The National Landscapes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Stable Trends in Black Populations and Late-Stage Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Gastric MALT Lymphoma Demographics and Overall Incidence Rates and Trends
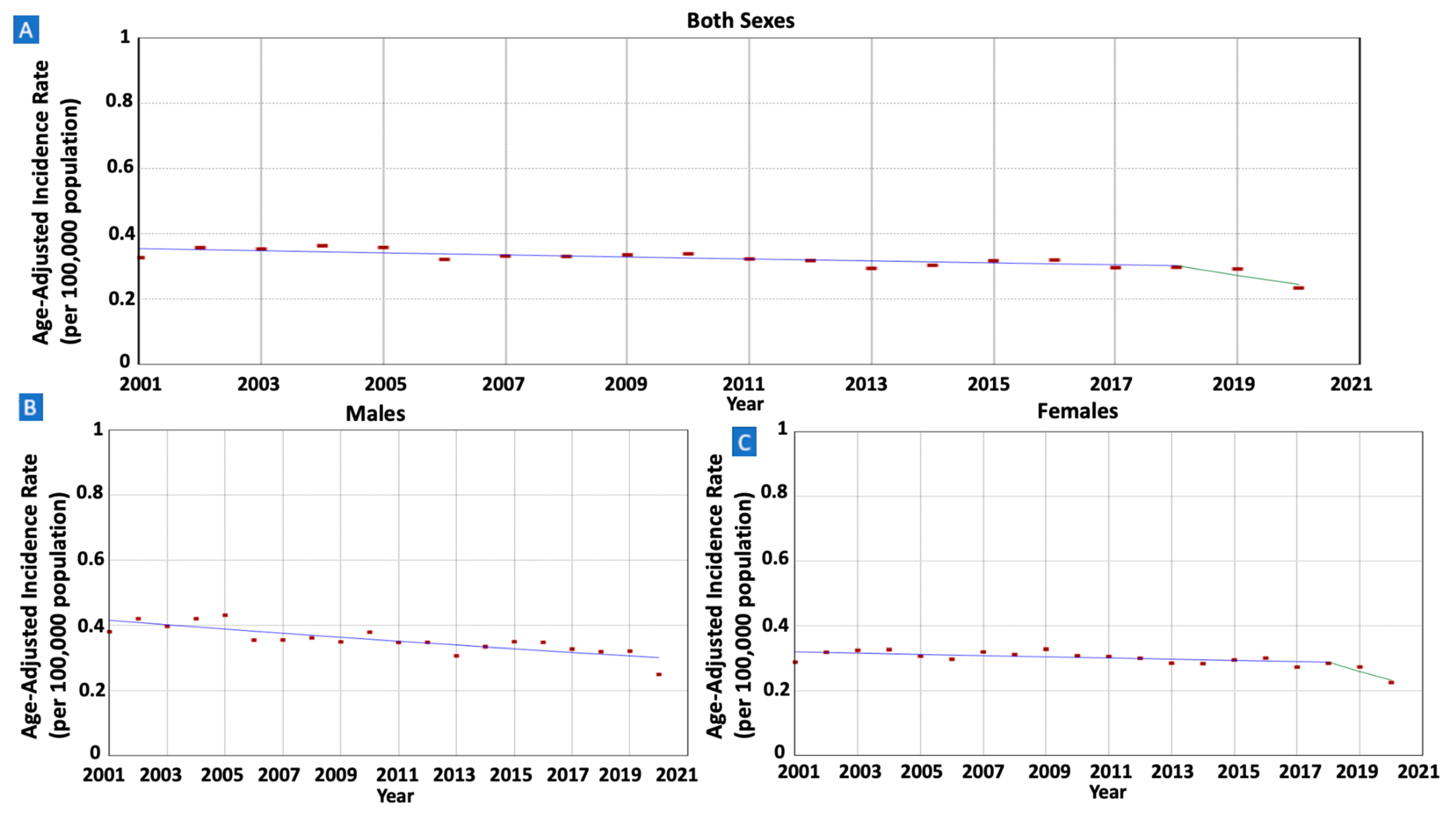
3.2. Gastric MALT Lymphoma Incidence Trend per Sex
3.3. Gastric MALT Lymphoma Incidence Rates and Trends per Age
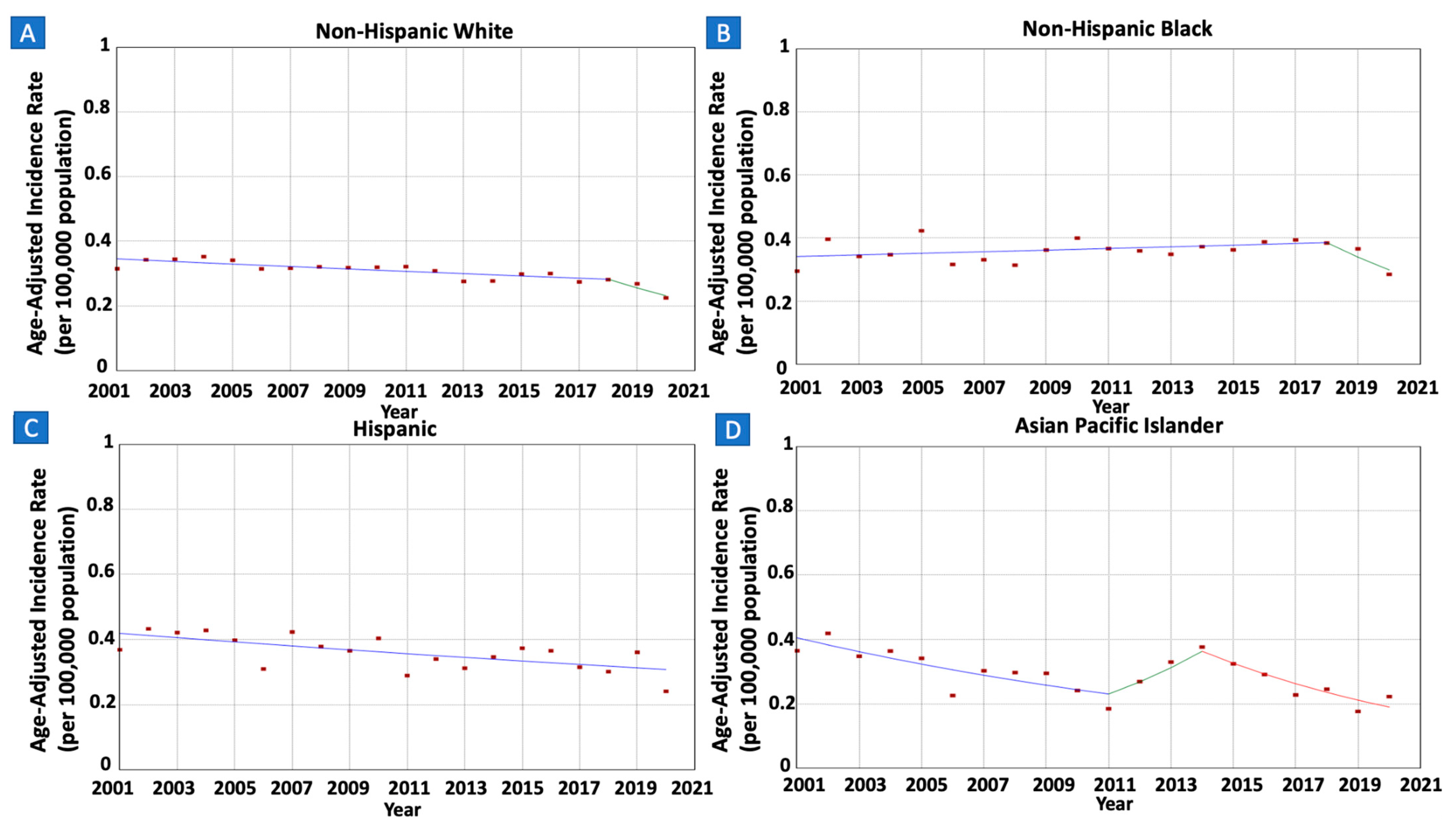
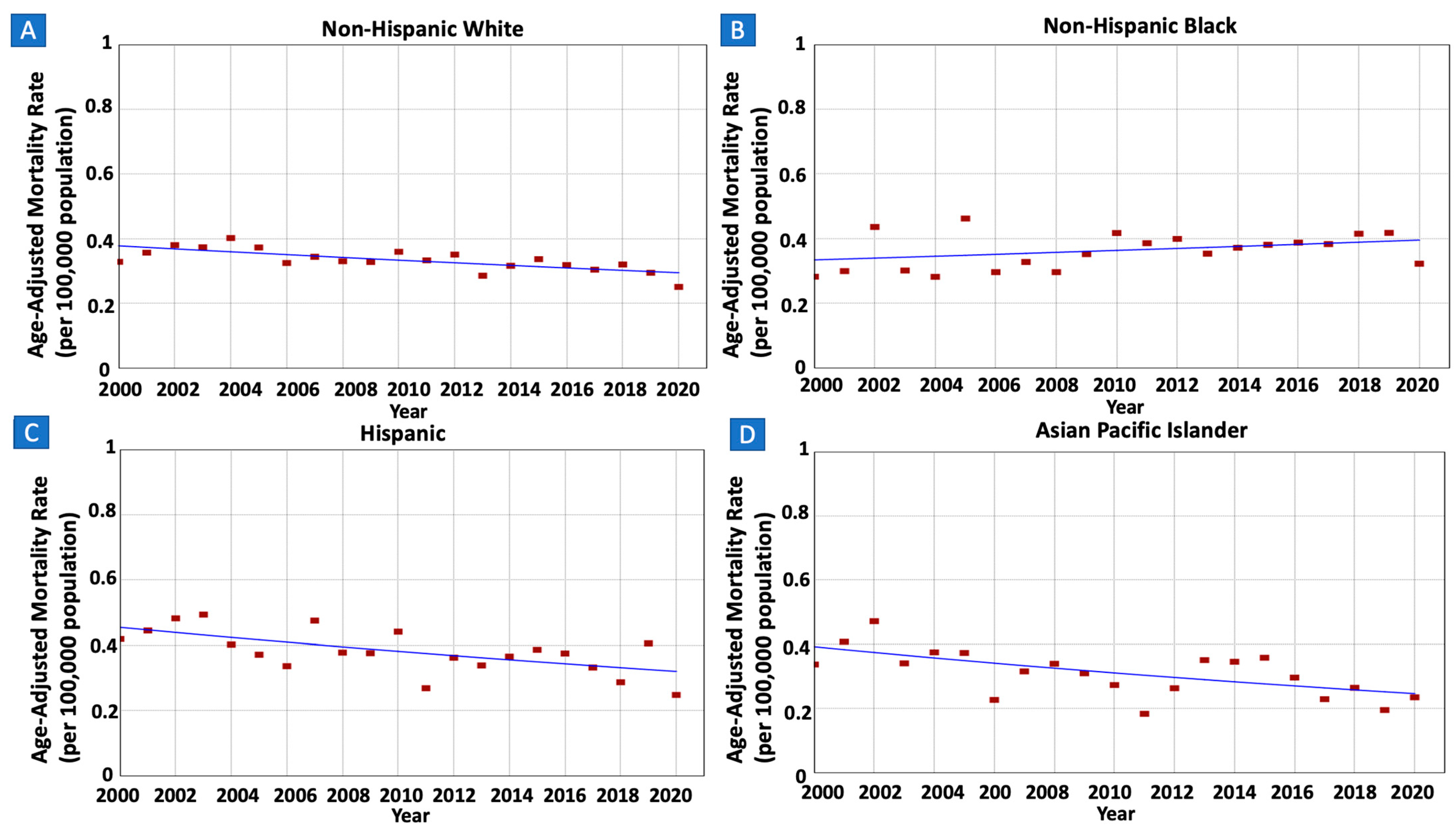
3.4. Gastric MALT Lymphoma Incidence Rates and Trends per Race/Ethnicity
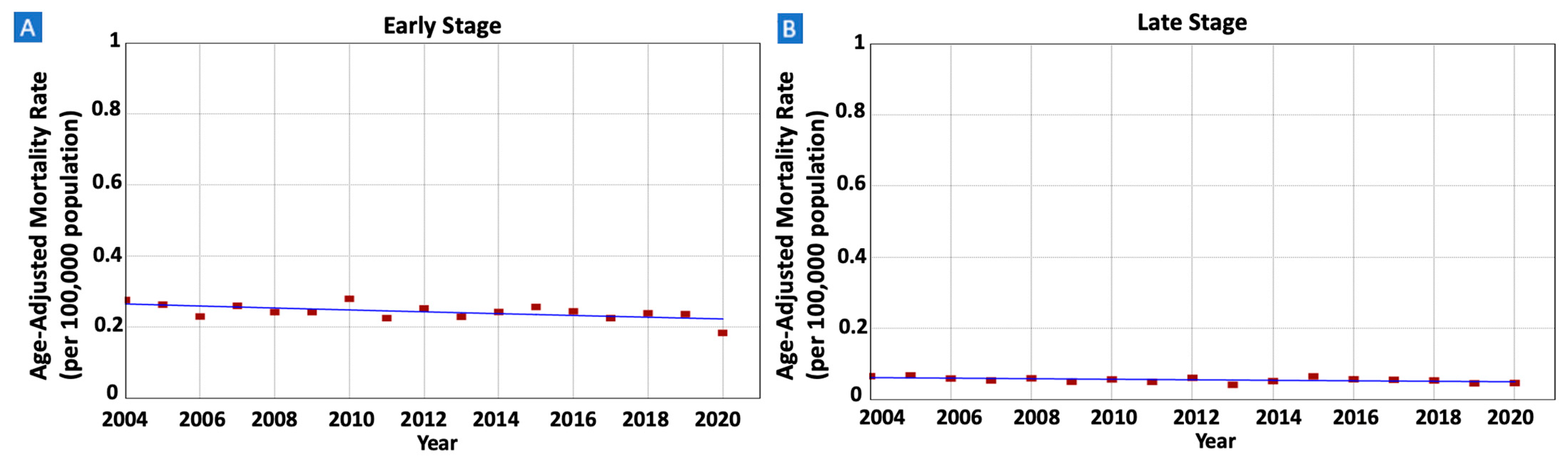
3.5. Gastric MALT Lymphoma Incidence Rates and Trends per Tumor Stage at Diagnosis
