Management and Clinical Outcomes of Breast Cancer in Women Diagnosed with Hereditary Cancer Syndromes in a Clinic-Based Sample from Colombia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
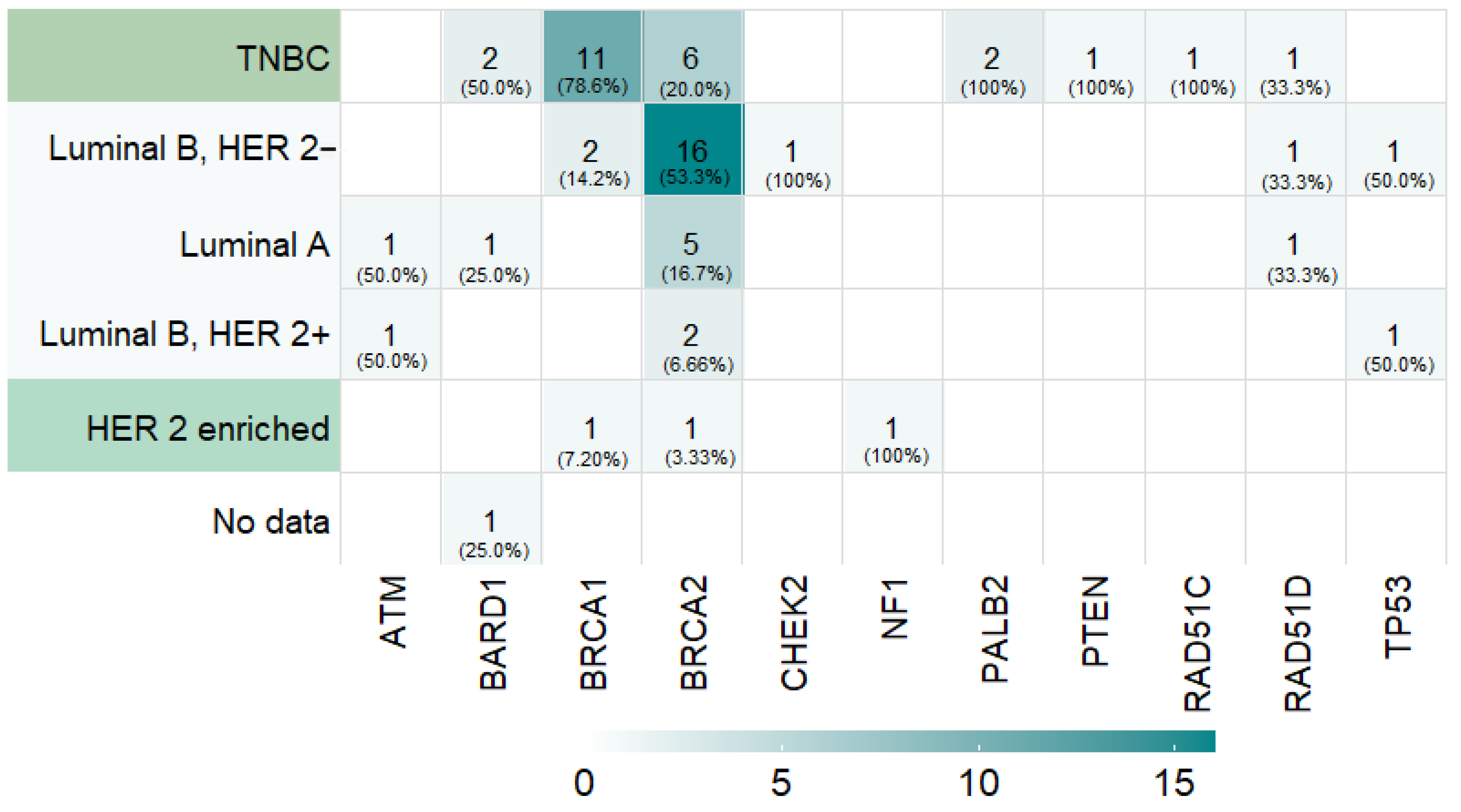
3.2. Prevalence of Germline PVs in Cancer-Predisposing Genes
