.; Li, X.I.; Beyrer, J.; Mahoui, M.; Oton, A.B. Sequential therapy with ramucirumab and/or checkpoint inhibitors for non-small-cell lung cancer in routine practice. Future Oncol. 2019, 15, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Arigami, T.; Matsushita, D.; Okubo, K.; Yanagita, S.; Ehi, K.; Sasaki, K.; Noda, M.; Kita, Y.; Mori, S.; Kurahara, H.; et al. Response rate and prognostic impact of salvage chemotherapy after nivolumab in patients with advanced gastric cancer. Oncology 2020, 98, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Kotake, M.; Murakami, H.; Kenmotsu, H.; Naito, T.; Takahashi, T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann. Oncol. 2017, 28, 669–670. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Kaira, K.; Kawasaki, T.; Mouri, A.; Hashimoto, K.; Shiono, A.; Shinomiya, S.; Miura, Y.; Nishihara, F.; Murayama, Y.; et al. Severe hepatotoxicity due to osimertinib after nivolumab therapy in patients with non-small cell lung cancer harboring EGFR mutation. Thorac. Cancer 2020, 11, 1045–1051. [Google Scholar] [CrossRef]
- Ohno, H.; Mano, S.; Katagiri, N.; Oguri, R.; Miyazaki, K.; Ito, K.; Sekiya, Y.; Inoue, K.; Masuda, A.; Tsuzuku, A.; et al. Influence of using history of immune checkpoint inhibitor therapy for neutropenia caused by combination therapy of ramucirumab and docetaxel. Pharmazie 2022, 77, 248–254. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Lyman, G.H.; Kuderer, N.M.; Crawford, J.; Wolff, D.A.; Culakova, E.; Poniewierski, M.S.; Dale, D.C. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 2011, 117, 1917–1927. [Google Scholar] [CrossRef]
- Kukec, R.R.; Grabnar, I.; Vovk, T.; Mrhar, A.; Kovac, V.; Cufer, T. Febrile neutropenia in chemotherapy treated small-cell lung cancer patients. Radiol. Oncol. 2015, 49, 173–180. [Google Scholar] [CrossRef]
- Yoh, K.; Hosomi, Y.; Kasahara, K.; Yamada, K.; Takahashi, T.; Yamamoto, N.; Nishio, M.; Ohe, Y.; Koue, T.; Nakamura, T.; et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 2016, 99, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Kawaguchi, T.; Crowley, J.; Moon, J.; Furuse, K.; Kawahara, M.; Teramukai, S.; Ohe, Y.; Kubota, K.; Williamson, S.K.; et al. Japanese. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: A model for assessing population-related pharmacogenomics. J. Clin. Oncol. 2009, 27, 3540–3546. [Google Scholar] [CrossRef]
- Kasahara, N.; Sunaga, N.; Kuwako, T.; Naruse, I.; Imai, H.; Jingu, A.; Tsukagoshi, Y.; Masuda, T.; Kitahara, S.; Tsurumaki, H.; et al. Administration of docetaxel plus ramucirumab with primary prophylactic pegylated-granulocyte colony-stimulating factor for pretreated non-small cell lung cancer: A phase II study. Support. Care Cancer 2020, 28, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Hanna, N.; Shepherd, F.A.; Fossella, F.V.; Pereira, J.R.; De Marinis, F.; von Pawel, J.; Gatzemeier, U.; Tsao, T.C.Y.; Pless, M.; Muller, T.; et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 2004, 22, 1589–1597. [Google Scholar] [CrossRef]