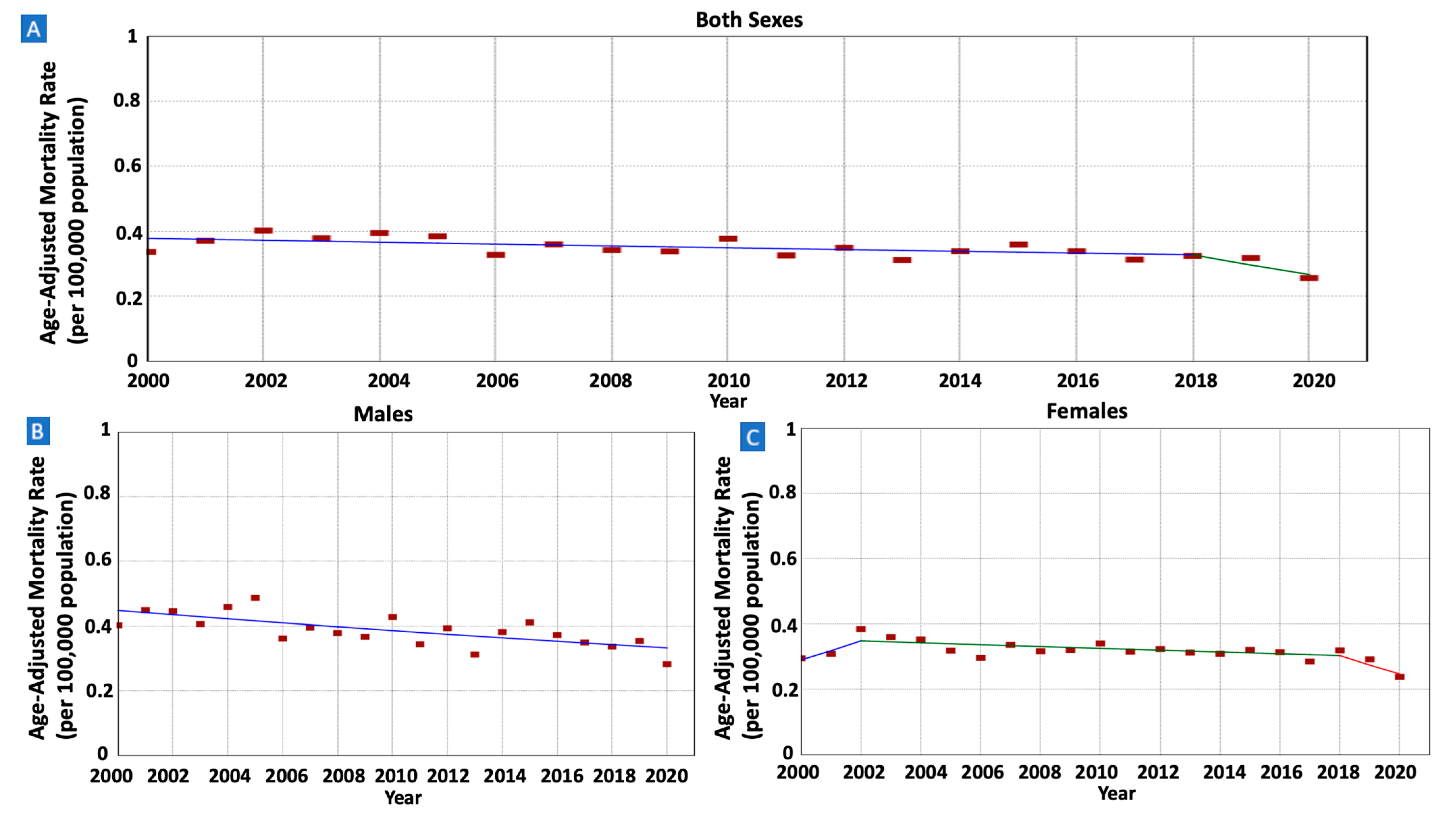
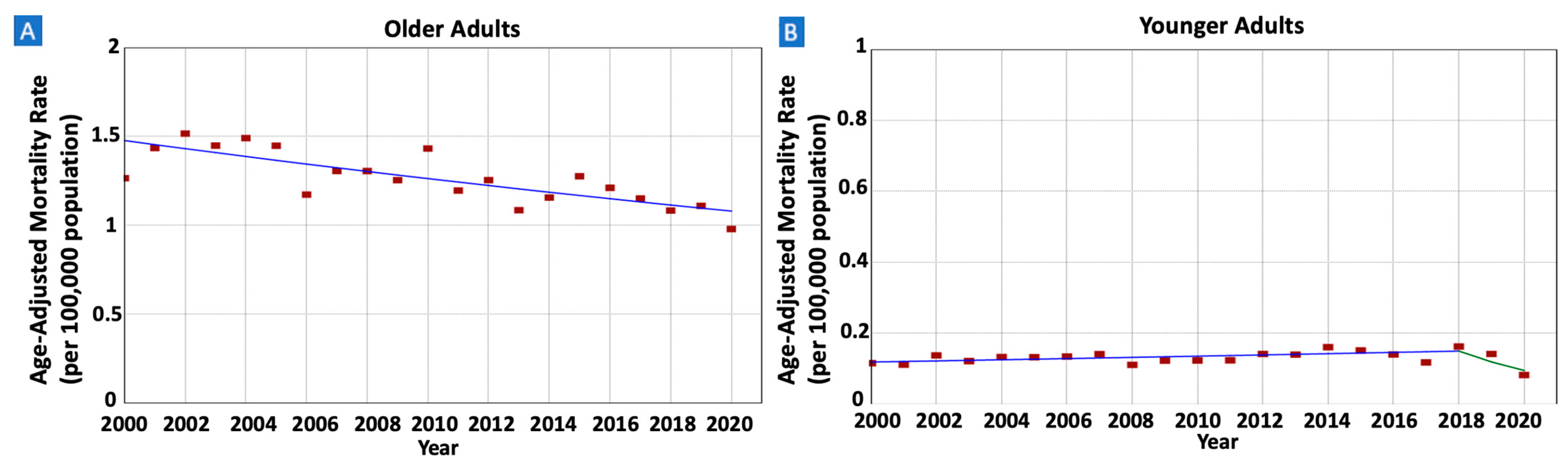
3.6. Gastric MALT Lymphoma IBM Rates and Trends
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zucca, E.; Bertoni, F.; Stathis, A.; Cavalli, F. Marginal zone lymphomas. Hematol. Oncol. Clin. N. Am. 2008, 22, 883–901. [Google Scholar] [CrossRef]
- Di Rocco, A.; Petrucci, L.; Assanto, G.M.; Martelli, M.; Pulsoni, A. Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers 2022, 14, 1742. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.O.; Morton, L.M.; Devesa, S.S.; Check, D.P.; Curtis, R.E.; Weisenburger, D.D.; Dores, G.M. Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br. J. Haematol. 