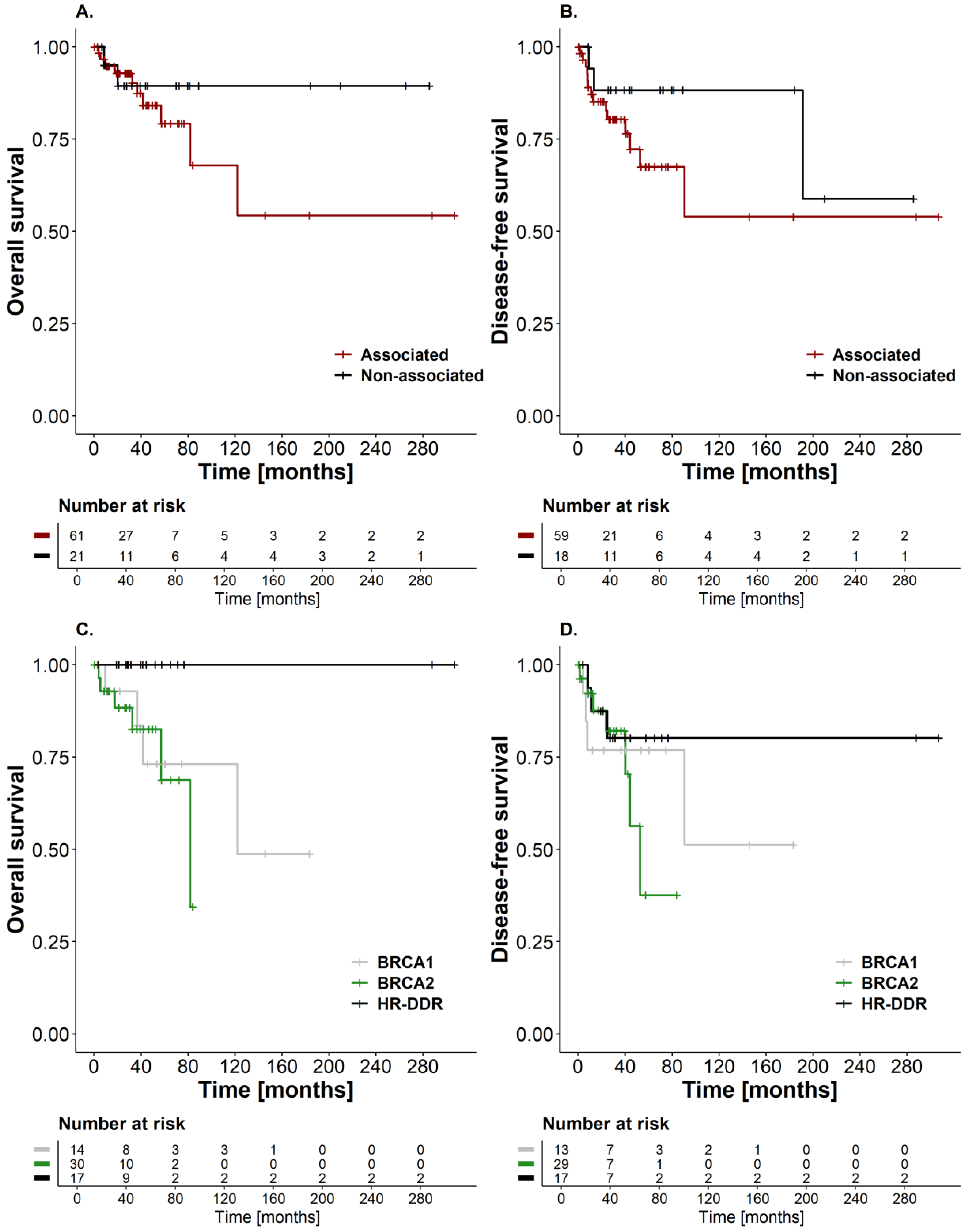
3.3. Survival Outcomes and Disease Recurrence in Breast Cancer Patients Carrying PVs in “Associated”and “Non-Associated” Breast Cancer Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.who.int/today (accessed on 1 April 2024).
