Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
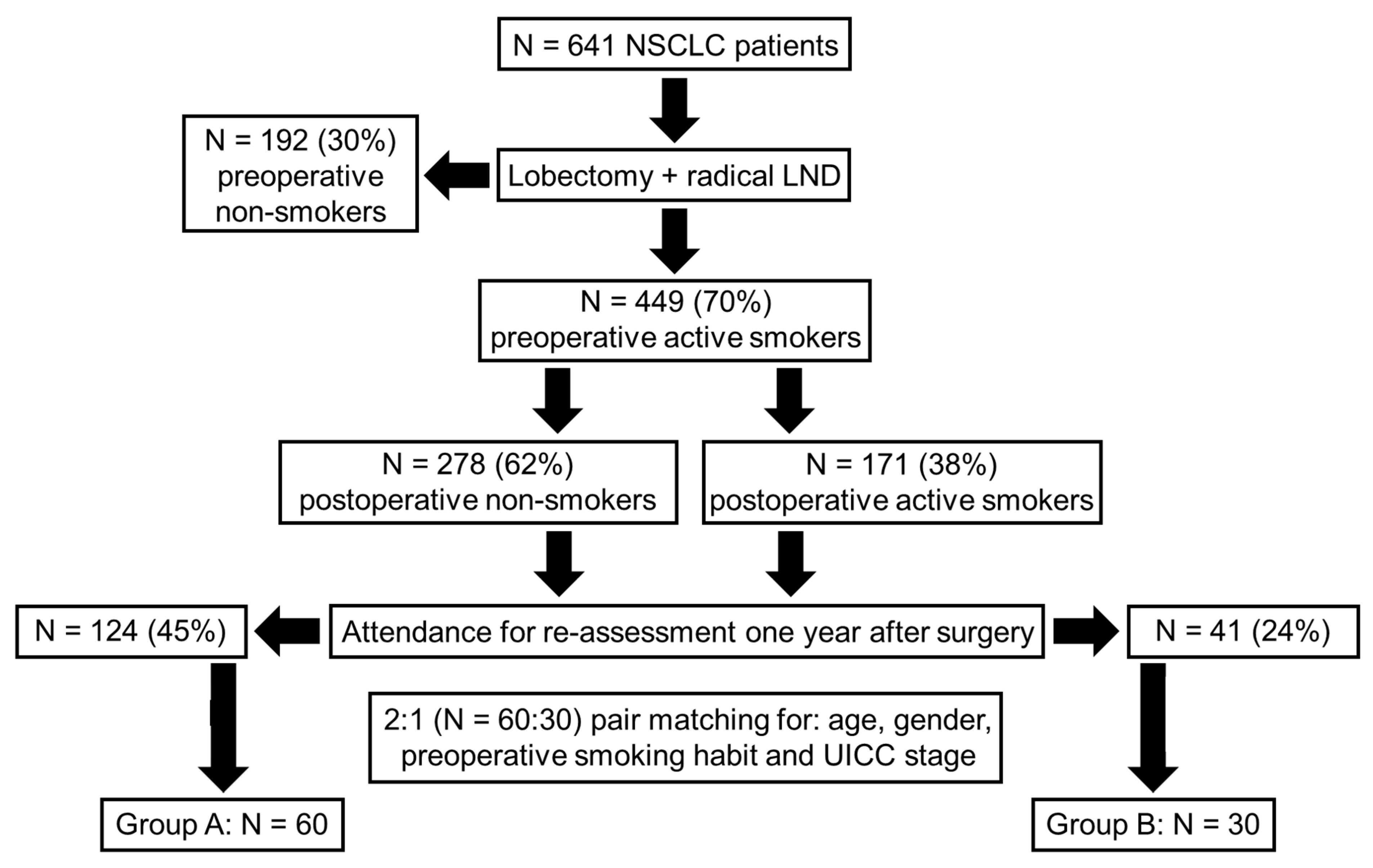
2.1. Patient Recruitment
2.2. Formation of Study Groups
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
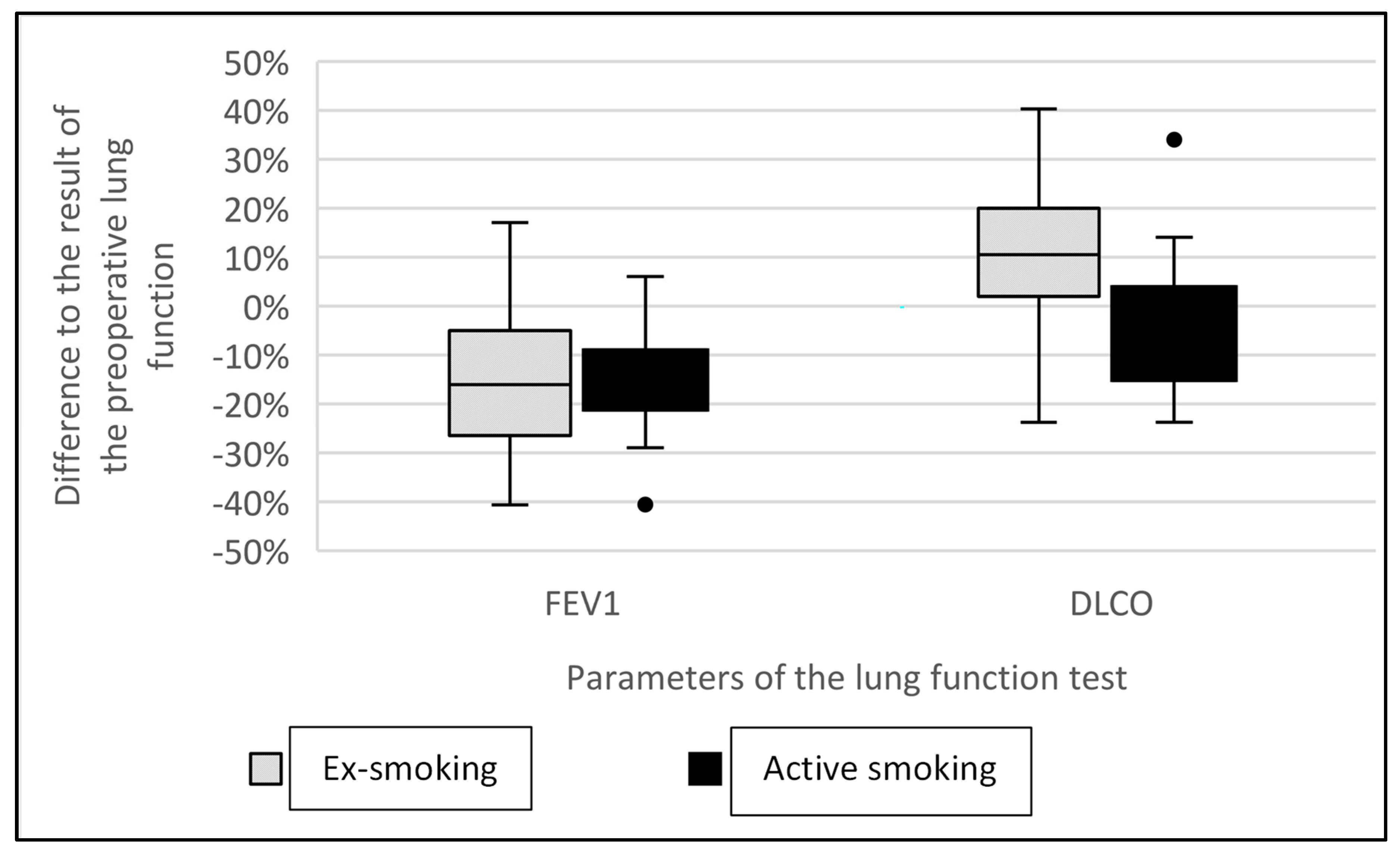
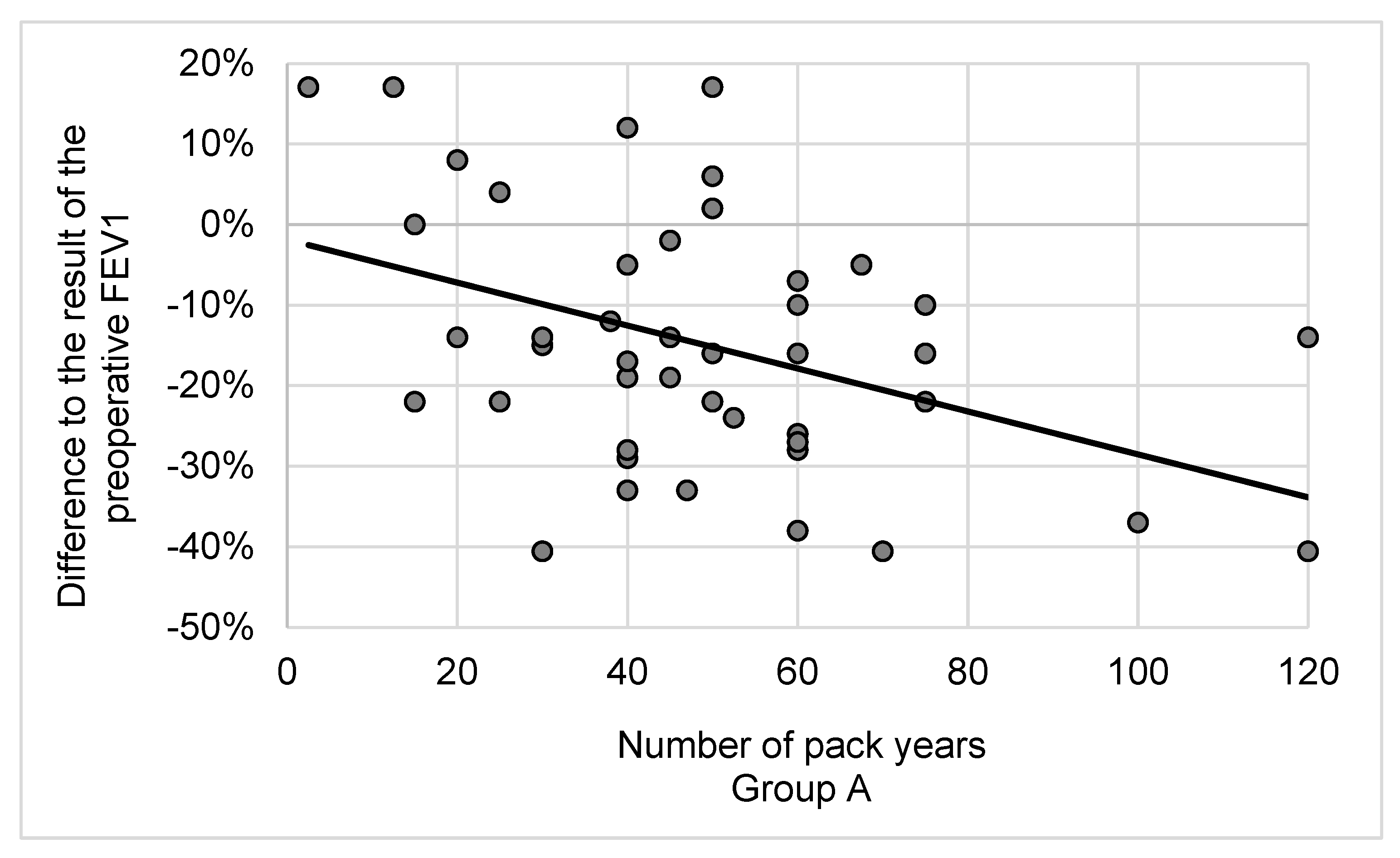
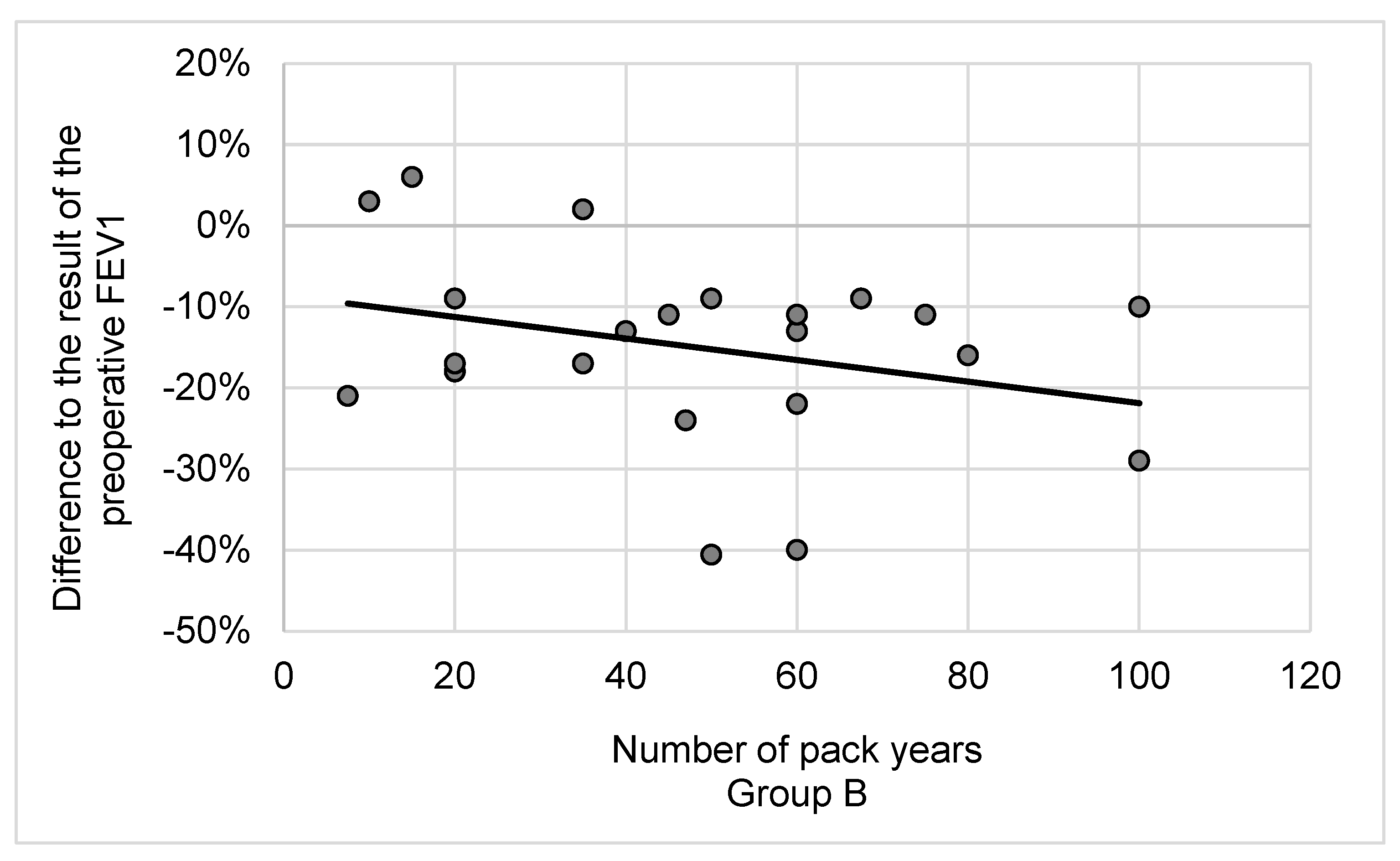
3.2. Lung Function and Cessation of Smoking after Lobectomy
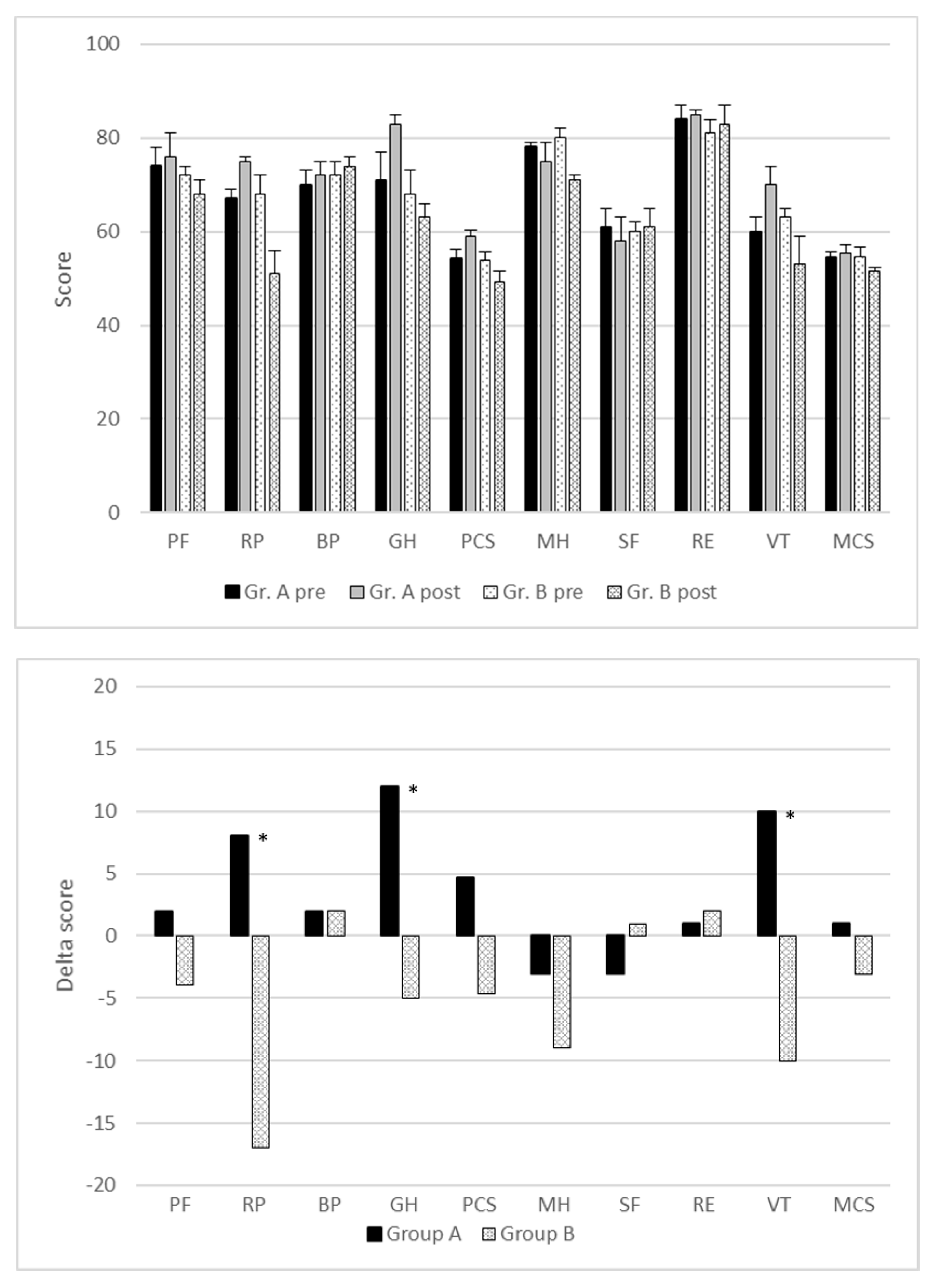
3.3. Quality of Life and Cessation of Smoking after Lobectomy
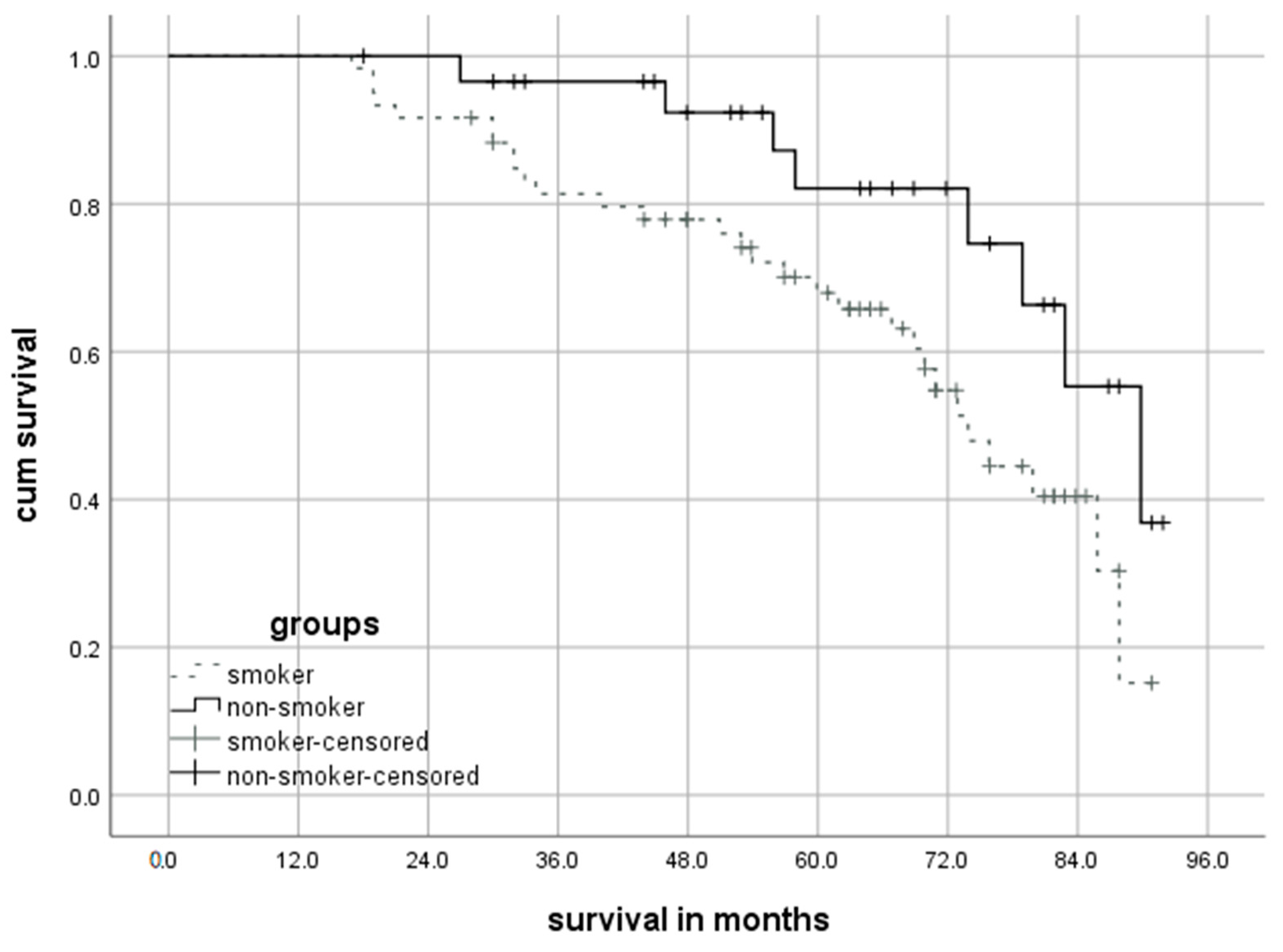
3.4. Long-Term Survival and Cessation of Smoking after Lobectomy
4. Discussion
4.1. New Potential for Non-Smokers
4.2. Lung Cancer Surgery, the Cessation of Smoking and Pulmonary Function
4.3. Influencing Quality of Life through Tobacco Cessation
4.4. Smoking Behavior Affects Long-Term Survival
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krebs in Deutschland für 2017/2018. 13. Ausgabe; Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg): Berlin, Germany, 2021.
- Vitzthum, K.; Thielke, L.; Deter, A.; Riemer, T.; Eggeling, S.; Pankow, W.; Mache, S. Smoking Lung Cancer Patients and Tobacco Cessation—Is the Current Treatment in Germany Sufficient? Pneumologie 2015, 69, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Chang, Y.; Regan, S.; Tindle, H.A.; Singer, D.E.; Rigotti, N.A. Improvements in health-related quality of life among smokers who quit after hospitalization. Prev. Med. 