2014, 165, 67–77. [Google Scholar] [CrossRef]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Zucca, E.; Bertoni, F.; Vannata, B.; Cavalli, F. Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin. Cancer Res. 2014, 20, 5207–5216. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Wotherspoon, A.; Diss, T.; Pan, L.; Isaacson, P.; Doglioni, C.; Moschini, A.; de Boni, M. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575–577. [Google Scholar] [CrossRef]
- Lemos, F.F.B.; de Castro, C.T.; Calmon, M.S.; Luz, M.S.; Pinheiro, S.L.R.; dos Santos, C.F.S.M.; Santos, G.L.C.; Marques, H.S.; Delgado, H.A.; Teixeira, K.N.; et al. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J. Gastroenterol. 2023, 29, 2202–2221. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Y.; Zhang, X.; Fu, K. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection: A review of current diagnosis and management. Biomark. Res. 2016, 4, 15. [Google Scholar] [CrossRef]
- Zucca, E.; Bertoni, F. The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood 2016, 127, 2082–2092. [Google Scholar] [CrossRef]
- Filip, P.V.; Cuciureanu, D.; Diaconu, L.S.; Vladareanu, A.M.; Pop, C.S. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J. Med. Life 2018, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, R.; Magam, S.G.; Joy, M.; Natt, D.; Patel, A.; Tadikonda, A.; Desai, J.; Bunting, S.; Yost, R.M.; Akande, O.; et al. Unpacking the Racial Gap: Helicobacter pylori Infection Clearance Among Different Racial Groups. Cureus 2023, 15, e43080. [Google Scholar] [CrossRef]
- National Program of Cancer Registries and Surveillance EaERPSSDNaSI-USCSPURD, 2022 Submission (2001–2020). United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2023. Available online: www.cdc.gov/cancer/uscs/public-use (accessed on 13 January 2024).
- Centers for Disease Control and Prevention. Software and Tools for Cancer Registries and Surveillance. Available online: https://www.cdc.gov/cancer/npcr/tools/index.htm (accessed on 13 January 2024).