- Chavarri-Guerra, Y.; Blazer, K.R.; Weitzel, J.N. Genetic Cancer Risk Assessment for Breast Cancer in Latin America. Rev. Investig. Clin. 2017, 69, 94–102. [Google Scholar] [CrossRef]
- Nagy, R.; Sweet, K.; Eng, C. Highly penetrant hereditary cancer syndromes. Oncogene 2004, 23, 6445–6470. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Maxwell, K.N.; Churpek, J.; Kohlmann, W.; AlHilli, Z.; Arun, B.; Buys, S.S.; Cheng, H.; Domchek, S.M.; et al. NCCN Guidelines(R) Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J. Natl. Compr. Canc Netw. 2023, 21, 1000–1010. [Google Scholar] [CrossRef]
- Huber-Keener, K.J. Cancer genetics and breast cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2022, 82, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Easton, D.F.; Stratton, M.; Narod, S.; Goldgar, D.; Devilee, P.; Bishop, D.T.; Weber, B.; Lenoir, G.; Chang-Claude, J.; et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1998, 62, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef]
- Greenup, R.; Buchanan, A.; Lorizio, W.; Rhoads, K.; Chan, S.; Leedom, T.; King, R.; McLennan, J.; Crawford, B.; Kelly Marcom, P.; et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann. Surg. Oncol. 2013, 20, 3254–3258. [Google Scholar] [CrossRef]
- Dos Santos Vidal, R.; Hawrysh, A.; Walia, J.S.; Davey, S.; Feilotter, H. Eligibility Criteria and Genetic Testing Results from a High-Risk Cohort for Hereditary Breast and Ovarian Cancer Syndrome in Southeastern Ontario. J. Mol. Diagn. 2016, 18, 362–369. [Google Scholar] [CrossRef]
- Grindedal, E.M.; Heramb, C.; Karsrud, I.; Ariansen, S.L.; Maehle, L.; Undlien, D.E.; Norum, J.; Schlichting, E. Current guidelines for BRCA testing of breast cancer patients are insufficient to detect all mutation carriers. BMC Cancer 2017, 17, 438. [Google Scholar] [CrossRef]
- Alemar, B.; Gregorio, C.; Herzog, J.; Matzenbacher Bittar, C.; Brinckmann Oliveira Netto, C.; Artigalas, O.; Schwartz, I.V.D.; Coffa, J.; Alves Camey, S.; Weitzel, J.; et al. BRCA1 and BRCA2 mutational profile and prevalence in hereditary breast and ovarian cancer (HBOC) probands from Southern Brazil: Are international testing criteria appropriate for this specific population? PLoS ONE 2017, 12, e0187630. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Rhiem, K.; Hahnen, E.; Loibl, S.; Weber, K.E.; Seiler, S.; Zachariae, S.; Hauke, J.; Wappenschmidt, B.; Waha, A.; et al. Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer 2018, 18, 265. [Google Scholar] [CrossRef] [PubMed]
- Eliade, M.; Skrzypski, J.; Baurand, A.; Jacquot, C.; Bertolone, G.; Loustalot, C.; Coutant, C.; Guy, F.; Fumoleau, P.; Duffourd, Y.; et al. The transfer of multigene panel testing for hereditary breast and ovarian cancer to healthcare: What are the implications for the management of patients and families? Oncotarget 2017, 8, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Cock-Rada, A.M.; Ossa, C.A.; Garcia, H.I.; Gomez, L.R. A multi-gene panel study in hereditary breast and ovarian cancer in Colombia. Fam. Cancer 2018, 17, 23–30. [Google Scholar] [CrossRef]
- Ossa Gomez, C.A.; Achatz, M.I.; Hurtado, M.; Sanabria-Salas, M.C.; Sullcahuaman, Y.; Chavarri-Guerra, Y.; Dutil, J.; Nielsen, S.M.; Esplin, E.D.; Michalski, S.T.; et al. Germline Pathogenic Variant Prevalence Among Latin American and US Hispanic Individuals Undergoing Testing for Hereditary Breast and Ovarian Cancer: A Cross-Sectional Study. JCO Glob. Oncol. 2022, 8, e2200104. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Quezada Urban, R.; Franco Cortes, C.A.; Diaz Velasquez, C.E.; Montealegre Paez, A.L.; Pacheco-Orozco, R.A.; Castro Rojas, C.; Garcia-Robles, R.; Lopez Rivera, J.J.; Gaitan Chaparro, S.; et al. Latin American Study of Hereditary Breast and Ovarian Cancer LACAM: A Genomic Epidemiology Approach. Front. Oncol. 