Simple Summary
Endometrial cancer is a complex disease with multiple risk factors, distinct histological subtypes, and a variety of available therapeutic options. The disease’s pathogenesis involves several molecular mechanisms, such as genetic mutations, defects in the DNA mismatch repair (MMR) pathway, hormonal signaling pathway imbalances, epigenetic changes, and disturbances in angiogenesis. On the other hand, molecular biomarkers seem to have an essential role in the diagnosis, prognosis, prediction of therapeutic response, and monitoring of disease progression in endometrial cancer. This review highlights the most significant dysregulated molecular mechanisms and reveals the relationship between specific biomarkers and targeted therapy approaches in the fight against endometrial cancer.
Abstract
Endometrial cancer is one the most prevalent gynecological cancers and, unfortunately, has a poor prognosis due to low response rates to traditional treatments. However, the progress in molecular biology and understanding the genetic mechanisms involved in tumor processes offers valuable information that has led to the current classification that describes four molecular subtypes of endometrial cancer. This review focuses on the molecular mechanisms involved in the pathogenesis of endometrial cancers, such as genetic mutations, defects in the DNA mismatch repair pathway, epigenetic changes, or dysregulation in angiogenic or hormonal signaling pathways. The preclinical genomic and molecular investigations presented allowed for the identification of some molecules that could be used as biomarkers to diagnose, predict, and monitor the progression of endometrial cancer. Besides the therapies known in clinical practice, targeted therapy is described as a new cancer treatment that involves identifying specific molecular targets in tumor cells. By selectively inhibiting these targets, key signaling pathways involved in cancer progression can be disrupted while normal cells are protected. The connection between molecular biomarkers and targeted therapy is vital in the fight against cancer. Ongoing research and clinical trials are exploring the use of standard therapy agents in combination with other treatment strategies like immunotherapy and anti-angiogenesis therapy to improve outcomes and personalize treatment for patients with endometrial cancer. This approach has the potential to transform the management of cancer patients. In conclusion, enhancing molecular tools is essential for stratifying the risk and guiding surgery, adjuvant therapy, and cancer treatment for women with endometrial cancer. In addition, the information from this review may have an essential value in the personalized therapy approach for endometrial cancer to improve the patient’s life.
1. Introduction
Endometrial cancer (EC) has its origin in the endometrium, which is the mucous membrane lining the uterus. It is one of the most frequent gynecologic cancers, and it predominantly affects postmenopausal women, although it can also be found in younger women (under 40 years of age) or in premenopausal women. EC usually presents with abnormal uterine bleeding, such as postmenopausal or irregular menstrual bleeding [1]. The etiology and pathology of EC are influenced by a combination of genetic, hormonal, and environmental factors [2]. According to the latest available data from the World Health Organization’s International Agency for Research on Cancer (IARC) and the European Cancer Information System (ECIS), the incidence of EC in Europe has been increasing over the past few decades, partly due to changes in obesity prevalence, hormonal factors, and improvements in cancer detection and diagnosis [3,4]. Mortality rates tend to be lower than incidence rates, reflecting improvements in treatment and outcomes for EC patients, particularly those diagnosed at early stages [5]. EC is a heterogeneous disease with multiple risk factors, histological subtypes, and treatment options. Early detection and complex treatment approaches are essential in optimizing results and improving survival rates for EC. The diagnosis is based on clinical evaluation, imaging techniques (such as transvaginal sonography or MRI), and histopathological examination, which implies prior tissue sampling (usually an endometrial biopsy/curettage) [6,7,8].
Menarche age, age at the last birth, breastfeeding, fertility treatments, and parity can represent a series of factors involved in the appearance of EC. Also, the use of nonsteroidal anti-inflammatory drugs, oral contraceptives, and IUDs or the existence of some medical conditions such as polycystic ovarian syndrome, metabolic syndrome, systemic lupus erythematosus, diabetes mellitus, hypertension, and obesity can lead to the occurrence and advancement of EC [9,10,11]. Unlike the aforementioned pathologies, the percentage of malignant pathologies of the endometrium and their impact on fertility are still unknown due to the lack of reports based on large groups of patients [12]. EC diagnosed in women under 40 years old is usually a very well-differentiated focal endometrioid tumor, restricted in the endometrium or with minimal myometrial invasion, with a high expression of estrogen and progesterone receptors. Clinical observation shows that 8% of patients under 40 years of age with EC are diagnosed with stage I (50–90%) [13,14].
Classification and staging of EC are important for guiding treatment decisions and generating the most realistic prognosis. The traditional histologic classification elaborated by Bokhman divides EC into estrogen-dependent carcinomas (EEC), which include the following subtypes: endometrioid adenocarcinoma and mucinous adenocarcinoma or type I, and non-endometrioid endometrial carcinomas (NEEC) or less frequent tumors (<20%), which encompass more aggressive subtypes, such as serous carcinoma, clear cell carcinoma, and carcinosarcoma (also known as malignant mixed Müllerian tumor or MMMT) [15].
The evolution of genetic and molecular studies has offered valuable information that has led to the current classification that describes four molecular subtypes of EC. The Cancer Genome Atlas (TCGA) has played a significant role in advancing our understanding of the molecular landscape of EC [16]. The TCGA described in 2022 four molecular subtypes of EC based on genomic characteristics: (1) POLE-mutated (ultra mutated, ≥100 mutations/Mb) tumors are characterized by mutations in the DNA polymerase epsilon (POLE) gene; these mutations lead to distinct characteristics such as high mutational burden, microsatellite stability, significant immune cell infiltration, and generally favorable prognosis [17], and they are often well-differentiated endometrioid carcinomas. (2) Microsatellite instability (MSI-H) tumors are characterized by defects in the DNA mismatch repair (MMR) system and when the errors were accumulated in microsatellite regions of the genome, leading to MSI; often, MSI-H is accompanied by a high mutational burden, and significant immune response due the infiltration of immune cells, into the tumor microenvironment. All of these factors influence the behavior, prognosis, and treatment response of these tumors. Because this subtype appears at an earlier stage, it is associated with a better prognosis and is more commonly found in endometrioid carcinomas [18,19]. This subtype is associated with a better prognosis and is more commonly found in endometrioid carcinomas. (3) Copy number low (CNL)/microsatellite stable (MSS) tumors have a relatively stable genome with fewer genetic alterations and lower mutation rates [20]. This subtype is enriched for endometrioid carcinomas and is linked with a moderately favorable prognosis. (4) Copy number high (CNH) tumors have a high level of chromosomal instability and numerous copy number alterations, especially in TP53 and PIK3CA; this subtype is enriched for serous carcinomas that are more aggressive and is associated with a poorer prognosis [21].
In this comprehensive review, we have thoroughly focused in the first part on the various molecular mechanisms responsible for the development and progression of ECs. This information helps to identify biomarkers that enhance the understanding of EC pathology and guide the development of effective treatments. So, in the second part of the review, we present the known diagnostic or prognostic biomarkers or those in different stages of research in the laboratory or in clinical trials. At the end of the article, some molecules are presented that can be considered molecular targets and allow the approach of targeted therapy strategies for endometrial cancer.
2. Molecular Mechanisms Involved in the Pathogenesis of Endometrial Cancers
The pathogenesis of endometrial cancer implies complex molecular mechanisms responsible for the appearance of genetic mutations, the activation of oncogenes accompanied by the inactivation of tumor suppressor genes, defects in the DNA mismatch repair (MMR) pathway, imbalance in hormonal signaling pathways, epigenetic modifications, or disorders in angiogenesis (Figure 1) [22,23].
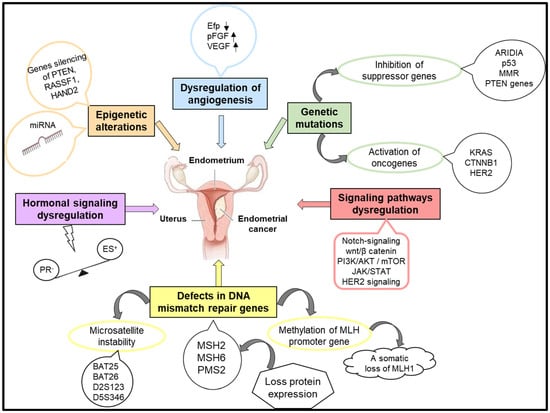
Figure 1.
The molecular mechanisms involved in the occurrence and evolution of endometrial cancer.
2.1. Genetic Mutations
Genetic mutations are essential factors in the development and progression of EC. These mutations can affect a series of genes involved in different cellular processes, such as the regulation of cellular cycle, DNA repair, apoptosis, and hormonal signaling. One of the most frequent genetic modifications in EC is that found on PTEN suppressor gene (phosphatase and tensin homolog), which regulates cell growth, survival, and proliferation. Many endometrial tumors exhibit a loss of PTEN function that can occur due to mutations, deletions, or epigenetic silencing. This loss of PTEN function leads to the dysregulation of the PI3K/AKT/mTOR pathway, which promotes abnormal cell growth and proliferation. This, in turn, contributes to the development and progression of EC [24]. PTEN status in EC patients can have prognostic and therapeutic implications. Tumors with PTEN alterations may exhibit more aggressive behavior and resistance to certain therapies. Additionally, targeting the PI3K/AKT/mTOR pathway, either directly or indirectly, represents a potential therapeutic strategy for ECs with PTEN mutations or loss of PTEN function. Understanding the role of PTEN in endometrial cancers is essential for developing targeted therapies and improving patient outcomes [25,26].
Furthermore, mutations in the TP53 gene determine the loss of its suppressive functions, allowing cells to avoid apoptosis and to accumulate genetic modifications. Thus, the presence of TP53 mutations in ECs is associated with high aggressiveness and a poor prognosis [27].
In endometrial cancers, especially in clear cell and endometrioid subtypes, mutations of ARID1A (AT-rich interaction domain 1A gene), which codes a subunit of the SWI/SNF chromatin remodeling complex, are very frequent. The presence of ARID1A mutations induces disorders in the process of chromatin remodeling and that of genetic expression, contributing to tumorigenesis and the progression of the tumor [28]. In high-grade endometrioid carcinomas, ARID1A mutations are present in over 40% of cases, while in low-grade endometrioid carcinomas, they are observed in only 25% of cases [29]. ARID1A interacts with numerous transcription factors and other signaling molecules, so the recorded disturbances induce changes at the level of several molecular pathways. Some studies have shown that changes in the ARID1A gene in EC influence PI3K and protein kinase B (AKT) signaling pathways [30,31]. Also, an association was found between ARID1A loss with microsatellite instability (MSI) and mismatch repair (MMR) deficiency in the case of uterine endometrioid carcinoma. In clear cell carcinoma, loss of ARID1A is associated with aberrant expression of the p53 protein or mutations of PTEN and PIK3CA [32]. In ARID1A-deficient endometrial cancer cells, the level of FOXO1 increased while the mitogen-activated protein kinase pathway (MAPK) and insulin-like growth factor-1 signaling pathway decreased [33]. In endometrial carcinogenesis, dysregulation of the ARID1A gene can influence several genes such as the skeletal phosphatase 2 subunit alpha gene (PPP2R1A), PTEN, PIK3CA, KRAS, catenin beta 1 gene (CTNNB1), TP53, proto-oncogene B-Raf, serine/threonine kinase gene (BRAF), and PPP2R5C. Thus, starting from the level of dysregulation of ARID1A, a classification was made based on the mutational profile of endometrial carcinomas [34]. The mechanism of action of ARID1A in the initiation and progression of cancer cells needs to be clarified in order to develop new diagnostic tools and to design new, more effective therapeutic approaches.