- Olszewski, A.J.; Castillo, J.J. Comparative outcomes of oncologic therapy in gastric extranodal marginal zone (MALT) lymphoma: Analysis of the SEER-Medicare database. Ann. Oncol. 2013, 24, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yu, B.; Feuer, E.J. Selecting the number of change-points in segmented line regression. Stat. Sin. 2009, 19, 597–609. [Google Scholar]
- Kim, J.; Kim, H.J. Consistent Model Selection in Segmented Line Regression. J. Stat. Plan. Inference 2016, 170, 106–116. [Google Scholar] [CrossRef]
- Statistical Research and Applications Branch NCI. Joinpoint Regression Program VM. 2021. Available online: https://surveillance.cancer.gov/help/joinpoint/tech-help/citation (accessed on 13 January 2024).
- Rustgi, S.D.; McKinley, M.; McBay, B.; Zylberberg, H.M.; Gomez, S.L.; Hur, C.; Kastrinos, F.; Gupta, S.; Kim, M.K.; Itzkowitz, S.H.; et al. Epidemiology of Gastric Malignancies 2000-2018 According to Histology: A Population-Based Analysis of Incidence and Temporal Trends. Clin. Gastroenterol. Hepatol. 2023, 21, 3285–3295.e8. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Lukas, J.; Dolak, W.; Simonitsch-Klupp, I.; Mayerhoefer, M.E.; Raderer, M. Gender Aspects in Extranodal Marginal Zone B-Cell Lymphoma of the Mucosa-Associated Lymphoid Tissue: Does Sex Matter? Oncology 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Min, G.-J.; Kang, D.; Lee, H.H.; Kim, S.-J.; Kim, T.Y.; Jeon, Y.-W.; O, J.H.; Choi, B.-O.; Park, G.; Cho, S.-G. Long-term clinical outcomes of gastric mucosa-associated lymphoid tissue lymphoma in real-world experience. Ann. Hematol. 2023, 102, 877–888. [Google Scholar] [CrossRef]
- Capelle, L.; de Vries, A.; Looman, C.; Casparie, M.; Boot, H.; Meijer, G.; Kuipers, E. Gastric MALT lymphoma: Epidemiology and high adenocarcinoma risk in a nation-wide study. Eur. J. Cancer 2008, 44, 2470–2476. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Andriani, A.; Cristofari, F.; Cardinale, V.; Spinelli, G.P.; Tomao, S.; Morini, S. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J. Clin. Gastroenterol. 2010, 44, 340–344. [Google Scholar] [CrossRef]
- Luminari, S.; Cesaretti, M.; Marcheselli, L.; Rashid, I.; Madrigali, S.; Maiorana, A.; Federico, M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: Results from a population-based study on extranodal marginal zone lymphomas. Ann. Oncol. 2010, 21, 855–859. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Yeh, K.-H.; Lin, C.-W.; Liou, J.-M.; Wu, M.-S.; Chen, L.-T.; Cheng, A.-L. Current Status of the Spectrum and Therapeutics of Helicobacter pylori-Negative Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers 2022, 14, 1005. [Google Scholar] [CrossRef]
- Brown, H.; Cantrell, S.; Tang, H.; Epplein, M.; Garman, K.S. Racial Differences in Helicobacter pylori Prevalence in the US: A Systematic Review. Gastro Hep Adv. 2022, 1, 857–868. [Google Scholar] [CrossRef]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7. [Google Scholar] [CrossRef] [PubMed]
- Peña-Galo, E.; Gotor, J.; Harb, Y.; Alonso, M.; Alcedo, J. Socioeconomic and demographic factors associated with failure in Helicobacter pylori eradication using the standard triple therapy. Gastroenterol. Hepatol. Bed Bench 2021, 14, 53–58. [Google Scholar]
- Reichstein, J.; Parish, A.; Garbarino, S.; Wilder, J.; Epplein, M.; Fisher, D.A.; Niedzwiecki, D.; Muir, A.J.; Leiman, D.A. S1332 Variations and Racial Disparities in Helicobacter pylori Guideline Adherence for Eradication Testing. Am. J. Gastroenterol. 2020, 115, S670–S671. [Google Scholar] [CrossRef]