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Simonato, L.; Agudo, A.; Ahrens, W.; Benhamou, E.; Benhamou, S.; Boffetta, P.; Brennan, P.; Darby, S.C.; Forastiere, F.; Fortes, C.; et al. Lung cancer and cigarette smoking in Europe: An update of risk estimates and an assessment of inter-country heterogeneity. Int. J. Cancer 2001, 91, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.S.; Africano, N.L.; Tercyak, K.P.; Taylor, K.L. Nicotine dependence treatment for patients with cancer. Cancer 2003, 98, 632–644. [Google Scholar] [CrossRef]
- Waller, L.L.; Weaver, K.E.; Petty, W.J.; Miller, A.A. Effects of continued tobacco use during treatment of lung cancer. Expert Rev. Anticancer Ther. 2010, 10, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Shi, W.; Amar, D.; Thaler, H.T.; Gabovich, N.; Bains, M.S.; White, D.A. Smoking and timing of cessation: Impact on pulmonary complications after thoracotomy. Chest 2005, 127, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Dresler, C.M. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer 2003, 39, 119–124. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Park, H.Y.; Choe, Y.R.; Oh, I.J.; Kim, M.S.; Kho, B.G.; Shin, H.J.; Park, C.K.; Kim, Y.I.; Kim, Y.C.; Ahn, H.R.; et al. Efficacy of an inpatient smoking cessation program at a single regional cancer center: A prospective observational study. Medicine 2021, 100, e24745. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Kosinski, M.; Gandek, B.; Aaronson, N.K.; Apolone, G.; Bech, P.; Brazier, J.; Bullinger, M.; Kaasa, S.; Leplège, A.; et al. The factor structure of the SF-36 Health Survey in 10 countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1159–1165. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Kosinski, M.; Bayliss, M.S.; McHorney, C.A.; Rogers, W.H.; Raczek, A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med. Care 1995, 33, As264–As279. [Google Scholar] [PubMed]
- Ellert, U.; Kurth, B.M. Methodological views on the SF-36 summary scores based on the adult German population. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004, 47, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Ellert, U.; Kurth, B.M. Health related quality of life in adults in Germany: Results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Groth, S.S.; Whitson, B.A.; Kuskowski, M.A.; Holmstrom, A.M.; Rubins, J.B.; Kelly, R.F. Impact