2019, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Salas, M.C.; Pedroza-Durán, A.; Rivera, A.L.; Gonzalez-Hurtado, D.; Cuadrado, D.; Quintero-Ortiz, M.; Suarez-Rodríguez, R.; Gómez, A.M.; Manotas, M.C.; Brugés-Maya, R.; et al. Criterios para la identificación de síndromes de cáncer de mama hereditarios. Revisión de la literatura y recomendaciones para el Instituto Nacional de Cancerología—Colombia. Rev. Colomb. Cancerol. 2023, 27, 26–41. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Manotas, M.C.; Rivera, A.L.; Gomez, A.M.; Abisambra, P.; Guevara, G.; Medina, V.; Tapiero, S.; Huertas, A.; Riano-Moreno, J.; Mejia, J.C.; et al. SDHB exon 1 deletion: A recurrent germline mutation in Colombian patients with pheochromocytomas and paragangliomas. Front. Genet. 2022, 13, 999329. [Google Scholar] [CrossRef] [PubMed]
- Manotas, M.C.; Rivera, A.L.; Sanabria-Salas, M.C. Variant curation and interpretation in hereditary cancer genes: An institutional experience in Latin America. Mol. Genet. Genomic Med. 2023, 11, e2141. [Google Scholar] [CrossRef]
- Wang, Y.A.; Jian, J.W.; Hung, C.F.; Peng, H.P.; Yang, C.F.; Cheng, H.S.; Yang, A.S. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer 2018, 18, 315. [Google Scholar] [CrossRef]
- Hu, C.; Polley, E.C.; Yadav, S.; Lilyquist, J.; Shimelis, H.; Na, J.; Hart, S.N.; Goldgar, D.E.; Shah, S.; Pesaran, T.; et al. The Contribution of Germline Predisposition Gene Mutations to Clinical Subtypes of Invasive Breast Cancer from a Clinical Genetic Testing Cohort. J. Natl. Cancer Inst. 2020, 112, 1231–1241. [Google Scholar] [CrossRef]
- Sun, J.; Meng, H.; Yao, L.; Lv, M.; Bai, J.; Zhang, J.; Wang, L.; Ouyang, T.; Li, J.; Wang, T.; et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin. Cancer Res. 2017, 23, 6113–6119. [Google Scholar] [CrossRef]
- De Silva, D.L.; Stafford, L.; Skandarajah, A.R.; Sinclair, M.; Devereux, L.; Hogg, K.; Kentwell, M.; Park, A.; Lal, L.; Zethoven, M.; et al. Universal genetic testing for women with newly diagnosed breast cancer in the context of multidisciplinary team care. Med. J. Aust. 2023, 218, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, P.W.; Beitsch, P.D.; Patel, R.; Rosen, B.; Compagnoni, G.; Baron, P.L.; Simmons, R.; Brown, E.A.; Gold, L.; Holmes, D.; et al. Clinical Utility of Universal Germline Genetic Testing for Patients with Breast Cancer. JAMA Netw. Open 2022, 5, e2232787. [Google Scholar] [CrossRef]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.S., Jr.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [CrossRef]
- Esplin, E.D.; Nielsen, S.M.; Bristow, S.L.; Garber, J.E.; Hampel, H.; Rana, H.Q.; Samadder, N.J.; Shore, N.D.; Nussbaum, R.L. Universal Germline Genetic Testing for Hereditary Cancer Syndromes in Patients with Solid Tumor Cancer. JCO Precis. Oncol. 2022, 6, e2100516. [Google Scholar] [CrossRef] [PubMed]
- Beitsch, P.D.; Whitworth, P.W.; Hughes, K.; Patel, R.; Rosen, B.; Compagnoni, G.; Baron, P.; Simmons, R.; Smith, L.A.; Grady, I.; et al. Underdiagnosis of Hereditary Breast Cancer: Are Genetic Testing Guidelines a Tool or an Obstacle? J. Clin. Oncol. 2019, 37, 453–460. [Google Scholar] [CrossRef]
- Mandelker, D.; Zhang, L.; Kemel, Y.; Stadler, Z.K.; Joseph, V.; Zehir, A.; Pradhan, N.; Arnold, A.; Walsh, M.F.; Li, Y.; et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA 2017, 318, 825–835. [Google Scholar] [CrossRef]
- Kuzbari, Z.; Bandlamudi, C.; Loveday, C.; Garrett, A.; Mehine, M.; George, A.; Hanson, H.; Snape, K.; Kulkarni, A.; Allen, S.; et al. Germline-focused analysis of tumour-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann. Oncol. 2023, 34, 215–227. [Google Scholar] [CrossRef]