Inactivation of the PTEN tumor suppressor gene is associated with the inactivation of other tumor suppressor genes, such as TP53 (p53) and ARID1A, in EC [35]. Loss of function of these genes removes important regulatory mechanisms that normally inhibit tumor growth and promote apoptosis [36]. Oncogenes, such as KRAS, CTNNB1 (β-catenin), and HER2/neu (human epidermal growth factor receptor 2), when mutated or activated, induce the stimulation of proliferation and extended survival of tumor cells, promoting the invasion and metastases of EC [37]. KRAS is a proto-oncogene involved in transmitting signals from the cell-surface receptors to the cell’s nucleus. KRAS mutations have been observed especially in the endometrioid subtype and are responsible for the activation of its MAPK/ERK signaling pathway, which contributes to the growth and progression of the tumor [38]. CTNNB1 codes β-catenin, which is involved in cellular adherence and in the regulation of the Wnt signaling pathway [39]. Mutations in CTNNB1 lead to the stabilization and accumulation of β-catenin in the cytoplasm, followed by its translocation in the nucleus, where it acts as a transcriptional co-activator, promoting the expression of genes, such as c-Myc, cyclin D1, surviving, and MMP7 (matrix metalloproteinase 7). Increased expression of these genes promotes cell cycle progression, inhibits apoptosis, and enhances cellular proliferation, contributing to tumor growth and progression [40,41].
HER2/neu is a tyrosine kinase receptor in the EGFR family (epidermal growth factor receptor). Amplification or overexpression of HER2/neu leads to the constitutive activation of its signaling pathways, PI3K/AKT/mTOR and MAPK/ERK, which promote proliferation and cell survival [42]. Targeting these oncogenic pathways represents a potential therapeutic strategy for subsets of EC patients with specific oncogene mutations [43].
2.2. Defects in DNA Mismatch Repair Genes
Defects in DNA mismatch repair genes, such as MLH1, MSH2, MSH6, and PMS2, are associated with microsatellite instability-high (MSI-H) endometrial cancers. MMR deficiency leads to the accumulation of DNA replication errors and genomic instability, predisposing cells to cancer development [44]. In some endometrial cancers, sporadic modifications of the MMR pathway can occur, without any inherited genetic predisposition. These modifications are the result of somatic mutations or epigenetic modifications that affect the MMR genes, leading to a dysfunction of MMR, accompanied by a growth in the levels of microsatellites (MSI-H). It is relevant to mention the significantly high risk of developing endometrial cancer in women with Lynch syndrome, which is an inherited disease caused by mutations of MMR genes (especially MLH1, MSH2, MSH6, and PMS2) [45]. Testing of the expression of MSI or MMR protein in tumoral tissue with the help of immunohistochemistry techniques is used to identify endometrial cancer patients with an MMR deficit. Identifying the MMR status can be useful in guiding therapeutic decisions because patients with endometrial cancer, and an MMR deficit can have a different response to treatment [46].
2.3. Hormonal Signaling
Hormonal signaling is an essential mechanism involved in the development and progression of EC. The endometrium is very sensitive to hormonal fluctuations, especially those of estrogen and progesterone. Imbalances in estrogen and progesterone levels, as well as alterations in hormone receptor expression, contribute to the pathogenesis of endometrial cancer [47].
The growth of estrogen levels (ES) due to endogen factors (such as obesity, polycystic ovarian syndrome, or estrogen-secreting tumors) or exogen factors (such as hormonal substitution therapy without any progestin) can lead to excessive growth of endometrial cells and can increase the risk for developing endometrial cancer [48]. ES exerts its effects through binding with estrogen specific receptors, ER-alfa and ER-beta, thus activating the signaling pathways responsible for cellular proliferation, angiogenesis stimulation, and apoptosis inhibition. A high expression of ER-alfa or the loss of ER-beta expression can contribute to the development of EC [49]. Furthermore, the disturbances that can occur in the metabolism of estrogen inside the body (hydroxylation, methylation) can lead to the formation of genotoxic metabolites or the accumulation of estrogenic compound (2-hydroxiestrone (2-OHEI) and 16α-hydroxy estrone (16α-OHEI)). An increased ratio between 16α-OHEI and 2-OHEI has been associated with a high risk of endometrial cancer [50,51]. Progesterone (PR), another ovarian hormone, acts to counterbalance the effects of estrogen on the endometrium. PR has anti-proliferative effects on the endometrial cells by inhibiting the progression of the cellular cycle and DNA synthesis [52]. Progesterone induces a decrease in the expression of the genes involved in the progression of the cellular cycle, such as cyclins and cyclin-dependent kinases (CDK), and it also regulates the expression of cellular cycle inhibitors, such as p21 and p27, and, thus, it inhibits cellular proliferation. A deficiency in progesterone signaling, which can occur due to the absence of ovulation or due to an inadequate luteal phase, can lead to estrogen stimulation and increase the risk for EC [53,54]. A dysregulation in hormonal signaling can induce abnormal proliferation, genomic instability, and angiogenesis inside the endometrium, thus predisposing to the development of EC [55]. Understanding the interactions between hormonal factors and the molecular pathways involved in the pathogenesis of EC is crucial to find new molecular targets, which can lead to new preventive or therapeutic strategies [56].
2.4. Epigenetic Modifications
Epigenetic modifications occur in EC, involving DNA methylation, histone modification, and micro-RNA (miRNA) dysregulation, leading to aberrant gene expressions [57]. An epigenetic modification that can appear in endometrial cancer is the aberrant methylation of DNA at the level of CpG islets, which is induced by the activation of DNA-methyltransferases (DNMT), and it determines gene expression modifications by inhibiting or activating transcription [58]. Also, DNA methylation involves the hypermethylation of the promotor regions of tumor suppressor genes, which can lead to their silencing, or the hypomethylation of oncogenes, which can lead to their overexpression, thus promoting tumorigenesis [59].
Modifications that can appear in histones include the acetylation, methylation, and phosphorylation processes, and they affect the structure of chromatin and the transcription of genes, contributing to the progression of EC [60,61]. The process of histone methylation can take place on lysine residues (mono-, di-, or tri-methylation) or arginine residues (mono- or di-methylation). This process is regulated by histone methyl-transferases (HMT) and histone demethylases (HMT). The abnormal modification of histones affects gene expression [62]. Histone acetylation is dependent on the balance between histone acetyltransferases (HAT) and histone deacetylases (HDAC), which influence access to transcription factors and thus influence gene expression [63]. Furthermore, histone acetylation/deacetylation influences the gene expression of non-histone proteins, which are involved in tumor progression and metastases. Thus, an increased level of HDAC (HDAC1, HDAC2, and HDAC3) found in EC is linked to poor prognosis [64].
2.5. Angiogenesis
Angiogenesis is a physiological process that ensures the formation of new blood vessels, and it contributes to wound healing and embryological development. In EC, the dysregulation of angiogenic signaling pathways leads to the abnormal formations of new blood vessels and so it contributes to tumor growth, invasion, and metastasis by providing oxygen and nutrients for tumor cells [65]. A series of factors contribute to angiogenic signaling in endometrial cancer. Inside the tumor, the proliferative capacity of tumor cells exceeds the process of blood vessel growth, and it determines the appearance of areas characterized by a lack of oxygen (hypoxia). Hypoxia-inducible factor-1α (HIF-1α) is a key transcription factor that regulates cellular response to hypoxia [66]. Hypoxia triggers the increased release of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) [67], fibroblast growth factor (FGF) [68], and platelet-derived growth factor (PDGF) [69]. Angiopoietins (Ang) and their receptor Tie2 play a crucial role in regulating the stability and remodeling of blood vessels. Overexpression of angiopoietins and the activation of Tie2 can promote abnormal angiogenesis in endometrial cancer [70].
Endometrial tumor cells secrete anti-angiogenic factors in small quantities, factors such as thrombospondin-1 and endostatin, which have a role in inhibiting angiogenesis. This imbalance between pro-angiogenic factors and anti-angiogenic factors promotes angiogenesis inside the tumor microenvironment. Dysregulation of the Notch signaling pathway is associated with the blood vessel maturation process and is involved in promoting angiogenesis and tumor progression in EC [71].
Another signaling pathway that is frequently deregulated in endometrial cancer is represented by the PI3K/AKT/mTOR pathway. The activation of this pathway can promote angiogenesis by positive regulation of the expression of VEGF, increasing endothelial cell proliferation and tumoral cell survival [72].
Also, epigenetic alterations, such as DNA methylation [73] and histone modification [74], can impact the expression of genes involved in angiogenesis and the formation of abnormal blood vessels in tumors. Furthermore, matrix metalloproteinases (MMP) facilitate tumoral angiogenesis by degrading the extracellular matrix, allowing endothelial cells to migrate and form new blood vessels [41].
In post-transcription regulation of gene expression in EC, a major role is played by micro-RNA (miARN). These are short, non-coding, single-stranded RNA molecules that regulate post-transcription gene expression through binding with mRNA [75]. miRNAs modulate processes such as cellular division, proliferation, differentiation, apoptosis, and blood vessel formation [76]. The modified expression of miRNAs has also been found in endometrial cancer, and it can influence angiogenesis processes by targeting genes involved in the functionality of endothelial cells, vascular permeability, and angiogenic signaling [77,78].
In conclusion, epigenetic modifications and the dysregulation of angiogenesis have an important role in the development and progression of EC. Deciphering and understanding these processes can lead to identifying new molecular biomarkers or therapeutic targets useful in finding more efficient treatments for EC.
3. Biomarkers in Endometrial Cancer
Molecular biomarkers are molecules that indicate a biological or pathological process or therapy response. They can be found in tissues, blood, urine, or other bodily fluids and analyzed using various molecular techniques, such as polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and next-generation sequencing (NGS).
Molecular biomarkers are essential in diagnosing, prognosis, predicting therapeutic response, and monitoring disease progression in endometrial cancer (Figure 2). Continuous advancements in molecular techniques and genomic profiling technologies are expanding the range of molecular biomarkers available for use in the management of endometrial cancer.
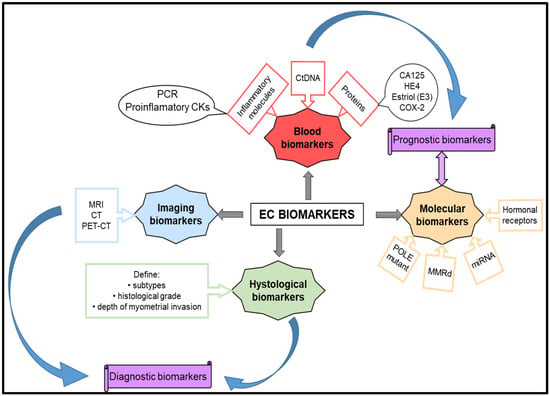
Figure 2.
Biomarkers for diagnosis and prognostic endometrial cancer.
Examining the endometrial tissue obtained through biopsy or surgical resection is still the most reliable method for diagnosing EC. Pathologists rely on histological features, such as glandular architecture, nuclear morphology, and mitotic activity to differentiate between benign conditions, hyperplasia, and malignancy [79].