of preoperative smoking status on postoperative complication rates and pulmonary function test results 1-year following pulmonary resection for non-small cell lung cancer. Lung Cancer 2009, 64, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Rapicetta, C.; Tenconi, S.; Voltolini, L.; Luzzi, L.; Scala, V.; Gotti, G. Impact of lobectomy for non-small-cell lung cancer on respiratory function in octogenarian patients with mild to moderate chronic obstructive pulmonary disease. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2011, 39, 555–559. [Google Scholar] [CrossRef]
- Fukui, M.; Suzuki, K.; Matsunaga, T.; Oh, S.; Takamochi, K. Importance of Smoking Cessation on Surgical Outcome in Primary Lung Cancer. Ann. Thorac. Surg. 2019, 107, 1005–1009. [Google Scholar] [CrossRef]
- Agostini, P.J.; Lugg, S.T.; Adams, K.; Smith, T.; Kalkat, M.S.; Rajesh, P.B.; Steyn, R.S.; Naidu, B.; Rushton, A.; Bishay, E. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J. Cardiothorac. Surg. 2018, 13, 28. [Google Scholar] [CrossRef]
- Lugg, S.T.; Tikka, T.; Agostini, P.J.; Kerr, A.; Adams, K.; Kalkat, M.S.; Steyn, R.S.; Rajesh, P.B.; Bishay, E.; Thickett, D.R.; et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery. J. Cardiothorac. Surg. 2017, 12, 52. [Google Scholar] [CrossRef]