- Nokihara, H.; Lu, S.; Mok, T.S.K.; Nakagawa, K.; Yamamoto, N.; Shi, Y.K.; Zhang, L.; Soo, R.A.; Yang, J.C.; Sugawara, S.; et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann. Oncol. 2017, 28, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Yoneshima, Y.; Morita, S.; Ando, M.; Nakamura, A.; Iwasawa, S.; Yoshioka, H.; Goto, Y.; Takeshita, M.; Harada, T.; Hirano, K.; et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Fossella, F.V.; DeVore, R.; Kerr, R.N.; Crawford, J.; Natale, R.R.; Dunphy, F.; Kalman, L.; Miller, V.; Lee, J.S.; Moore, M.; et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J. Clin. Oncol. 2000, 18, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Dancey, J.; Ramlau, R.; Mattson, K.; Gralla, R.; O’Rourke, M.; Levitan, N.; Gressot, L.; Vincent, M.; Burkes, R.; et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 2000, 18, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, Y.; Takahashi, K.; Kanuma, A.; Kondo, Y.; Nakata, Y.; Sakajo, Y. Risk factors of severe neutropenia induced by ramucirumab plus paclitaxel combination therapy. Jpn. J. Pharm. Health Care Sci. 2018, 44, 128–135. [Google Scholar] [CrossRef]
- Lin, J.J.; Chin, E.; Yeap, B.Y.; Ferris, L.A.; Kamesan, V.; Lennes, I.T.; Sequist, L.V.; Heist, R.S.; Mino-Kenudson, M.; Gainor, J.F.; et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small cell lung cancer. J. Thorac. Oncol. 2019, 14, 135–140. [Google Scholar] [CrossRef]
- Sonoda, T.; Umeda, Y.; Demura, Y.; Tada, T.; Nakashima, K.; Anzai, M.; Yamaguchi, M.; Shimada, A.; Ohi, M.; Honjo, C.; et al. Efficacy and safety of nanoparticle albumin-bound paclitaxel monotherapy after immune checkpoint inhibitor administration for advanced non-small cell lung cancer: A multicenter phase 2 clinical trial. Cancer Med. 2023, 12, 13041–13053. [Google Scholar] [CrossRef] [PubMed]
- Foundation for Promotion of Cancer Research. Cancer Statistics in Japan-2019. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2019_en.html (accessed on 14 March 2024).
- Tozuka, T.; Kitazono, S.; Sakamoto, H.; Yoshida, H.; Amino, Y.; Uematsu, S.; Yoshizawa, T.; Hasegawa, T.; Ariyasu, R.; Uchibori, K.; et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer 2020, 144, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Shiono, A.; Kaira, K.; Mouri, A.; Yamaguchi, O.; Hashimoto, K.; Uchida, T.; Miura, Y.; Nishihara, F.; Murayama, Y.; Kobayashi, K.; et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac. Cancer 2019, 10, 775–781. [Google Scholar] [CrossRef]
- Takahara, Y.; Abe, R.; Nagae, S.; Tanaka, T.; Ishige, Y.; Shionoya, I.; Yamamura, K.; Nishiki, K.; Nojiri, M.; Kato, R.; et al. Investigation of response of patients with non-small cell lung cancer to docetaxel (plus ramucirumab) therapy in second-line treatment. Thorac. Cancer 2023, 14, 3549–3555. [Google Scholar] [CrossRef]
- Katayama, Y.; Yamada, T.; Sawada, R.; Kawachi, H.; Morimoto, K.; Watanabe, S.; Watanabe, K.; Takeda, T.; Chihara, Y.; Shiotsu, S.; et al. Prospective Observational Study of Ramucirumab Plus Docetaxel After Combined Chemoimmunotherapy in Patients With Non-Small-Cell Lung Cancer. Oncologist 2024, 29, e681–e689. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, S.; Matsumoto, K.; Tamiya, A.; Taniguchi, Y.; Matsuda, Y.; Uchida, J.; Ueno, K.; Kawachi, H.; Tamiya, M.; Yanase, T.; et al. Sequencing strategies with ramucirumab and docetaxel following prior treatments for advanced non-small cell lung cancer: A multicenter retrospective cohort study. Eur. J. Clin. Pharmacol. 