Imaging biomarkers are a crucial factor in assessing the response of EC to treatment. Two imaging techniques, namely, magnetic resonance imaging (MRI) and computed tomography (CT) scans, help monitor changes in tumor size, volume, and enhancement patterns revealed by imaging studies, indicating response or progression during or after treatment [80,81]. Positron emission tomography-computed tomography (PET-CT) scans, on the other hand, use radioactive tracers to detect changes in tumor metabolic activity over time, thus being particularly useful for evaluating treatment response in advanced or recurrent EC [82].
Serum biomarkers facilitate early detection, precise diagnosis, prognostic evaluation, and treatment response monitoring. Clinical evaluation of serum biomarkers alongside imaging and clinical data can ensure an accurate diagnosis and the selection of an optimal treatment option [83]. HE4 (human epididymis protein 4), together with other serum biomarkers, such as CA-125, CA724, CA19-9 [84,85,86], and YKL-40, also known as chitinase-3-like 1 protein (CHI3L1) and DKK-3 [87], can contribute to the detection and monitoring of endometrial cancer. High levels of HE4 have been associated with advanced-stage disease and can indicate the aggressiveness of the tumor. Thus, high serum levels of HE4 are associated with advanced stage disease, as well as with the implication of the endocervical stroma and deep myometrial invasion [88]. A serum HE4 value higher than 201.3 pmol/L indicates a high risk for recurrence for endometrioid cancers [89,90]. In conclusion, monitoring the modifications of HE4 levels can, in time, offer additional information about disease progression and the risk of recurrence.
A series of serum proteins are being studied as potential biomarkers for EC, but the so far obtained results have not been enough to be validated in clinical practice. Such a protein is Dickkopf-1 (DKK-1), which is involved in embryological development and has been found in high concentrations in the serum of patients with advanced-stage EC (stages III–IV) [91]. Also, there are studies that have identified high serum levels of DJ-1 protein (part of the peptidase C56 family) in patients with EC, especially in non-endometrioid subtypes [92,93]. Other protein biomarkers such as osteopontin, leptin, and tumor-associated glycoprotein 72 (TAG-72) are under study. Osteopontin is a glycoprotein found in increased quantity in different cancers, including EC. Its expression is linked to the advancement of the tumor, metastasis, and unfavorable results in EC patients. It assists in promoting tumor cell survival, invasion, and angiogenesis by interacting with cell surface receptors and signaling pathways involved in these processes. Furthermore, osteopontin can impact the immune response in the tumor microenvironment, influencing the anti-tumor immune response and immune evasion mechanisms. The osteopontin levels in serum or tumor tissue have been studied as a possible biomarker for predicting disease severity, treatment response, and patient outcomes in endometrial cancer [94]. Leptin is a hormone mainly produced by adipose tissue. It has pro-inflammatory and pro-angiogenic properties and is linked to cancer development and progression, including EC [95]. Leptin receptors are present in endometrial cancer cells, and leptin signaling pathways can promote the proliferation, migration, and invasion of tumor cells. High levels of circulating leptin are linked to an increased risk of EC, particularly in postmenopausal women and obese individuals [96].
Tumor-associated glycoprotein 72 (TAG-72) is a glycoprotein present on the surface of cells that is often found in higher levels in EC and other types of cancers. TAG-72 can be detected in tumor tissue or serum and has been studied as a potential biomarker to help diagnose and track cancer progression. In endometrial cancer patients, TAG-72 expression has been linked to more advanced stages of disease, lymph node metastases, and poorer prognosis [97].
Hormonal biomarkers such as estrogen and progesterone receptors and biomarkers associated with inflammation and immune response such as C-reactive protein (CRP) and some cytokines can be detected in the serum of patients with EC [98]. These biomarkers can provide information regarding tumor–host interactions and immune response, and they may hold prognostic significance [99].
Biomarkers associated with angiogenesis, such as VEGF and its soluble receptors (RVEGF), can be measured in the serum of patients with EC [100]. These biomarkers are indicative of tumor angiogenesis and can provide insights into disease aggressiveness and the efficacy of anti-angiogenic therapies.
Tumor cells release fragments of tumor-derived DNA into the bloodstream, known as circulating tumor DNA (ctDNA). Analysis of ctDNA can be performed using various techniques, such as digital PCR (dPCR), next-generation sequencing (NGS), and allele-specific PCR (AS-PCR). These techniques allow for detecting and quantifying specific mutations or genetic changes in ctDNA. By analyzing ctDNA, we can obtain information about the genetic mutations, changes in the number of copies, and methylation patterns of the tumor [101]. These data are helpful for early detection, monitoring the response to treatment, and detecting minimal residual disease or recurrence in EC patients [102].
Prognostic Biomarkers Are Indicators That Predict the Course or Outcome of a Disease
They aid clinicians in identifying the risk of recurrence or overall survival, regardless of the treatment. In EC, numerous biomarkers have been discovered as prognostic indicators. Histopathological examination of tissue or biopsy samples is used to identify biomarkers that aid in the diagnosis and prognosis of endometrial cancers [103]. Histological grade is a strong prognostic factor in EC and is determined by the tumor’s degree of cellular differentiation and architectural features. Tumors are typically categorized as Grade 1 (well-differentiated), Grade 2 (moderately differentiated), or Grade 3 (poorly differentiated). Higher-grade tumors are associated with a worse prognosis and an increased risk of recurrence. The stage of a tumor describes how far it has spread and is a critical factor in predicting the outcome of endometrial cancer. The International Federation of Gynecology and Obstetrics (FIGO) staging system determines the tumor stage based on various factors, such as tumor size, depth of invasion, involvement of nearby structures, and the presence of metastases. Patients with advanced-stage tumors (FIGO stage III or IV) generally have worse outcomes than those with early stage disease [104]. Lymphovascular invasion (LVI) is a significant prognostic factor in EC. It refers to the presence of tumor cells within lymphatic or blood vessels. LVI indicates a higher risk of lymph node metastasis and distant recurrence, which leads to a poorer prognosis [105]. Tumor size is another important prognostic factor in EC. Tumors with diameters greater than 2 cm indicate a worse prognosis [106]. The depth of myometrial invasion, which is determined by the extent of tumor infiltration into the uterine wall, is also an important prognostic factor. Deep myometrial invasion, defined as greater than 50% of myometrial thickness, is associated with a higher risk of lymph node involvement and distant metastasis, leading to a poorer prognosis [107].
Immunohistochemical staining for p53 protein expression or molecular analysis to test TP53 gene mutations can assess p53 status. Alterations in TP53, which encodes the p53 protein, are associated with high-grade tumors, aggressive tumor behavior, and poorer prognosis in EC [108]. Ki-67 expression is another marker of cellular proliferation, and high levels of its expression are associated with aggressive tumor behavior and a worse prognosis in EC [109].
Immunohistochemical staining can be used to analyze the expression of MMR proteins (such as MLH1, MSH2, MSH6, and PMS2), which can help define deficient DNA mismatch repair (dMMR). These proteins are associated with a better prognosis and improved response to immunotherapy in EC [110,111].
MicroRNAs (miRNAs) are small non-coding RNAs that play important roles in the regulation of gene expression. Dysregulation of miRNA expression has been implicated in the pathogenesis, progression, and prognosis of various cancers, including endometrial cancer. Several studies have investigated the potential of miRNAs as molecular biomarkers for prognosis in EC [112]. Overexpression of miR-21 and miR-183-5p have been associated with tumor aggressiveness, lymph node metastasis, and poorer prognosis in endometrial cancer [77]. Reduced expression of miR-205 has been associated with advanced-stage disease and poorer prognosis in endometrial cancer. Low levels of miR-192, miR-194, miR-215, miR-99a, and miR-100 expression has been correlated with tumor aggressiveness and metastasis in endometrial cancer [113]. Dysregulation of the miR-200 family members (miR-200a, miR-200b, miR-200c, miR-141, miR-429) has been associated with epithelial-to-mesenchymal transition (EMT), tumor aggressiveness, and metastasis in EC [114]. The miR-34 family members are known tumor suppressor miRNAs, and the decrease in their expression has been associated with tumor aggressiveness, high-grade tumors, and poorer prognosis in EC [115]. These miRNAs have shown promise as potential prognostic biomarkers. However, further validation in larger, well-designed studies is needed to confirm their clinical utility and to establish standardized protocols for their assessment in routine clinical practice.
Tumor-infiltrating lymphocytes (TILs) are immune cells that have migrated into the tumor microenvironment and can provide information about the tumor’s biology and the patient’s immune response [116]. In patients with endometrial tumors, a higher density of TILs is correlated with a better prognosis, including more prolonged overall survival and progression-free survival. TILs, especially cytotoxic CD8+ T cells, are indicative of a robust anti-tumor immune response, which can help control tumor growth and spread. Thus, TILs can be considered prognostic biomarkers in endometrial cancers, but their prognostic value varies among the different molecular subtypes of endometrial cancer. POLE ultramutated and MSI-H subtypes, which are associated with high mutation rates and increased neoantigen load, tend to have higher levels of TILs and generally better outcomes. On the contrary, the copy number high (CN-H) subtype, which is associated with more aggressive disease, often shows lower levels of TILs and poorer prognosis [117]. Also, assessing TILs in endometrial cancer can help identify patients who might benefit from immunotherapy, making them a valuable tool for personalized treatment approaches [118]. Combining TIL assessment with other biomarkers, such as PD-L1 expression, mutational load, and molecular subtyping, can enhance the prognostic and predictive power of endometrial cancer management.
The prognostic biomarkers help clinicians stratify patients into different risk groups and guide treatment decisions, such as the intensity of adjuvant therapy and the frequency of surveillance. Integrating prognostic biomarkers into clinical practice enables personalized management of endometrial cancer patients based on their individual risk factors.
4. Endometrial Cancer Therapy between Standard Approaches and Future Directions
4.1. Standard Therapy
Therapeutic approaches for treating endometrial cancer depend on various factors, such as the stage and grade of the tumor, the depth of myometrial invasion, the status of lymphovascular space involvement, the presence of specific biomarkers, and the patient’s overall health. Management of endometrial cancer at diagnosis involves surgical methods. Thus, minimally invasive surgery, such as laparoscopic or robot-assisted surgery, can be used for early stage endometrial cancer, which can lead to good results with less morbidity and a shorter postoperative recovery period [119]. Patients with high-risk features, such as deep myometrial invasion, high-grade tumors, or lymphovascular invasion, may be recommended to undergo postoperative radiation therapy. External beam radiation therapy or brachytherapy can be used to kill cancer cells [120]. Radiation therapy can be used as neoadjuvant therapy to shrink the tumor before surgery or as adjuvant therapy to kill any remaining cancer cells after surgery [121].
In the case when the tumors cannot undergo surgery, chemotherapy may be used as the primary treatment to kill cancer cells. Often, chemotherapy may be applied after surgery to destroy any remaining cancer cells. Chemotherapy is a standard treatment for endometrial cancer. The chemotherapy drugs used for this treatment are Paclitaxel, Carboplatin or Cisplatin, Doxorubicin (Adriamycin), Epirubicin, and Ifosfamide [122]. These drugs belong to different categories: taxane, platinum-based, anthracycline, and alkylating agent chemotherapy drugs. These chemotherapy drugs interfere with the growth and division of cancer cells by damaging the DNA of cancer cells and may be used alone or in various combinations [123,124]. The dosage and specific regimen of these chemotherapeutic drugs depend on the stage and characteristics of the endometrial cancer. A multidisciplinary team of healthcare professionals determines the appropriate combination of medications for a particular patient [125].