- Rodriguez, M.; Gómez-Hernandez, M.T.; Novoa, N.; Jiménez, M.F.; Aranda, J.L.; Varela, G. Refraining from smoking shortly before lobectomy has no influence on the risk of pulmonary complications: A case-control study on a matched population. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 51, 498–503. [Google Scholar] [CrossRef]
- Martínez, Ú.; Brandon, K.O.; Sutton, S.K.; Brandon, T.H.; Simmons, V.N. Does smoking abstinence predict cancer patients’ quality of life over time? Psychooncology 2019, 28, 1702–1711. [Google Scholar] [CrossRef]
- Andreas, S.; Rittmeyer, A.; Hinterthaner, M.; Huber, R.M. Smoking cessation in lung cancer-achievable and effective. Dtsch. Arztebl. Int. 2013, 110, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Bloom, E.L.; Oliver, J.A.; Sutton, S.K.; Brandon, T.H.; Jacobsen, P.B.; Simmons, V.N. Post-operative smoking status in lung and head and neck cancer patients: Association with depressive symptomatology, pain, and fatigue. Psychooncology 2015, 24, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.J.; Rowland, C.; Rowe, R.; Ellis, S.; Crabtree, C.; Horsman, J.M.; Wadsley, J.; Hatton, M.Q.; Woll, P.J.; Eiser, C. The relationship between smoking and quality