2023, 79, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Yamada, T.; Okuma, Y.; Kitadai, R.; Takeda, T.; Kanematsu, T.; Goto, H.; Yoneda, H.; Harada, T.; Kubota, Y.; et al. Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients. Transl. Lung Cancer Res. 2019, 8, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Brueckl, W.M.; Reck, M.; Rittmeyer, A.; Kollmeier, J.; Wesseler, C.; Wiest, G.H.; Christopoulos, P.; Tufman, A.; Hoffknecht, P.; Ulm, B.; et al. Efficacy of docetaxel plus ramucirumab as palliative third-line therapy following second-line immune-checkpoint-inhibitor treatment in patients with non-small-cell lung cancer stage IV. Clin. Med. Insights Oncol. 2020, 14, 1179554920951358. [Google Scholar] [CrossRef]
- Ishida, M.; Morimoto, K.; Yamada, T.; Shiotsu, S.; Chihara, Y.; Yamada, T.; Hiranuma, O.; Morimoto, Y.; Iwasaku, M.; Tokuda, S.; et al. Impact of docetaxel plus ramucirumab in a second-line setting after chemoimmunotherapy in patients with non-small-cell lung cancer: A retrospective study. Thorac. Cancer 2022, 13, 173–181. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Mori, K.; Takata, S.; Shibata, K.; Chikamori, K.; Kimura, N.; Nagai, Y.; Nakagawa, T.; Igawa, S.; Harada, T.; et al. Extended ICI treatment after first-line chemoimmunotherapy could predict the clinical benefit of ramucirumab plus docetaxel in advanced non-small lung cancer: Post hoc analysis from NEJ051 (REACTIVE study). Thorac. Cancer 2024, 15, 163–171. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Lin, F.; Chen, H.; Jiang, T.; Zheng, J.; Liu, Q.; Yang, B.; Wang, X.; Lin, X. The effect of low-dose chemotherapy on the tumor microenvironment and its antitumor activity combined with anti-PD-1 antibody. Immunotherapy 2022, 14, 283–294. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Morise, M.; Ito, K.; Hataji, O.; Takahashi, K.; Koyama, J.; Kuwatsuka, Y.; Goto, Y.; Imaizumi, K.; Itani, H.; et al. Efficacy and safety of second-line therapy of docetaxel plus ramucirumab after first-line platinum-based chemotherapy plus immune checkpoint inhibitors in non-small cell lung cancer (SCORPION): A multicenter, open-label, single-arm, phase 2 trial. EClinicalMedicine 2023, 66, 102303. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- The Japan Lung Cancer Society Guidelines for Diagnosis and Treatment of the Lung Cancer/Malignant Pleural Mesothelioma/Thymic Tumors-2018, Version 1.1. 2018. Available online: https://www.haigan.gr.jp/guideline/2018/1/2/180102070100.html#cq67 (accessed on 26 May 2024).

| ICI (+) Group (n = 50) | ICI (−) Group (n = 39) | p-Value | |
|---|---|---|---|
| Age (years) | 67 (61–72) | 68 (65–74) | 0.097 |
| Body weight (kg) | 58.5 (53.0–67.5) | 62.0 (55.2–68.0) | 0.307 |
| Body surface area (m2) | 1.60 (1.52–1.73) | 1.63 (1.53–1.72) | 0.869 |
| Male/female (n) | 41/9 | 31/8 | 0.765 |
| Performance status: 3 or 4 (n) | 1 | 1 | 1.000 |
| WHO classification (n) | |||
| Squamous cell carcinoma | 13 | 7 | 0.373 |
| Non-squamous cell carcinoma | 32 | 30 | |
| Unknown | 5 | 2 | |
| Stage (n) | |||
| III | 9 | 5 | 0.480 |
| IV | 40 | 34 | |
| Unknown | 1 | 0 | |
| Chemotherapy | |||
| Number of courses of RAM + DTX therapy | 4.0 (2.0–7.8) | 4.0 (2.0–5.5) | 0.582 |