Hormonal therapy is a treatment option that has been used for a long time to treat recurrent endometrial cancer. It is particularly useful for patients with slow-growing, low-grade tumors or for patients for whom other treatments may be too toxic. Hormonal therapy can be used for women with estrogen-receptor-positive advanced or recurrent endometrial cancer. It involves using medications to block the estrogen effects on cancer cells.
Although progestins and megestrol acetate therapy are FDA-approved for treating advanced and recurrent endometrial cancer patients, recent studies have presented contradictory results [126,127]. Thus, some studies have highlighted a correlation between the expression level of ER and PR receptors in primary tumors compared to recurrent tumors. In contrast, other studies deny this correlation [128,129]. These findings have opened new directions for hormone therapy. Studies in molecular biology have revealed that the MAPK pathway is involved in ER alpha signaling and activates other molecules such as ERK and AKT [130,131]. In the case of PR signaling, the PI3K/AKT/mTOR pathway is also activated in addition to the MAPK pathway. All this led to the approach of some studies that combine endocrine therapy with mTOR inhibitor. Endometrial cancer preclinical studies have demonstrated that inhibiting the PI3K/Akt pathway can reverse progestin resistance in EC models. This approach has been pursued in clinical trials [131].
Luteinizing-hormone-releasing hormone agonists (LHRH agonists) lower estrogen levels in women who are premenopausal and could be used in the therapy of EC [132]. It has been found that the use of aromatase inhibitors can be effective in treating endometrioid tumors that have a positive estrogen receptor status. Aromatase is an enzyme that is responsible for estrogen biosynthesis and has been found in malignant endometrial tissue. Aromatase inhibitors that can regulate estrogen levels and exert antitumor effects in endometrial carcinoma by blocking aromatase have been developed. Studies have shown that aromatase inhibitors such as letrozole or anastrozole can be used to treat EC, particularly in postmenopausal women with hormone-receptor-positive tumors [133]. These drugs are still being studied for how to use them best to treat EC.
4.2. Targeted Therapy Strategies in Endometrial Cancer
Targeted therapy refers to a specific type of cancer treatment that involves identifying specific molecular targets in cancer cells responsible for tumor formation and growth. These molecular targets are then selectively inhibited or modulated by targeted therapies, which disrupt key signaling pathways involved in cancer progression while protecting normal cells. This link between molecular markers and targeted therapy is crucial in the fight against cancer.
Targeted therapy is a recent approach to treating EC, and only a few validated drugs are currently being used. These drugs are primarily used to treat high-risk endometrial cancers that have metastasized or relapsed after previous treatment. Some of the therapeutic strategies presented are still part of a preclinical or clinical study. The molecular profiling of endometrial tumors is essential for identifying potential biomarkers and guiding targeted therapy decisions [134].
4.2.1. Targeting the PI3K/AKT/mTOR Signaling Pathway
The PI3K/AKT/mTOR signaling pathway is often disrupted in EC, making it a target for therapeutic intervention. PI3K/AKT/mTOR inhibitors are a class of targeted therapies that act by inhibiting various components of the PI3K/AKT/mTOR signaling pathway. PI3K inhibitors, such as Alpelisib, Taselisib, and Buparlisib, block the activity of the PI3K enzyme (phosphoinositide 3-kinase) that is responsible for converting phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), which in turn activates the downstream AKT and mTOR (mammalian target of rapamycin) signaling pathways [135]. Capivasertib and Ipatasertib act as AKT inhibitors to block the activation of AKT (protein kinase B), thereby preventing the phosphorylation and inactivation of pro-apoptotic proteins and promoting cell cycle arrest and apoptosis in cancer cells. Everolimus and temsirolimus are used as mTOR inhibitors. They block the activity of mTOR complex 1 (mTORC1), leading to decreased protein synthesis, cell cycle arrest, and autophagy in cancer cells. Tumors with PIK3CA mutations or other PI3K pathway alterations are more likely to respond to PI3K/AKT/mTOR inhibitors. Everolimus is a medication approved for clinical use in treating advanced or recurrent EC. It can be used alone or in combination with hormonal therapy like exemestane. This treatment is specifically designed for selected EC patients who have hormone-receptor-positive, HER2-negative disease [136].
PI3K inhibitors like Alpelisib, Taselisib, Buparlisib, and an AKT inhibitor known as Capivasertib are being evaluated in clinical trials as single agents or in combination with other targeted therapies or chemotherapy. The effectiveness of using single-agent PI3K inhibitors for the treatment of EC has been limited due to drug resistance and toxicity. However, combining PI3K inhibitors with standard chemotherapy or radiation therapy may improve the therapeutic response and lead to better outcomes, especially in cases of aggressive or advanced-stage disease [137]. Ongoing clinical trials are currently evaluating the effectiveness, safety, and optimal combinations of PI3K/AKT/mTOR inhibitors in different settings of EC to enhance treatment outcomes and patient care [138,139]. Alpelisib is currently undergoing clinical trials as a standalone treatment or in combination with hormonal therapy or other targeted therapies to effectively treat advanced or recurrent EC. This drug has shown exceptional results in treating tumors with PIK3CA mutations or other alterations in the PI3K pathway [140]. Also, the combination of PI3K/AKT inhibitors with hormonal therapies, such as progesterone or aromatase inhibitors (such as exemestane), may synergistically inhibit tumor growth and enhance response rates in hormone-receptor-positive endometrial cancers [47,141,142].
Combining PI3K/AKT/mTOR inhibitors with immune checkpoint inhibitors like Pembrolizumab or Nivolumab offers a promising approach regarding the improvement of antitumor immune response and enhancement of therapeutic outcomes in EC. This strategy is particularly effective for tumors with microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) [143,144].
4.2.2. Targeting Angiogenesis
Angiogenesis inhibitors are a type of medication that hinders the growth of new blood vessels and is responsible for supplying cancer cells with nutrients. Antiangiogenic agents can target the vascular endothelial growth factor (VEGF). VEGF plays a crucial role in angiogenesis, and Bevacizumab, a monoclonal antibody, blocks its action, thereby preventing the formation of new blood vessels that supply cancer cells with nutrients. Aflibercept (VEGF Trap-Eye) is a fusion protein with a high binding affinity to VEGF-A, VEGF-B, and placental growth factor, thus acting as an angiogenesis inhibitor [145,146]. Thalidomide also has antiangiogenic properties, although its exact mechanism of action is not yet fully understood [147]. There are several drugs that inhibit angiogenesis, and they are currently being tested in clinical trials to determine their effectiveness against the recurrence or metastasis of endometrial cancer. These drugs are called multi-targeted tyrosine kinase inhibitors and include Axitinib, which targets VEGF receptors (VEGFR-1, VEGFR-2, VEGFR-3); Pazopanib and Sunitinib, which target VEGF receptors (VEGFR-1, VEGFR-2, VEGFR-3) and platelet-derived growth factor receptors (PDGFR-α, PDGFR-β); and Nintedanib, which targets VEGF and PDGFR receptors, as well as fibroblast growth factor receptors (FGFR-1, FGFR-2, FGFR-3). One inhibitor of the angiopoietin-Tie2 receptor, which is being tested in endometrial cancer, is Trebananib [148].
Antiangiogenic therapy has emerged as a promising treatment option for endometrial cancer. These targeted therapies can benefit patients with advanced or recurrent EC by blocking angiogenesis, which can disrupt tumor growth and slow down disease progression [149]. Ongoing research and clinical trials are exploring the use of antiangiogenic agents in combination with other treatment strategies to improve outcomes and personalize treatment for patients with EC [150,151,152].
4.2.3. Targeting HER2-Receptors
HER2-positive EC can be treated with HER2-targeted therapy, which has shown great promise. The first and currently the only approved HER2-targeted agent for this indication is trastuzumab, a monoclonal antibody that targets the HER2 receptor. By inhibiting HER2-mediated signaling pathways, trastuzumab leads to decreased proliferation and survival of HER2-positive cancer cells. In clinical practice, trastuzumab (Herceptin) has been approved for selected patients with HER2-positive EC in combination with chemotherapy. For HER2-positive advanced or recurrent EC patients who are not suitable for curative surgery or radiation therapy, this treatment approach is recommended [153,154]. Ongoing research and clinical trials are currently exploring the effectiveness and safety of various HER2-targeted agents, including pertuzumab, lapatinib, and T-DM1. These agents are being studied both alone and in combination with other treatment options to optimize treatment outcomes and create personalized treatment approaches for patients who have HER2-positive EC [155]. It is crucial to identify HER2 overexpression or amplification in endometrial tumors to select appropriate HER2-targeted therapies and to guide personalized treatment decisions in the management of EC [124,156,157].
4.2.4. Immunotherapy
In March 2020, molecular typing of EC was achieved for the first time, and it was included in the guidelines for diagnosis and treatment of EC, which indicates the era of immunotherapy based on tumor microenvironment and genotype. The study of the pathogenesis of EC has revealed the presence of several types of immune system cells in tumor tissue. These cells secrete specific cytokines that modulate the anti-tumor immune response [157]. Immunotherapy is a promising cancer treatment that stimulates the immune system to target tumor cells specifically. Immunotherapy may be used for advanced or recurrent EC with MSI-H or dMMR tumors [158]. Immunotherapy methods such as immune checkpoint inhibitors (ICIs), cancer vaccines, adoptive cell transfer (ACT), lymphocyte-promoting cytokines, and oncolytic viruses are currently being investigated for the treatment of EC [159,160]. One approach involves targeting immune checkpoints, which are molecules that control how the immune system responds to cancer cells. When these checkpoints are overexpressed in cancer cells, they can inhibit the immune system’s ability to identify and attack the tumor [161]. The programmed cell death protein 1 (PD-1) pathway is an important immune checkpoint that has been researched in relation to EC. PD-1 is a protein on the surface of certain immune cells, including T cells. When PD-1 interacts with its ligands, PD-L1 and PD-L2, which are usually present in tumor cells, it can inhibit T cells’ activity, thereby preventing them from attacking the cancer cells. These immune checkpoint signals are considered important targets for immunotherapies that have recently been developed [162,163].
Clinical trials have investigated several immunotherapy drugs designed to target the PD-1 pathway for EC [164,165,166]. In January 2022, Pembrolizumab (Keytruda) showed efficacy in clinical trials and has received FDA (U.S. Food and Drug Administration) approval for specific indications in the treatment of EC. Pembrolizumab (Keytruda) is a checkpoint inhibitor targeting the programmed death receptor-1 (PD-1) protein on T cells, which helps the immune system recognize and attack cancer cells. It is approved for the treatment of advanced or recurrent EC with specific biomarker characteristics, such as microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR) that has progressed following prior treatment [167]. In one study report, two cases of recurrent POLE ultra-mutated and MSH6 hyper-mutated endometrial carcinoma refractory to conventional surgical and chemotherapeutic treatments were effectively treated with the anti-PD-1 monoclonal antibody Nivolumab [168].
Numerous other inhibitors are tested in preclinical studies and clinical trials. Aside from Avelumab and Durvalumab, there are promising immune checkpoint inhibitors targeting PD-L1 in EC. Studies of Avelumab effects in patients with recurrent/persistent EC were performed in a trial study (NCT02912572) [169]. The effects of Durvalumab were analyzed in MSI ECs in a phase II PHAE DRA trial (ANZGOG1601) [170].
Another method of immunotherapy involves the use of retroviruses or lentiviruses to modify T cells by introducing chimeric antigen receptors (CARs). Research on CAR T-cell therapy in EC is only carried out in preclinical studies, and the validation of the results, even if they are encouraging, requires further studies [171].