- White, B.; Patel, P.; Schwartz, M.; Hunter, K.; DeSipio, J.; Phadtare, S. S1642 Factors Associated With Failure to Complete Helicobacter pylori Eradication Testing in an Urban Tertiary Care Center. Am. J. Gastroenterol. 2022, 117, e1175–e1176. [Google Scholar] [CrossRef]
- Epplein, M.; Cohen, S.S.; Sonderman, J.S.; Zheng, W.; Williams, S.M.; Blot, W.J.; Signorello, L.B. Neighborhood socio-economic characteristics, African ancestry, and Helicobacter pylori sero-prevalence. Cancer Causes Control 2012, 23, 897–906. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.C.; Lee, J.Y.; Ahn, J.Y.; Kang, S.H.; Yang, H.-J.; Kim, S.J.; Joo, M.K.; Park, J.M. Long-Term Clinical Outcomes of Gastric MALT Lymphoma: A Nationwide Multicenter Study in Korea. Front. Oncol. 2021, 11, 681689. [Google Scholar] [CrossRef] [PubMed]
- Gillis, D.; Edwards, B.P.M. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon 2019, 5, e02515. [Google Scholar] [CrossRef]
- Park, H.S.; Lloyd, S.; Decker, R.H.; Wilson, L.D.; Yu, J.B. Limitations and biases of the Surveillance, Epidemiology, and End Results database. Curr. Probl. Cancer 2012, 36, 216–224. [Google Scholar] [CrossRef] [PubMed]


| Demographic Variable | Cases (N = 21,625) a | N in 2001 (2020) | Age-Adjusted Incidence Rate (Crude Rate) in 2001 | Age-Adjusted Incidence Rate (Crude Rate) in 2020 | Time Period | APC b (95% CI) | AAPC (95% CI) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Both Sexes (All Ages) | 21,625 (100%) | 913 (938) | 0.33 (0.32) | 0.23 (0.29) | 2001–2018 | −0.93 * (−1.36 to −0.50) | −1.93 * (−3.18 to −0.66) |
| 2018–2020 | −10.01 (−20.75 to 2.20) | ||||||
| Males | 10,676 (49.4%) | 458 (455) | 0.38 (0.33) | 0.25 (0.29) | 2001–2020 | −1.67 * (−2.24 to −1.09) | −1.67 * (−2.24 to −1.09) |
| Females | 10,949 (50.6%) | 455 (483) | 0.29 (0.32) | 0.23 (0.30) | 2001–2018 | −0.61 * (−1.07 to –0.14) | −1.66 * (−3.02 to −0.28) |
| 2018–2020 | −10.17 (−21.78 to 3.17) | ||||||
| Age | |||||||
| Older Adults | 17,317 (80.1%) | 737 (800) | 1.24 (1.23) | 0.87 (0.84) | 2001–2020 | −1.66 * (−2.13 to –1.18) | −1.66 * (−2.13 to –1.18) |
| Younger Adults | 4297 (19.9%) | 175 (138) | 0.11 (0.11) | 0.08 (0.08) | 2001–2018 | −0.73 * (0.16 to 1.54) | −1.38 * (−2.24 to −0.46) |
| 2018–2020 | −17.64 * (−24.46 to −5.71) | ||||||
| Race/Ethnicity | |||||||
| White | 15,390 (71.2%) | 700 (627) | 0.31 (0.37) | 0.22 (0.33) | 2001–2018 | −1.18 (−1.57 to 0.36) | −2.09 * (−2.72 to –1.30) |
| 2018–2020 | −9.50 * (−15.13 to −1.97) | ||||||
| Black | 2493 (11.5%) | 78 (121) | 0.30 (0.23) | 0.28 (0.29) | 2001–2018 | 0.71 (−0.22 to 5.03) | −0.71 (−1.67 to 0.80) |
| 2018–2020 | −11.97 (−20.35 to 0.08) | ||||||
| Hispanic | 2147 (9.9%) | 74 (105) | 0.37 (0.20) | 0.24 (0.18) | 2001–2020 | −1.61 (−2.70 to −0.32) | −1.61 * (−2.70 to −0.32) |
| API | 832 (3.8%) | 30 (48) | 0.37 (0.25) | 0.22 (0.23) | 2001–2011 | −5.49 * (−9.99 to −3.23) | −3.92 * (−5.14 to 2.96) |
| 2011–2014 | 16.40 * (3.05 to 23.78) | ||||||
| 2014–2020 | −10.27 * (−15.49 to −7.12) | ||||||
| AI/AN | 104 (0.5%) | ^ | ^ | ||||
| Stage at Diagnosis | |||||||
| Early Stage | 15,125 (69.9%) | 609 (649) | 0.22 (0.22) | 0.16 (0.20) | 2001–2004 | 4.67 * (0.10 to 13.29) | −1.10 * (−1.84 to −0.31) |
| 2004–2018 | −0.88 * (−1.77 to −0.02) | ||||||
| 2018–2020 | −10.58 * (−16.20 to −3.36) | ||||||
| Late Stage | 3859 (17.8%) | 139 (190) | 0.05 (0.05) | 0.05 (0.06) | 2001–2003 | 16.33 * (1.66 to 30.12) | −0.56 (−1.70 to 0.53) |