| Prophylactic G-CSF administration (%) | 40.0 | 43.6 | 0.733 |
| RDI | |||
| RAM | 96.4 ± 5.2 | 95.5 ± 5.3 | 0.421 |
| DTX | 91.5 ± 8.7 | 91.9 ± 8.8 | 0.825 |
| Blood biochemical parameter at baseline | |||
| WBC (/μL) | 6250 (5225–9100) | 6500 (5500–8300) | 0.960 |
| AST (IU/L) | 23 (16–29) | 23 (20–28) | 0.571 |
| ALT (IU/L) | 16 (11–26) | 16 (11–22) | 0.859 |
| T-Bil (mg/dL) | 0.43 (0.30–0.60) | 0.48 (0.40–0.60) | 0.289 |
| Ccr (mL/min) | 69.0 (54.3–88.8) | 66.2 (55.9–79.9) | 0.350 |
| Medication | |||
| Immunosuppressives | 10 | 6 | 0.782 |
| Adverse Event | Number of Patients | p-Value | |
|---|---|---|---|
| ICI (+) Group (n = 50) | ICI (−) Group (n = 39) | ||
| Leukopenia | 23 | 21 | 0.525 |
| Anaemia | 5 | 3 | 0.732 |
| Thrombocytopenia | 2 | 3 | 0.650 |
| AST | 3 | 1 | 0.628 |
| ALT | 2 | 0 | 0.502 |
| T-Bil | 0 | 0 | 1.000 |
| Hypertension | 18 | 17 | 0.516 |
| Proteinuria | 3 | 4 | 0.695 |
| Neutropenia | 27 | 33 | 0.002 |
| Factor | Univariate Analysis Hazard Ratio (95% CI) | p-Value | Multivariate Analysis Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.012 (0.981–1.045) | 0.446 | ||
| Male | 0.989 (0.512–1.913) | 0.989 | ||
| Squamous cell carcinoma | 1.302 (0.720–2.353) | 0.383 | ||
| Stage IV | 1.465 (0.693–3.095) | 0.317 | ||
| Usage history of immune checkpoint inhibitor | 0.540 (0.323–0.903) | 0.019 | 0.538 (0.322–0.900) | 0.018 |
| Course number of RAM + DTX therapy | 0.968 (0.803–1.168) | 0.736 | ||
| With prophylactic G-CSF | 0.263 (0.139–0.499) | <0.001 | 0.264 (0.140–0.499) | <0.001 |
| ICI (+) Group (n = 50) | ICI (−) Group (n = 39) | p-Value | |
|---|---|---|---|
| Pretreatment with cytotoxic anticancer drugs | 49 | 37 | 0.579 |
| Interval (days) from pretreatment with cytotoxic anticancer drugs | 137 (7–79) | 61 (22–1637) | 0.754 |
| Medical history of immediate prior chemotherapy | |||
| Chemotherapy | |||
| Platinum | 22 | 24 | 0.135 |
| Taxane | 10 | 9 | 0.797 |
| Medical history | |||
| Radiotherapy | 31 | 23 | 0.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohno, H.; Hayashi, T.; Torii, S.; Niwa, M.; Katagiri, N.; Nakao, Y.; Mano, S.; Takimoto, N.; Hirashita, T. Influence of Previous Therapy for Neutropenia Caused by Combination Therapy of Ramucirumab and Docetaxel. Cancers 2024, 16, 2076. https://doi.org/10.3390/cancers16112076
Ohno H, Hayashi T, Torii S, Niwa M, Katagiri N, Nakao Y, Mano S, Takimoto N, Hirashita T. Influence of Previous Therapy for Neutropenia Caused by Combination Therapy of Ramucirumab and Docetaxel. Cancers. 2024; 16(11):2076. https://doi.org/10.3390/cancers16112076
Chicago/Turabian StyleOhno, Hiroyuki, Takahiro Hayashi, Shota Torii, Miduki Niwa, Nanae Katagiri, Yuri Nakao, Shota Mano, Norio Takimoto, and Tomoyuki Hirashita. 2024. "Influence of Previous Therapy for Neutropenia Caused by Combination Therapy of Ramucirumab and Docetaxel" Cancers 16, no. 11: 2076. https://doi.org/10.3390/cancers16112076
APA StyleOhno, H., Hayashi, T., Torii, S., Niwa, M., Katagiri, N., Nakao, Y., Mano, S., Takimoto, N., & Hirashita, T. (2024). Influence of Previous Therapy for Neutropenia Caused by Combination Therapy of Ramucirumab and Docetaxel. Cancers, 16(11), 2076. https://doi.org/10.3390/cancers16112076