Cancer vaccines are a form of immunotherapy that aims to activate the immune system to recognize and destroy cancer cells. In the case of EC, clinical trials are exploring various cancer vaccines to assess their safety, effectiveness, and potential use in treating the disease. Therapeutic cancer vaccines are composed of tumor/immune cell vaccines; peptide vaccines; and genetic vaccines that consist of DNA, RNA and viral vaccines. There are various peptide-based cancer vaccines that target tumor-associated antigens like NY-ESO-1, MAGE-A, and WT1. These vaccines are currently under clinical trial for treating EC [172,173]. Cancer vaccines are a promising approach for treating EC. Ongoing research and clinical trials are working towards optimizing the design and delivery of these vaccines, identifying predictive biomarkers and developing personalized treatment strategies to improve patient care and outcomes in EC. It is crucial to evaluate cancer vaccines in combination with other immunotherapies, targeted therapies, or standard treatments to explore their potential synergistic effects and develop effective treatment approaches for patients with EC. In order to address the challenges that arise in immunotherapy, it is essential to create personalized treatment strategies to achieve individualized precision treatment. Additionally, it is important to reduce the immunosuppressive environment in patients, particularly the immunosuppressive microenvironment within tumor tissues.
Therapy combinations involving immune checkpoint inhibitors, targeted therapies, chemotherapy, and other immunomodulatory agents are being explored in clinical trials for EC treatment. These approaches aim to improve the antitumor immune response, overcome immunotherapy resistance, and enhance treatment outcomes for patients with advanced or recurrent EC. Ongoing research and clinical trials are crucial to improve outcomes and patient care in EC by optimizing the selection of appropriate combination therapies, identifying predictive biomarkers, and developing personalized treatment strategies (Table 1) [174].

Table 1.
Therapeutic combinations strategies in endometrial cancer.
With increased clinical experience and supported by advanced scientific research, tumor immunotherapy can be better understood, leading to an innovative and safe approach to treating cancer patients.
4.2.5. Targeting miRNAs
MicroRNAs (miRNAs) are small, non-coding RNA molecules that play an important role in the regulation of gene expression. Aberrant expression of specific miRNAs has been identified in endometrial cancer, and researchers are currently exploring the potential of using miRNA-based therapies as a treatment approach. There are several approaches to the development and use of miRNA-based therapies. Firstly, synthetic miRNA mimics can be used to restore the expression of tumor-suppressive miRNAs that are downregulated in EC. Secondly, miRNA inhibitors, such as antisense oligonucleotides or antagomirs, can suppress the expression of oncogenic miRNAs that are overexpressed in EC. Lastly, miRNA delivery vehicles, such as lipid nanoparticles or viral vectors, can deliver miRNA mimics or inhibitors to tumor cells in vivo. These delivery systems can enhance the stability and specificity of miRNA-based therapies, improving their efficacy in treating EC [184,185].
Several miRNAs, including miR-34a, miR-182 and miR-182, are highly expressed in endometrial tumor cells. Studies have shown that restoring the expression of miR-34a, a tumor suppressor miRNA, can prevent cell proliferation, induce apoptosis, and suppress tumor growth in experimental EC models [186]. Additionally, inhibiting the expression of miR-182 suppresses cell proliferation, migration, and invasion, while inhibiting miR-21 expression can enhance apoptosis and increase the sensitivity of endometrial tumor cells to chemotherapy. Ongoing preclinical and clinical studies are exploring the use of miR-21 inhibitors alone or in combination with standard therapies [187,188]. However, it is important to acknowledge that while miRNA-based therapies show promise, further research is needed to optimize their efficacy, safety, and clinical utility in the treatment of EC.
In conclusion, targeted therapy drugs are commonly used alongside additional treatments such as chemotherapy, hormonal therapy, or radiation therapy, depending on the specific cancer characteristics and individual patient needs. The selection of targeted therapy drugs and treatment plans is based on biomarker testing results, tumor characteristics, and clinical trial data. The aim is to create a personalized treatment plan that provides the best possible outcome for the patient.
Ongoing research may lead to the approval of new targeted therapy drugs for treating endometrial cancer. Clinical trials provide an opportunity to evaluate new drugs, treatment combinations, and strategies that are not yet widely accessible.
5. Conclusions
This review describes the complexity of endometrial cancers, including various molecular mechanisms, a series of biomarkers, and their critical role in guiding targeted therapies. Multiple subclones in a single tumor can complicate treatment, as different subclones may respond differently to therapies. Therefore, knowing the genomic and epigenomic profile of tumors is essential in guiding treatment plans. Also, combining targeted therapies based on molecular profiles (e.g., PI3K inhibitors with hormone therapy in the CNL subtype) may improve treatment efficacy and overcome resistance mechanisms. In addition, treatment plans can be monitored based on changes in biomarkers and emerging resistance patterns to optimize therapeutic outcomes. Thus, understanding this complex landscape enables the development of personalized treatment strategies and opens up new future research directions in order to improve EC management and increase the quality of life of patients.
Author Contributions
M.B. and I.-S.B. created the outline and drafted the manuscript. M.M. and N.R. designed the figures and tables, and V.R. searched the literature for inclusion in the study, which was then checked and reviewed by C.M. and M.T.N., along with the critical revision of the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Felix, A.S.; Brinton, L.A. Cancer Progress and Priorities: Uterine Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.F. Pathology of Endometrial Carcinoma. In Molecular Genetics of Endometrial Carcinoma, Advances in Experimental Medicine and Biology; Ellenson, H.L., Ed.; Springer: Cham, Switzerland, 2017; Volume 943, pp. 75–96. [Google Scholar]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Mortality Database. Health Statistics and Information Systems. Geneva, Switzerland. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 12 September 2023).
- Frias-Gomez, J.; Benavente, Y.; Ponce, J.; Brunet, J.; Ibáñez, R.; Peremiquel-Trillas, P.; Baixeras, N.; Zanca, A.; Piulats, J.M.; Aytés, Á.; et al. Sensitivity of cervico-vaginal cytology in endometrial carcinoma: A systematic review and meta-analysis. Cancer Cytopathol. 2020, 128, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Fischerova, D.; Valentin, L.; Testa, A.C.; Franchi, D.; Sladkevicius, P.; Frühauf, F.; Lindqvist, P.G.; Mascilini, F.; Fruscio, R.; et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: Prospective multicenter study. Ultrasound Obstet. Gynecol. 2018, 51, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Madár, I.; Szabó, A.; Vleskó, G.; Hegyi, P.; Ács, N.; Fehérvári, P.; Kói, T.; Kálovics, E.; Szabó, G. Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 907. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, 477. [Google Scholar] [CrossRef] [PubMed]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of metaanalyses of observational studies. BMJ 2015, 350, 7607. [Google Scholar] [CrossRef]
- Kalliala, I.; Markozannes, G.; Gunter, M.; Paraskevaidis, E.; Gabra, H.; Mitra, A.; Terzidou, V.; Bennett, P.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Obesity and gynaecological and obstetrical conditions: An umbrella review of the literature. BMJ 2017, 359, 4511. [Google Scholar] [CrossRef] [PubMed]
- Lavafian, A.; Pezeshki, P.S.; Rezaei, N. Investigation of the female infertility risk associated with anti-cancer therapy. Clin. Transl. Oncol. 2023, 25, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Chudecka-Głaz, A.; Pityński, K.; Baranowski, W.; Markowska, J.; Sawicki, W. Endometrial Cancer Management in Young Women. Cancers 2022, 14, 1922. [Google Scholar] [CrossRef] [PubMed]
- Britton, H.; Huang, L.; Lum, A.; Leong, S.; Shum, K.; Kale, M.; Burleigh, A.; Senz, J.; Yang, W.; McConechy, M.; et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol. Oncol. 2019, 153, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Două tipuri patogenetice de carcinom endometrial. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA Classification of endometrial cancer: The place of carcinosarcoma. Pathol. Oncol. Res. 2020, 26, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, H.; Banno, K.; Yanokura, M.; Takeda, T.; Iijima, M.; Nakamura, K.; Iida, M.; Adachi, M.; Watanabe, K.; Matoba, Y.; et al. New use of microsatellite instability analysis in endometrial cancer. Oncol. Lett. 2017, 14, 3297–3301. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Yang, X.; Guo, L.; Liu, B.; Lin, J.; Liang, H.; Sun, J.; Zhang, C.; Ye, K. MSIsensor-pro: Fast, accurate, and matched-normal-sample-free detection of microsatellite instability. Genom. Proteom. Bioinf. 2020, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Benz, C.C.; Yau, C.; Laird, P.W.; Ding, L.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Snez, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; De Marco, M.P.; Besharat, A.R.; Costanzi, F. Endocrine Disruptors and Endometrial Cancer: Molecular Mechanisms of Action and Clinical Implications, a Systematic Review. Int. J. Mol. Sci. 2022, 23, 2956. [Google Scholar] [CrossRef] [PubMed]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Saccone, G.; Campanino, M.R.; Mollo, A.; De Placido, G.; Insabato, L.; Zullo, F. Loss of PTEN expression as diagnostic marker of endometrial precancer: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2019, 98, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Cacalano, N.; Zhu, H.; Liu, Q.; Xie, M.; Kamrava, M.; Konecny, G.; Jin, S. Oncogenic Y68 frame shift mutation of PTEN represents a mechanism of docetaxel resistance in endometrial cancer cell lines. Sci. Rep. 2019, 9, 2111. [Google Scholar] [CrossRef] [PubMed]