| 2003–2013 | −3.70 * (−9.97 to −2.67) | ||||||
| 2013–2016 | 12.07 * (3.02 to 17.23) | ||||||
| 2016–2020 | −8.93 * (−16.39 to −5.24) | ||||||
| Demographic Variable | Deaths (N = 11,036) a | N in 2000 (2020) | Age-Adjusted Mortality Rate (Crude Rate) in 2000 | Age-Adjusted Mortality Rate (Crude Rate) in 2020 | Time Period | APC b (95% CI) | AAPC (95% CI) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Both Sexes (All Ages) | 11,036 (100%) | 423 (480) | 0.34 (0.31) | 0.26 (0.31) | 2000–2018 | −0.79 (−3.83 to 15.34) | −1.72 (−3.01 to 0.32) |
| 2018–2020 | −9.68 (−21.93 to 0.06) | ||||||
| Males | 5479 (49.6%) | 215 (240) | 0.40 (0.32) | 0.28 (0.31) | 2000–2020 | −1.47 * (−2.22 to −0.69) | −1.47 * (−2.22 to −0.69) |
| Females | 5557 (50.4%) | 208 (240) | 0.30 (0.30) | 0.24 (0.30) | 2000–2002 | 9.56 * (0.49 to 19.02) | −0.80 (−1.63 to 0.15) |
| 2002–2018 | −0.86 (−2.01 to 0.15) | ||||||
| 2018–2020 | −9.74 * (−16.16 to −1.63) | ||||||
| Age | |||||||
| Older Adults | 8762 (79.4%) | 335 (412) | 1.27 (1.26) | 0.98 (0.93) | 2000–2020 | −1.55 * (−2.13 to −0.97) | −1.55 * (−2.13 to −0.97) |
| Younger Adults | 2271 (20.6%) | 88 (68) | 0.12 (0.11) | 0.08 (0.08) | 2000–2018 | 1.28 * (0.31 to 6.29) | −1.12 (−2.96 to 1.34) |
| 2018–2020 | −20.32 * (−35.07 to −0.34) | ||||||
| Race/Ethnicity | |||||||
| White | 7412 (67.2%) | 313 (295) | 0.33 (0.38) | 0.25 (0.36) | 2000–2020 | −1.23 * (−1.85 to −0.65) | −1.23 * (−1.85 to −0.65) |
| Black | 1176 (10.7%) | 33 (62) | 0.28 (0.21) | 0.32 (0.32) | 2000–2020 | 0.85 (−0.33 to 2.16) | 0.85 (−0.33 to 2.16) |
| Hispanic | 1611 (14.6%) | 50 (76) | 0.42 (0.19) | 0.25 (0.19) | 2001–2020 | −1.73 * (−2.99 to −0.33) | −1.73 * (−2.99 to −0.33) |
| API | 692 (6.3%) | 21 (39) | 0.34 (0.24) | 0.24 (0.26) | 2001–2020 | −2.30 * (−3.66 to −0.73) | −2.30 * (−3.66 to −0.73) |
| AI/AN | 38 (0.3%) | 1 (2) | 0.10 (0.11) | 0.20 (0.21) | ^ | ||
| Stage at Diagnosis | |||||||
| Early Stage | 7282 (66.0%) | 371 (185) | 0.28 (0.26) | 0.18 (0.22) | 2004–2020 | −1.08 * (−1.89 to −0.25) | −1.08 * (−1.89 to −0.25) |
| Late Stage | 1681 (15.2%) | 89 (42) | 0.07 (0.06) | 0.05 (0.06) | 2004–2003 | −1.31 (−2.81 to 0.33) | −1.31 (−2.81 to 0.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, Y.; Pirquet, C.; Timmons, K.; Abboud, I.; Awadallah, M.; Al-Khazraji, A.; Hajifathalian, K. The National Landscapes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Stable Trends in Black Populations and Late-Stage Tumors. Cancers 2024, 16, 2024. https://doi.org/10.3390/cancers16112024
Abboud Y, Pirquet C, Timmons K, Abboud I, Awadallah M, Al-Khazraji A, Hajifathalian K. The National Landscapes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Stable Trends in Black Populations and Late-Stage Tumors. Cancers. 2024; 16(11):2024. https://doi.org/10.3390/cancers16112024
Chicago/Turabian StyleAbboud, Yazan, Charlotte Pirquet, Kiley Timmons, Ibrahim Abboud, Mina Awadallah, Ahmed Al-Khazraji, and Kaveh Hajifathalian. 2024. "The National Landscapes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Stable Trends in Black Populations and Late-Stage Tumors" Cancers 16, no. 11: 2024. https://doi.org/10.3390/cancers16112024
APA StyleAbboud, Y., Pirquet, C., Timmons, K., Abboud, I., Awadallah, M., Al-Khazraji, A., & Hajifathalian, K. (2024). The National Landscapes of Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Stable Trends in Black Populations and Late-Stage Tumors. Cancers, 16(11), 2024. https://doi.org/10.3390/cancers16112024