- Mandelker, D.; Donoghue, M.; Talukdar, S.; Bandlamudi, C.; Srinivasan, P.; Vivek, M.; Jezdic, S.; Hanson, H.; Snape, K.; Kulkarni, A.; et al. Germline-focussed analysis of tumour-only sequencing: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2019, 30, 1221–1231. [Google Scholar] [CrossRef]
- Mannan, A.U.; Singh, J.; Lakshmikeshava, R.; Thota, N.; Singh, S.; Sowmya, T.S.; Mishra, A.; Sinha, A.; Deshwal, S.; Soni, M.R.; et al. Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: Implications of embracing a multi-gene panel in molecular diagnosis in India. J. Hum. Genet. 2016, 61, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Yadav, S.; Hu, C.; Wunderle, M.; Haberle, L.; Hart, S.N.; Rubner, M.; Polley, E.C.; Lee, K.Y.; Gnanaolivu, R.D.; et al. Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer -Association With Patient and Disease Characteristics and Effect on Prognosis. J. Clin. Oncol. 2021, 39, 1619–1630. [Google Scholar] [CrossRef]
- Dutil, J.; Colon-Colon, J.L.; Matta, J.L.; Sutphen, R.; Echenique, M. Identification of the prevalent BRCA1 and BRCA2 mutations in the female population of Puerto Rico. Cancer Genet. 2012, 205, 242–248. [Google Scholar] [CrossRef]
- Rodriguez, R.C.; Esperon, A.A.; Ropero, R.; Rubio, M.C.; Rodriguez, R.; Ortiz, R.M.; Anta, J.J.; de los Rios, M.; Carnesolta, D.; del Olivera, M.C.; et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Cuba. Fam. Cancer 2008, 7, 275–279. [Google Scholar] [CrossRef]
- Weitzel, J.N.; Lagos, V.; Blazer, K.R.; Nelson, R.; Ricker, C.; Herzog, J.; McGuire, C.; Neuhausen, S. Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1666–1671. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, A.L.; Ruiz-Flores, P.; Cerda-Flores, R.M.; Barrera-Saldana, H.A. Clinical follow up of mexican women with early onset of breast cancer and mutations in the BRCA1 and BRCA2 genes. Salud Publica Mex. 2005, 47, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Solano, A.R.; Aceto, G.M.; Delettieres, D.; Veschi, S.; Neuman, M.I.; Alonso, E.; Chialina, S.; Chacon, R.D.; Renato, M.C.; Podesta, E.J. BRCA1 And BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 2012, 1, 20. [Google Scholar] [CrossRef]
- Gomes, M.C.; Costa, M.M.; Borojevic, R.; Monteiro, A.N.; Vieira, R.; Koifman, S.; Koifman, R.J.; Li, S.; Royer, R.; Zhang, S.; et al. Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res. Treat. 2007, 103, 349–353. [Google Scholar] [CrossRef]
- Ferreyra, Y.; Rosas, G.; Cock-Rada, A.M.; Araujo, J.; Bravo, L.; Doimi, F.; Casas, J.; Clavo, M.L.A.; Pinto, J.A.; Belmar-Lopez, C. Landscape of germline BRCA1/BRCA2 variants in breast and ovarian cancer in Peru. Front. Oncol. 2023, 13, 1227864. [Google Scholar] [CrossRef]
- Bono, M.; Fanale, D.; Incorvaia, L.; Cancelliere, D.; Fiorino, A.; Calo, V.; Dimino, A.; Filorizzo, C.; Corsini, L.R.; Brando, C.; et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: Looking over the hedge. ESMO Open 2021, 6, 100235. [Google Scholar] [CrossRef]
- McGuire, K.P.; Mamounas, E.P. Management of Hereditary Breast Cancer: ASCO, ASTRO, and SSO Guideline. Ann. Surg. Oncol. 2020, 27, 1721–1723. [Google Scholar] [CrossRef]
- Tung, N.M.; Boughey, J.C.; Pierce, L.J.; Robson, M.E.; Bedrosian, I.; Dietz, J.R.; Dragun, A.; Gelpi, J.B.; Hofstatter, E.W.; Isaacs, C.J.; et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J. Clin. Oncol. 2020, 38, 2080–2106. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Friebel, T.; Lynch, H.T.; Neuhausen, S.L.; van’t Veer, L.; Garber, J.E.; Evans, G.R.; Narod, S.A.; Isaacs, C.; Matloff, E.; et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J. Clin. Oncol. 2004, 22, 1055–1062. [Google Scholar] [CrossRef]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collee, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef]