- Tresa, A.; Sambasivan, S.; Rema, P.; Dinesh, D.; Sivaranjith, J.; Nair, S.P.; Mathew, A.; Ammu, J.V.; Kumar, A. Clinical Profile and Survival Outcome of Endometrial Cancer with p53 Mutation. Indian J. Surg. Oncol. 2022, 13, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.L.; Ardighieri, L.; Ayhan, A.; Kuo, K.T.; Wu, C.H.; Wang, T.L.; Shih, I.M. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am. J. Surg. Pathol. 2013, 37, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Gutsche, K.; Dedes, K.J.; Fink, D.; Stucki, M.; Imesch, P. Loss of ARID1A expression sensitizes cancer cells to PI3K and AKT inhibition. Oncotarget 2014, 5, 5295–5303. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; McConechy, M.K.; Meng, B.; McIntyre, J.B.; Ewanowich, C.; Gilks, C.B.; Huntsman, D.G.; Köbel, M.; Lee, C.H. Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology 2015, 66, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; ter Haar, N.T.; Seeber, L.M.; v Diest, P.J.; Hes, F.J.; Vasen, H.F.; Nout, R.A.; Creutzberg, C.L.; Morreau, H.; Smit, V.T. Loss of ARID1A expression and its relationship with PI3K-AKT pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod. Pathol. 2013, 26, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bao, W.; Sang, Z.; Yang, Y.; Lu, M.; Xi, X. Microarray pathway analysis indicated that mitogen-activated protein kinase/extracellular signal-regulated kinase and insulin growth factor 1 signaling pathways were inhibited by small interfering RNA against AT-rich interactive domain 1A in endometrial cancer. Oncol. Lett. 2018, 15, 1829–1838. [Google Scholar] [PubMed]
- McConechy, M.K.; Ding, J.; Cheang, M.C.; Wiegand, K.; Senz, J.; Tone, A.; Yang, W.; Prentice, L.; Tse, K.; Zeng, T.; et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012, 228, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Guerini Rocco, E.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Colonna, F.; Calapà, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Byron, S.A.; Gartside, M.; Powell, M.A.; Wellens, C.L.; Gao, F.; Mutch, D.G.; Goodfellow, P.J.; Pollock, P.M. FGFR2 Point Mutations in 466 Endometrioid Endometrial Tumors: Relationship with MSI, KRAS, PIK3CA, CTNNB1 Mutations and Clinicopathological Features. PLoS ONE 2012, 7, 30801. [Google Scholar] [CrossRef]
- Ma, X.; Ma, C.; Wang, J. Endometrial Carcinogenesis and Molecular Signaling Pathways. Am. J. Mol. Biol. 2014, 4, 134–149. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Mascolo, M.; D’Alessandro, P.; Arduino, B.; Mollo, A.; Insabato, L.; Zullo, F. Nuclear Expression of β-Catenin in Endometrial Hyperplasia as Marker of Premalignancy. APMIS 2019, 127, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Płoska, A. Metalloproteinases in Endometrial Cancer-Are They Worth Measuring? Int. J. Mol. Sci. 2021, 22, 12472. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.K.; Najjar, O.; Damast, S.; Blakaj, A.; Tymon-Rosario, J.; Shahi, M.; Santin, A.; Klein, M.; Dolan, M.; Cimino-Mathews, A.; et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: A multi-institutional cohort study. Gynecol. Oncol. 2020, 159, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, M.; Janssens, V. Targeted Therapies in Type II Endometrial Cancers: Too Little, but Not Too Late. Int. J. Mol. Sci. 2018, 19, 2380. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.M.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, P.P.; Ghozali, S.A.S.; Buchanan, D.D.; Pisano, M.I.; Reece, J.C. Risk of cancer in individuals with Lynch-like syndrome and their families: A systematic review. J. Cancer Res. Clin. Oncol. 2023, 149, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Pearlman, R.; de la Chapelle, A.; Pritchard, C.C.; Zhao, W.; Jones, D.; Yilmaz, A.; Chen, W.; Frankel, W.L.; Suarez, A.A.; et al. Double somatic mismatch repair gene pathogenic variants as common as Lynch syndrome among endometrial cancer patients. Gynecol. Oncol. 2021, 160, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Dao, F.; Levine, D.A. Angiogenesis in endometrial carcinoma: Therapies and biomarkers, current options, and future perspectives. Gynecol. Oncol. 2021, 160, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; O’Donnell, A.L.; Mohamed, S.; Mousa, S.; Dandona, P. Overexpression of estrogen receptor-alpha in the endometrial carcinoma cell line Ishikawa: Inhibition of growth and angiogenic factors. Gynecol. Oncol. 2004, 95, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Dallal, C.M.; Lacey, J.V., Jr.; Pfeiffer, R.M.; Bauer, D.C.; Falk, R.T.; Buist, D.S.; Cauley, J.A.; Hue, T.F.; LaCroix, A.Z.; Tice, J.A.; et al. Estrogen Metabolism and Risk of Postmenopausal Endometrial and Ovarian Cancer: The B∼FIT Cohort. Horm. Cancer 2016, 7, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Zeleniuch-Jacquotte, A.; Shore, R.E.; Afanasyeva, Y.; Lukanova, A.; Sieri, S.; Koenig, K.L.; Idahl, A.; Krogh, V.; Liu, M.; Ohlson, N.; et al. Postmenopausal circulating levels of 2- and 16α-hydroxyestrone and risk of endometrial cancer. Br. J. Cancer 2011, 105, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Gompel, A. Progesterone and endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Utsunomiya, H.; Yaegashi, N.; Sasano, H. Biological roles of estrogen and progesterone in human endometrial carcinoma—New developments in potential endocrine therapy for endometrial cancer. Endocr. J. 2007, 54, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, D.; Gong, C.; Zhang, F.; He, J.; Zhang, W.; Zhao, Y.; Sun, J. Prognostic role of hormone receptors in endometrial cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2015, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Yetkin-Arik, B.; Kastelein, A.W.; Klaassen, I.; Jansen, C.; Latul, Y.P.; Vittori, M.; Biri, A.; Kahraman, K.; Griffioen, A.W.; Amant, F.; et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, Y.; Sun, X.; Wang, Y. New insights for gynecological cancer therapies: From molecular mechanisms and clinical evidence to future directions. Cancer Metastasis Rev. 2023, 42, 891–925. [Google Scholar] [CrossRef] [PubMed]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Cornel, K.M.C.; Wouters, K.; Van de Vijver, K.K.; van der Wurff, A.A.M.; van Engeland, M.; Kruitwagen, R.F.P.; Pijnenborg, J.M.A. Gene promoter methylation in endometrial carcinogenesis. Pathol. Oncol. Res. 2019, 25, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Multinu, F.; Chen, J.; Madison, J.D.; Torres, M.; Casarin, J.; Visscher, D.; Shridhar, V.; Bakkum-Gamez, J.; Sherman, M.; Wentzensen, N.; et al. Analysis of DNA methylation in endometrial biopsies to predict risk of endometrial cancer. Gynecol. Oncol. 2020, 156, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Nagasaka, K.; et al. Oncogenic histone methyltransferase EZH2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Filiaci, V.L.; Walker, J.L.; Holman, L.L.; Hill, E.K.; Moore, R.G.; Ring, K.L.; Pearl, M.L.; Muller, C.Y.; Kushnir, C.L.; et al. A surgical window trial evaluating medroxyprogesterone acetate with or without entinostat in patients with endometrial cancer and validation of biomarkers of cellular response. Clin. Cancer Res. 2021, 27, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Liu, M.; Yao, B.; Jiang, H.; Yang, D.; Li, Q.; Zeng, X.; Wang, Y.; Cao, J.; Deng, Y.; et al. TCF3 is epigenetically silenced by EZH2 and DNMT3B and functions as a tumor suppressor in endometrial cancer. Cell Death Differ. 2021, 28, 3316–3328. [Google Scholar] [CrossRef] [PubMed]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Zhang, G.; Hwa, Y.L.; Li, J.; Dowdy, S.C.; Jiang, S.W. Nonhistone protein acetylation as cancer therapy targets. Expert Rev. Anticancer Ther. 2010, 10, 935–954. [Google Scholar] [CrossRef] [PubMed]
- Żyła, M.M.; Kostrzewa, M.; Litwińska, E.; Szpakowski, A.; Wilczyński, J.R.; Stetkiewicz, T. The role of angiogenic factors in endometrial cancer. Prz. Menopauzalny 2014, 13, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Vera, Y.M.; Gallardo-Rincón, D.; Ruíz-García, E.; Silva-Cázares, M.B.; de la Peña-Cruz, C.S.; López-Camarillo, C. The Role of Hypoxia in Endometrial Cancer. Curr. Pharm. Biotechnol. 2022, 23, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Taylor, A.; Showeil, R.; Trivedi, P.; Horimoto, Y.; Bagwan, I.; Ewington, L.; Lam, E.W.-F.; El-Bahrawy, M.A. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine 2014, 68, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kuang, K.; Du, J.; Eymin, B.; Jia, T. Far beyond anti-angiogenesis: Benefits for anti-basicFGF therapy in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, H.; Hu, W.; Li, S.; Yu, J. Over-Expression of Platelet-Derived Growth Factor-D Promotes Tumor Growth and Invasion in Endometrial Cancer. Int. J. Mol. Sci. 2014, 15, 4780–4794. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Mikelis, C.M. Targeting the Angiopoietin/Tie Pathway: Prospects for Treatment of Retinal and Respiratory Disorders. Drugs 2021, 81, 1731–1749. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Kaftanovskaya, E.M.; Manresa, C.; Barbara, A.M.; Poppiti, R.J.; Tan, Y.; Agoulnik, A.I. Constitutive Notch Signaling Causes Abnormal Development of the Oviducts, Abnormal Angiogenesis, and Cyst Formation in Mouse Female Reproductive Tract. Biol. Reprod. 2016, 94, 1–12. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, H.; Chen, J.; Lei, J.; Zhao, M.; Ji, B.; Chen, Y.; Qin, S.; Gao, Q. Research Progress of DNA Methylation in Endometrial Cancer. Biomolecules 2022, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Markouli, M.; Strepkos, D.; Basdra, E.K.; Papavassiliou, A.G.; Piperi, C. Prominent Role of Histone Modifications in the Regulation of Tumor Metastasis. Int. J. Mol. Sci. 2021, 22, 2778. [Google Scholar] [CrossRef] [PubMed]
- Indumati, S.; Apurva, B.; Gaurav, G.; Nehakumari, S.; Nishant, V. The Role of MicroRNAs in Development of Endometrial Cancer: A Literature Review. J. Reprod. Infertil. 2023, 24, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Favier, A.; Rocher, G.; Larsen, A.K.; Delangle, R.; Uzan, C.; Sabbah, M.; Castela, M.; Duval, A.; Mehats, C.; Canlorbe, G. MicroRNA as epigenetic modifiers in endometrial cancer: A systematic review. Cancers 2021, 13, 1137. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Barkley, J.E.; Bhattarai, B.; Firouzi, K.; Monk, B.J.; Coonrod, D.V.; Zenhausern, F. Identification of Endometrial Cancer-Specific microRNA Biomarkers in Endometrial Fluid. Int. J. Mol. Sci. 2023, 24, 8683. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Gullo, G.; Basile, G.; Gulia, C.; Porrello, A.; Cucinella, G.; Marinelli, E.; Zaami, S. Circulating miRNAs as a Tool for Early Diagnosis of Endometrial Cancer-Implications for the Fertility-Sparing Process: Clinical, Biological, and Legal Aspects. Int. J. Mol. Sci. 2023, 24, 11356. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Dobrotwir, A.; McNally, O.; Abu-Rustum, N.R.; Narayan, K. Role of imaging in the routine management of endometrial cancer. Int. J. Gynaecol. Obstet. 2018, 143, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Blomqvist, L. Imaging in endometrial cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Simcock, B.; Narayan, K.; Drummond, E.; Bernshaw, D.; Wells, E.; Hicks, R.J. The Role of Positron Emission Tomography/Computed Tomography in Planning Radiotherapy in Endometrial Cancer. Int. J. Gynecol. Cancer 2015, 25, 645–649. [Google Scholar] [CrossRef]