of life in advanced lung cancer patients: A prospective longitudinal study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Liptay, M.J.; Basu, S.; Hoaglin, M.C.; Freedman, N.; Faber, L.P.; Warren, W.H.; Hammoud, Z.T.; Kim, A.W. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J. Surg. Oncol. 2009, 100, 703–707. [Google Scholar] [CrossRef]
- Ozeki, N.; Kawaguchi, K.; Fukui, T.; Fukumoto, K.; Nakamura, S.; Hakiri, S.; Kato, T.; Hirakawa, A.; Okasaka, T.; Yokoi, K. The diffusing capacity of the lung for carbon monoxide is associated with the histopathological aggressiveness of lung adenocarcinoma. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 52, 969–974. [Google Scholar] [CrossRef]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, b5569. [Google Scholar] [CrossRef]
- Florou, A.N.; Gkiozos, I.C.; Tsagouli, S.K.; Souliotis, K.N.; Syrigos, K.N. Clinical significance of smoking cessation in subjects with cancer: A 30-year review. Respir. Care 2014, 59, 1924–1936. [Google Scholar] [CrossRef]
| Preoperative Smokers (n = 449) | Group A (n = 60) | Group B (n = 30) | SMD | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic data | ||||||||
| Age (Years) | 63.7 ± 11.3 | 62.6 ± 12.5 | 64.3 ± 9.7 | 0.14 | 0.82 | |||