- Njoku, K.; Barr, C.E.; Crosbie, E.J. Current and Emerging Prognostic Biomarkers in Endometrial Cancer. Front. Oncol. 2022, 12, 890908. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Barr, C.E.; Crosbie, E.J. HE4 as a Biomarker for Endometrial Cancer. Cancers 2021, 13, 4764. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Sun, X.; Li, B.; Ming, L. Clinical significance of serum HE4, CA125, CA724, and CA19-9 in patients with endometrial cancer. Technol. Cancer Res. Treat. 2017, 16, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Lopez-Gil, C.; Campoy, I.; Vallve, J.; Coll, E.; Cabrera, S.; Ramon, Y.; Cajal, S.R.Y.; Matias-Guiu, X.; Van Oostrum, J.; et al. Advances in endometrial cancer protein biomarkers for use in the clinic. Expert. Rev. Proteom. 2018, 15, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Kemik, P.; Saatli, B.; Yıldırım, N.; Kemik, V.D.; Deveci, B.; Terek, M.C.; Koçtürk, S.; Koyuncuoğlu, M.; Saygılı, U. Diagnostic and prognostic values of preoperative serum levels ofYKL-40, HE-4 and DKK-3 in endometrial cancer. Gynecol. Oncol. 2016, 140, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Capriglione, S.; Plotti, F.; Miranda, A.; Ricciardi, R.; Scaletta, G.; Aloisi, A.; Guzzo, F.; Montera, R.; Angioli, R. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: A single-center controlled study. Tumor Biol. 2015, 36, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Dewan, R.; Dewan, A.; Hare, S.; Bhardwaj, M.; Mehrotra, K. Diagnostic performance of serum human epididymis protein 4 in endometrial carcinoma: A pilot study. J. Clin. Diagn. Res. 2017, 11, XC01–XC05. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Capriglione, S.; Scaletta, G.; Aloisi, A.; Miranda, A.; Nardone, C.D.C.; Terranova, C.; Plotti, F. The role ofHE4 in endo metrial cancer recurrence: How to choose the optimal follow-up program. Tumor Biol. 2016, 37, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, S.; Huang, L.; Zhang, S. Clinical significance of serum DKK-1 in patients with gynecological cancer. Int. J. Gynecol. Cancer 2009, 19, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Cello, A.D.; Sanzo, M.D.; Perrone, F.M.; Santamaria, G.; Rania, E.; Angotti, E.; Venturella, R.; Mancuso, S.; Zullo, F.; Cuda, G.; et al. DJ-1 is a reliable serum biomarker for discriminating high-risk endometrial cancer. Tumor Biol. 2017, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benati, M.; Montagnana, M.; Danese, E.; Paviati, E.; Giudici, S.; Ruzzenente, O.; Franchi, M.; Lippi, G. The clinical significance of DJ-1 and HE4 in patients with endometrial cancer. J. Clin. Lab. Anal. 2018, 32, e22223. [Google Scholar] [CrossRef] [PubMed]
- Al Maghrabi, H.; Gomaa, W.; Al-Maghrabi, J. Increased osteopontin expression in endometrial carcinoma is associated with better survival outcome. Ginekol. Pol. 2020, 91, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Słabuszewska-Jóźwiak, A.; Lukaszuk, A.; Janicka-Kośnik, M.; Wdowiak, A.; Jakiel, G. Role of Leptin and Adiponectin in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 5307. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Fornsaglio, J.; Dogan, S.; Hedau, S.; Naik, D.; De, A. Gynaecological cancers and leptin: A focus on the endometrium and ovary. Facts Views Vis. Obgyn 2018, 10, 5–18. [Google Scholar] [PubMed]
- Kristofic, I.; Redzovic, A.; Laskarin, G.; Eminovic, S.; Haller, H.; Rukavina, D. Role of tumor-associated glycoprotein-72 in the progression of endometrial adenocarcinoma: A proposed study. Med. Hypotheses 2015, 84, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef] [PubMed]
- McCallion, A.; Nasirzadeh, Y.; Lingegowda, H.; Miller, J.E.; Khalaj, K.; Ahn, S.; Monsanto, S.P.; Bidarimath, M.; Sisnett, D.J.; Craig, A.W.; et al. Estrogen mediates inflammatory role of mast cells in endometriosis pathophysiology. Front. Immunol. 2022, 13, 961599. [Google Scholar] [CrossRef] [PubMed]
- Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, R.; Sienkiewicz, A.; Kinalski, M.; Terlikowski, S.J. Clinical value of selected markers of angiogenesis, inflammation, insulin resistance and obesity in type 1 endometrial cancer. BMC Cancer 2020, 20, 921. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Gorsia, D.N.; Collins, A.; Sandhu, P.; Foreman, N.; Gore, A.; Gore, A.; Wood, J.; Kent, C.; Silcock, L.; et al. Utility of Circulating Tumor DNA for Detection and Monitoring of Endometrial Cancer Recurrence and Progression. Cancers 2020, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, A.M.; Luthra, R.; Mehrotra, M.; Chen, W.; Barkoh, B.A.; Hu, P.; Zhang, W.; Broaddus, R.R. Targeted Next-Generation Sequencing of Endometrial Cancer and Matched Circulating Tumor DNA: Identification of Plasma-Based, Tumor-Associated Mutations in Early Stage Patients. Mod. Pathol. 2019, 32, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Capasso, I.; Garzon, S.; Kumar, S.; Weaver, A.L.; Gree, M.M.; De Vitis, L.A.; Uccella, S.; Petersen, I.; Glaser, G.; Langstraat, C.; et al. Prognostic factors in patients with endometrial cancer with isolated lymphatic recurrence. Int. J. Gynecol. Cancer 2023, 33, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.E.; Yoo, J.G.; Lee, S.J.; Yoon, J.H.; Park, D.C.; Kim, S.I. Prognostic significance of histological grade in low-risk endometrial cancer. Int. J. Med. Sci. 2022, 19, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, L.; Restaino, S.; Zannoni, G.F.; Vizzielli, G.; Chiantera, V.; Cappuccio, S.; Gioè, A.; La Fera, E.; Dinoi, G.; Angelico, G.; et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J. Gynecol. Oncol. 2021, 32, e11. [Google Scholar] [CrossRef] [PubMed]
- Çakır, C.; Kılıç, İ.Ç.; Yüksel, D.; Karyal, Y.A.; Üreyen, I.; Boyraz, G.; Durmuş, Y.; Gültekin, M.; Özgül, N.; Karalök, M.A.; et al. Does tumor size have prognostic value in patients undergoing lymphadenectomy in endometrioid-type endometrial cancer confined to the uterine corpus? Turk. J. Med. Sci. 2019, 49, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Yang, X.; Yu, Q.; Xu, X.; Zou, G.; Zhang, X. Association of Myometrial Invasion with Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies with 68,870 Patients. Front. Oncol. 2021, 11, 762329. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, A.; Minaguchi, T.; Fujieda, K.; Hosokawa, Y.; Nishida, K.; Shikama, A.; Tasaka, N.; Sakurai, M.; Ochi, H.; Satoh, T. Abnormal accumulation of p53 predicts radioresistance leading to poor survival in patients with endometrial carcinoma. Oncol. Lett. 2019, 18, 5952–5958. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.; Sivalingam, V.N.; Bolton, J.; McVey, R.; Nickkho-Amiry, M.; Powell, M.E.; Leary, A.; Nijman, H.W.; Nout, R.A.; Bosse, T.; et al. Ki-67 in endometrial cancer: Scoring optimization and prognostic relevance for window studies. Mod. Pathol. 2017, 30, 459–468. [Google Scholar] [CrossRef]
- Kato, M.; Takano, M.; Miyamoto, M.; Sasaki, N.; Goto, T.; Tsuda, H.; Furuya, K. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J. Gynecol. Oncol. 2015, 26, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Addante, F.; d’Amati, A.; Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Travaglino, A.; Raffone, A.; D’Alessandris, N.; Scaglione, G.; et al. Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 1056. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-de Lara, S.A.; Garza-Veloz, I.; Berthaud-González, B.; Martinez-Fierro, M.L. Circulating and Endometrial Tissue microRNA Markers Associated with Endometrial Cancer Diagnosis, Prognosis, and Response to Treatment. Cancers 2023, 15, 2686. [Google Scholar] [CrossRef]
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azaïs, H.; Bendifallah, S.; Daraï, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The Use of microRNAs in the Management of Endometrial Cancer: A Meta-Analysis. Cancers 2019, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor sup-pressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.T.; Hsu, S.T.; Sun, L.; Hwang, S.F.; Liu, C.K.; Shih, Y.H.; Chen, M.J.; Li, H.N.; Wang, J.S.; Wen, M.C.; et al. Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer. J. Clin. Med. 2023, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-infiltrating lymphocytes: From prognosis to treatment selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef]
- Presti, D.; Dall’Olio, F.G.; Besse, B.; Ribeiro, J.M.; Di Meglio, A.; Soldato, D. Tumor infil trating lymphocytes (TILs) as a predictive biomarker of response to checkpoint blockers in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2022, 177, 103773. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, A.; Radeva, M.; Rose, P.G. Surgical lymph node assessment influences adjuvant therapy in clinically apparent stage I endometrioid endometrial carcinoma, meeting Mayo criteria for lymphadenectomy. J. Surg. Oncol. 2021, 123, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, D.M.; Cope, A.; Riaz, I.B.; Petersen, I.; Cilby, W.; Langstraat, C.; Glaser, G.; Kumar, A.; Cappuccio, S.; Murad, M.H.; et al. External beam radiotherapy versus vaginal brachytherapy in patients with stage II endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2020, 30, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Yuce Sari, S.; Yavas, G.; Oymak, E.; Birgi, S.D.; Yigit, E.; Guler, O.C.; Gultekin, M.; Akyurek, S.; Yildiz, F. Outcome and safety analysis of endometrial cancer patients treated with postoperative 3D-conformal radiotherapy or intensity modulated radiotherapy. Acta Oncol. 2021, 60, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Spirtos, N.M.; Enserro, D.; Homesley, H.D.; Gibbons, S.K.; Cella, D.; Morris, R.T.; DeGeest, K.; Lee, R.B.; Miller, D.S. The addition of paclitaxel to doxorubicin and cisplatin and volume-directed radiation does not improve overall survival (OS) or long-term recurrence-free survival (RFS) in advanced endometrial cancer (EC): A randomized phase III NRG/Gynecologic Oncology Group (GOG) study. Gynecol. Oncol. 2019, 154, 13–21. [Google Scholar] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III–IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020, 26, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.N.; Amir, E.; MacKay, H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Soliman, P.T.; Westin, S.N.; Iglesias, D.A.; Fellman, B.M.; Yuan, Y.; Zhang, Q.; Yates, M.S.; Broaddus, R.R.; Slomovitz, B.M.; Lu, K.H.; et al. Everolimus, Letrozole, and Metformin in Women with Advanced or Recurrent Endometrioid Endometrial Cancer: A Multi-Center, Single Arm, Phase II Study. Clin. Cancer Res. 2020, 26, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Tangen, I.L.; Onyango, T.B.; Kopperud, R.; Berg, A.; Halle, M.K.; Øyan, A.M.; Werner, H.M.; Trovik, J.; Kalland, K.H.; Salvesen, H.B.; et al. Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget 2016, 7, 49289–49298. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Habu, Y.; Kobayashi, T.; Kawarai, Y.; Ishikawa, H.; Usui, H.; Shozu, M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J. Gynecol. Oncol. 2019, 30, e90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cheng, Y.; Zhang, Q.; Li, X.; Zhou, J.; Wang, J.; Wei, L. ATX-LPA axis facilitates estrogen-induced endometrial cancer cell proliferation via MAPK/ERK signaling pathway. Mol. Med. Rep. 2018, 17, 4245–4252. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Filiaci, V.L.; Coleman, R.L.; Walker, J.L.; Taub, M.C.; Finkelstein, K.A.; Moroney, J.W.; Fleury, A.C.; Muller, C.Y.; Holman, L.L.; et al. A randomized phase II (RP2) trial of everolimus and letrozole (EL) or hormonal therapy (medroxyprogesterone acetate/tamoxifen, PT) in women with advanced, persistent or recurrent endometrial carcinoma (EC): A GOG Foundation study. Gynecol. Oncol. 