| Male (%) | 65 | 64 | 62 | 0.46 | ||||
| Comorbidity | ||||||||
| COPD (%) | 35 | 38 | 29 | 0.31 | ||||
| BMI (kg/m²) | 26.1 ± 5.2 | 27.1 ± 5.3 | 23.7 ± 3.9 | 0.26 | 0.11 | |||
| Prev. oncol. disease (%) | 4 | 3 | 5 | 0.18 | ||||
| Smoking habit | ||||||||
| Pack years | 45 ± 34 | 47 ± 31 | 49 ± 27 | 0.04 | 0.87 | |||
| Lobectomy | ||||||||
| RUL (%) | 26 | 25 | 28 | |||||
| ML (%) | 5 | 5 | 7 | |||||
| RLL (%) | 19 | 22 | 16 | 0.21 | ||||
| LUL (%) | 26 | 23 | 28 | |||||
| LLL (%) | 24 | 25 | 21 | |||||
| Mean numb. of res. seg. | 4.0 ± 0.5 | 4.1 ± 0.4 | 4.0 ± 0.4 | 0.2 | 0.91 | |||
| Histologic subtype | ||||||||
| Adenocarcinoma (%) | 46 | 52 | 46 | |||||
| Squ. cell carcinoma (%) | 41 | 38 | 39 | 0.23 | ||||
| Large cell carcinoma (%) | 9 | 8 | 11 | |||||
| Others (%) | 4 | 2 | 4 | |||||
| NSCLC stage | ||||||||
| n | % | n | % | n | % | |||
| IA1 | 72 | 16 | 8 | 13 | 5 | 17 | ||
| IA2 | 22 | 5 | 7 | 12 | 2 | 7 | ||
| IA3 | 41 | 9 | 5 | 8 | 3 | 10 | ||
| IB | 63 | 14 | 7 | 12 | 4 | 13 | 0.41 | |
| IIA | 81 | 18 | 11 | 18 | 5 | 17 | ||
| IIB | 85 | 19 | 11 | 18 | 6 | 20 | ||
| IIIA | 67 | 15 | 10 | 17 | 4 | 13 | ||
| >IIIA | 18 | 4 | 1 | 2 | 1 | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doerr, F.; Leschczyk, T.; Grapatsas, K.; Menghesha, H.; Baldes, N.; Schlachtenberger, G.; Heldwein, M.B.; Michel, M.; Quaas, A.; Hagmeyer, L.; et al. Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients. Cancers 2024, 16, 465. https://doi.org/10.3390/cancers16020465
Doerr F, Leschczyk T, Grapatsas K, Menghesha H, Baldes N, Schlachtenberger G, Heldwein MB, Michel M, Quaas A, Hagmeyer L, et al. Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients. Cancers. 2024; 16(2):465. https://doi.org/10.3390/cancers16020465
Chicago/Turabian StyleDoerr, Fabian, Tobias Leschczyk, Konstantinos Grapatsas, Hruy Menghesha, Natalie Baldes, Georg Schlachtenberger, Matthias B. Heldwein, Maximilian Michel, Alexander Quaas, Lars Hagmeyer, and et al. 2024. "Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients" Cancers 16, no. 2: 465. https://doi.org/10.3390/cancers16020465
APA StyleDoerr, F., Leschczyk, T., Grapatsas, K., Menghesha, H., Baldes, N., Schlachtenberger, G., Heldwein, M. B., Michel, M., Quaas, A., Hagmeyer, L., Höpker, K., Wahlers, T., Darwiche, K., Taube, C., Schuler, M., Hekmat, K., & Bölükbas, S. (2024). Postoperative Tobacco Cessation Improves Quality of Life, Lung Function and Long-Term Survival in Non-Small-Cell Lung Cancer Patients. Cancers, 16(2), 465. https://doi.org/10.3390/cancers16020465