2022, 164, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Gorchev, G.; Harter, P.; Wimberger, P.; Stähle, A.; Hanker, L.; Hilpert, F.; Beckmann, M.W.; Dall, P.; Gründker, C.; et al. Efficacy and safety of AEZS-108 (LHRH agonist linked to doxorubicin) in women with advanced or recurrent endometrial cancer expressing LHRH receptors: A multicenter phase 2 trial (AGO-GYN5). Int. J. Gynecol. Cancer 2014, 24, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Schottinger, J.E.; Shi, J.; Chung, J.; Haque, R. Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer 2015, 121, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Sig Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Curigliano, G.; Delord, J.P.; Harb, W.; Azaro, A.; Han, Y.; Wilke, C.; Donnet, V.; Sellami, D.; Beck, T. A Phase Ib, open-label, dose-finding study of Alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Jiang, Y.; Yates, M.S.; Soliman, P.T.; Johnston, T.; Nowakowski, M.; Levenback, C.; Zhang, Q.; Ring, K.; Munsell, M.F.; et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015, 33, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Filiaci, V.; Dizon, D.S.; Carlson, J.W.; Powell, M.A.; Secord, A.A.; Tewari, K.S.; Bender, D.P.; O’Malley, D.M.; Stuckey, A.; et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018, 150, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hanker, A.B.; Kaklamani, V.; Arteaga, C.L. Challenges for the Clinical Development of PI3K Inhibitors: Strategies to Improve Their Impact in Solid Tumors. Cancer Discov. 2019, 9, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Roncolato, F.; Lindemann, K.; Willson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019, 10, CD012160. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.M.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.F.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): Results of the Phase 3 SOLAR-1 trial. Ann. Oncol. 2018, 29, viii709. [Google Scholar] [CrossRef]
- Heudel, P.; Frenel, J.S.; Dalban, C.; Bazan, F.; Joly, F.; Arnaud, A.; Abdeddaim, C.; Chevalier-Place, A.; Augereau, P.; Pautier, P.; et al. Safety and Efficacy of the mTOR Inhibitor, Vistusertib, Combined with Anastrozole in Patients with Hormone Receptor-Positive Recurrent or Metastatic Endometrial Cancer: The VICTORIA Multicenter, Open-label, Phase 1/2 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.M.; Backes, F.J. Do Not Forget about Hormonal Therapy for Recurrent Endometrial Cancer: A Review of Options, Updates, and New Combinations. Cancers 2023, 15, 1799. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Mirza, M.R.; Lortholary, A.; Cibula, D.; Walther, A.; Savarese, A.; Barretina-Ginesta, M.P.; Ortaç, F.; Papadimitriou, C.; Bodnar, L.; et al. ENGOT-en11/GOG-3053/KEYNOTE-B21: Phase 3 study of pembrolizumab or placebo in combination with adjuvant chemotherapy with/without radiotherapy in patients with newly diagnosed high-risk endometrial cancer. J. Clin. Oncol. 2021, 39, TPS5608. [Google Scholar] [CrossRef]
- Bradley, H.W.; Hayes, M.P.; Taylor, N.; Rader, J.; Bishop, E.; Hopp, E.; Mcalarnen, L.A.; Liegl, M.; Simpson, P.; Uyar, D. An open label, nonrandomized, multisite phase II trial combining bevacizumab, atezolizumab, and rucaparib for the treatment of previously treated recurrent and progressive endometrial cancer. J. Clin. Oncol. 2022, 40, 5510. [Google Scholar] [CrossRef]
- Oza, A.M.; Selle, F.; Davidenko, I.; Korach, J.; Mendiola, C.; Pautier, P.; Chmielowska, E.; Bamias, A.; DeCensi, A.; Zvirbule, Z.; et al. Efficacy and Safety of Bevacizumab-Containing Therapy in Newly Diagnosed Ovarian Cancer: ROSiA Single-Arm Phase 3B Study. Int. J. Gynecol. Cancer 2016, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Sill, M.W.; Lankes, H.A.; Fader, A.N.; Finkler, N.J.; Hoffman, J.S.; Rose, P.G.; Sutton, G.P.; Drescher, C.W.; McMeekin, D.S.; et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2012, 127, 538–543. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.S.; Sill, M.W.; Benbrook, D.; Darcy, K.M.; Stearns-Kurosawa, D.J.; Eaton, L.; Yamada, S.D. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: A Gynecologic Oncology Group study. Gynecol. Oncol. 2007, 105, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lheureux, S.; Oza, A.M. Treatment strategies for endometrial cancer: Current practice and perspective. Curr. Opin. Obstet. Gynecol. 2017, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Roškar, L.; Roškar, I.; Rižner, T.L.; Smrkolj, Š. Diagnostic and Therapeutic Values of Angiogenic Factors in Endometrial Cancer. Biomolecules 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Moughan, J.; Miller, B.E.; Xiao, Y.; Jhingran, A.; Portelance, L.; Bosch, W.R.; Matulonis, U.A.; Horowitz, N.S.; Mannel, R.S.; et al. NRG Oncology/RTOG 0921: A phase 2 study of postoperative intensity-modulated radiotherapy with concurrent cisplatin and bevacizumab followed by carboplatin and paclitaxel for patients with endometrial cancer. Cancer 2015, 121, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Taylor, M.H.; Lee, C.H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Gray, C.; Argáez, C. Trastuzumab Combination and Monotherapy for HER2 Advanced or Recurrent Uterine or Endometrial Cancer: A Review of Clinical Effectiveness and Cost-Effectiveness; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020.
- Mao, S.; Desravines, N.; Zarei, S.; Viswanathan, A.N.; Fader, A.N. Combined trastuzumab and radiation therapy for HER2-positive uterine serous carcinoma: A case report. Gynecol. Oncol. Rep. 2023, 49, 101250. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Anderson, S.J.; Siziopikou, K.P.; Arthur, D.W.; Rabinovitch, R.; Julian, T.B.; Parda, D.S.; Seaward, S.A.; Carter, D.L.; Lyons, J.A.; et al. Comparison of Radiation with or without Concurrent Trastuzumab for HER2-Positive Ductal Carcinoma In Situ Resected by Lumpectomy: A Phase III Clinical Trial. J. Clin. Oncol. 2021, 39, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.K.; Tangen, I.L.; Berg, H.F.; Hoivik, E.A.; Mauland, K.K.; Kusonmano, K.; Berg, A.; Hurtado, A.; Kalland, K.H.; Øyan, A.M.; et al. HER2 expression patterns in paired primary and metastatic endometrial cancer lesions. Br. J. Cancer 2018, 118, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The immune system in the normal endometrium and implications for endometrial cancer development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Iwata, T.; Aoki, D. Current status of cancer immunotherapy for gynecologic malignancies. Jpn. J. Clin. Oncol. 2021, 51, 167–172. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- Di Tucci, C.; Schiavi, M.C.; Faiano, P.; D’Oria, O.; Prata, G.; Sciuga, V.; Giannini, A.; Palaia, I.; Muzii, L.; Panici, P.B. Therapeutic vaccines and immune checkpoints inhibition options for gynecological cancers. Crit. Rev. Oncol. Hematol. 2018, 128, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Duffy, C.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.W.; Ellenson, L.H. Molecular Genetics of Endometrial Carcinoma. Annu. Rev. Pathol. 2019, 14, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudakis, D.; Karopoulou, E.; Matsas, A.; Katsampoula, G.A.; Tsarna, E.; Stamoula, E.; Christopoulos, P. Immunotherapy in Cervical and Endometrial Cancer: Current Landscape and Future Directions. Life 2024, 14, 344. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; Roque, D.M.; Siegel, E.R.; Buza, N.; Hui, P.; Bonazzoli, E.; Guglielmi, A.; Zammataro, L.; Nagarkatti, N.; Zaidi, S.; et al. A phase II evaluation of pembrolizumab in recurrent microsatellite instability-high (MSI-H) endometrial cancer patients with Lynch-like versus MLH-1 methylated characteristics (NCT02899793). Ann. Oncol. 2021, 32, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Bellone, S.; Buza, N.; Choi, J.; Schwartz, P.E.; Schlessinger, J.; Lifton, R.P. Regression of chemotherapy-resistant polymerase ε (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin. Cancer Res. 2016, 22, 5682–5687. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Antill, A.; Kok, P.S.; Stockler, M.R.; Robledo, K.; Yip, S.; Parry, M.; Smith, D.; Spurdle, A.; Barnes, E.; Friedlander, M.L.; et al. Updated results of activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). Ann. Oncol. 2019, 30, ix192. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Sharma, P.; Poussin, M.; Boesteanu, A.C.; Minutolo, N.G.; Gitto, S.B.; Omran, D.K.; Robinson, M.K.; Adams, G.P.; Simpkins, F.; et al. CAR T Cells Targeting MISIIR for the Treatment of Ovarian Cancer and Other Gynecologic Malignancies. Mol. Ther. 2020, 28, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Jäger, E.; Karbach, J.; Gnjatic, S.; Neumann, A.; Bender, A.; Valmori, D.; Ayyoub, M.; Ritter, E.; Ritter, G.; Jäger, D.; et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc. Natl. Acad. Sci. USA 2006, 103, 14453–14458. [Google Scholar] [CrossRef] [PubMed]
- Kaumaya, P.T.; Foy, K.C.; Garrett, J.; Rawale, S.V.; Vicari, D.; Thurmond, J.M.; Lamb, T.; Mani, A.; Kane, Y.; Balint, C.R.; et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J. Clin. Oncol. 2009, 27, 5270–5277. [Google Scholar] [CrossRef]
- Soberanis, P.P.; Lheureux, S. Novel Molecular Targets in Endometrial Cancer: Mechanisms and Perspectives for Therapy. Biologics 2024, 18, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Perez, M.; Madrid, L.F.; Redondo, A.; Rodriguez, J. A phase II study of pembrolizumab in combination with doxorubicin in advanced, recurrent or metastatic endometrial cancer. Ann. Oncol. 2019, 30, v432–v433. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Ferrandina, G.; Colombo, N.; Pignata, S.; Pietragalla, A.; Sonetto, C.; Pisano, C.; Lapresa, M.T.; Savarese, A.; Tagliaferri, P.; et al. Carboplatin-paclitaxel compared to carboplatin-paclitaxel-bevacizumab in advanced or recurrent endometrial cancer: MITO END-2—A randomized phase II trial. Gynecol. Oncol. 2019, 155, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Zhang, B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: A promising strategy for cancer treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Staropoli, N.; Salvino, A.; Falcone, F.; Farenza, V.; Costa, M.; Rossini, G.; Manti, F.; Crispino, A.; Riillo, C.; Ciliberto, D.; et al. Pembrolizumab plus lenvatinib in advanced endometrial cancer: Case report and systematic review of lung toxicity. Front. Oncol. 2023, 13, 1145986. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.Y.; Thomes, P.J.; Sundborg, M.; Shai, A.; De la Garza, J.; Nishio, S.; Gold, M.A.; et al. DUO-E Investigators. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab with or without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, L.; Caruso, G.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Attademo, L.; Tambaro, R.; Russo, D.; Califano, D.; Palaia, I.; et al. PARP Inhibitors in Endometrial Cancer: Current Status and Perspectives. Cancer Manag. Res. 2020, 12, 6123–6135. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as biomarkers in disease: Latest findings regarding their role in diagnosis and prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Therapeutic advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Donkers, H.; Bekkers, R.; Galaal, K. Diagnostic value of microRNA panel in endometrial cancer: A systematic review. Oncotarget 2020, 11, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Bogaczyk, A.; Zawlik, I.; Zuzak, T.; Kluz, M.; Potocka, N.; Kluz, T. The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 11489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).