- Wong, S.M.; Apostolova, C.; Eisenberg, E.; Foulkes, W.D. Counselling Framework for Germline BRCA1/2 and PALB2 Carriers Considering Risk-Reducing Mastectomy. Curr. Oncol. 2024, 31, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Boddicker, N.J.; Na, J.; Polley, E.C.; Hu, C.; Hart, S.N.; Gnanaolivu, R.D.; Larson, N.; Holtegaard, S.; Huang, H.; et al. Contralateral Breast Cancer Risk among Carriers of Germline Pathogenic Variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J. Clin. Oncol. 2023, 41, 1703–1713. [Google Scholar] [CrossRef]
- Giannakeas, V.; Lim, D.W.; Narod, S.A. The risk of contralateral breast cancer: A SEER-based analysis. Br. J. Cancer 2021, 125, 601–610. [Google Scholar] [CrossRef]
- Liederbach, E.; Piro, R.; Hughes, K.; Watkin, R.; Wang, C.H.; Yao, K. Clinicopathologic features and time interval analysis of contralateral breast cancers. Surgery 2015, 158, 676–685. [Google Scholar] [CrossRef]
- Metcalfe, K.; Gershman, S.; Ghadirian, P.; Lynch, H.T.; Snyder, C.; Tung, N.; Kim-Sing, C.; Eisen, A.; Foulkes, W.D.; Rosen, B.; et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: Retrospective analysis. BMJ 2014, 348, g226. [Google Scholar] [CrossRef]
- Vasconcelos de Matos, L.; Fernandes, L.; Louro, P.; Placido, A.; Barros, M.; Vaz, F. Challenges and Considerations on Risk-Reducing Surgery in BRCA1/2 Patients with Advanced Breast Cancer. Curr. Oncol. 2021, 28, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Mau, C.; Untch, M. Prophylactic Surgery: For Whom, When and How? Breast Care 2017, 12, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, Y.; Zhu, H.; Zhou, Y.; Mao, F.; Huang, X.; Zhou, X.; Cao, X.; Sun, Q. Breast-conserving therapy for breast cancer with BRCA mutations: A meta-analysis. Breast Cancer 2022, 29, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Losurdo, A.; Sagona, A.; Zuradelli, M.; Gatzemeier, W.; Barbieri, E.; Testori, A.; Errico, V.; Bianchi, P.; Biondi, E.; et al. Surgical management of BRCA-mutation carriers: A single institution experience. Eur. J. Surg. Oncol. 2022, 48, 1706–1712. [Google Scholar] [CrossRef]
- van den Broek, A.J.; Schmidt, M.K.; van’t Veer, L.J.; Tollenaar, R.A.; van Leeuwen, F.E. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: What’s the evidence? A systematic review with meta-analysis. PLoS ONE 2015, 10, e0120189. [Google Scholar] [CrossRef]
| Characteristics | Count (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| ≤40 | 26 | 31.7 |
| 41–50 | 26 | 31.7 |
| 51–60 | 23 | 28.0 |
| >60 | 7 | 8.5 |
| Indications for referral to genetics | ||
| Age ≤ 45 years | 40 | 48.7 |
| TNBC ≤ 60 years | 26 | 31.7 |
| ≥2 breast cancer cases in FDR or SDR | 38 | 46.3 |
| Metachronous and/or synchronous cancers | 11 | 13.4 |
| Family history of ovarian cancer | 4 | 4.8 |
| Male family member with breast cancer | 2 | 2.4 |
| Family history of prostate cancer * | 2 | 2.4 |
| Clinical stage | ||
| In situ | 2 | 2.4 |
| IA | 8 | 9.8 |
| IB | 1 | 1.2 |
| IIA | 14 | 17.1 |
| IIB | 13 | 15.9 |
| IIIA | 7 | 8.5 |
| IIIB | 27 | 32.9 |
| IIIC | 4 | 4.9 |
| IV | 5 | 6.1 |
| Histological type | ||
| Ductal (NST) | 74 | 90.2 |
| Lobular | 2 | 2.4 |
| Medullary | 2 | 2.4 |
| Metaplastic | 1 | 1.2 |
| Other (papillary) | 1 | 1.2 |
| No data | 2 | 2.4 |
| Histological grade | ||
| I | 3 | 3.7 |
| II | 33 | 40.2 |
| III | 39 | 47.6 |
| No data | 7 | 8.5 |
| St. Gallen 2013 surrogate classification | ||
| Luminal A | 13 | 15.8 |
| Luminal B, HER 2− | 25 | 30.5 |
| Luminal B, HER 2+ | 6 | 7.3 |
| TNBC | 29 | 35.4 |
| HER 2 enriched | 5 | 6.1 |
| No data | 4 | 4.9 |
| Treatment | Count (n) | Percentage (%) |
|---|---|---|
| Neoadjuvant chemotherapy | 59 | 71.9 |
| AC-T | 40 | 48.8 |
| AC-TCb | 6 | 7.3 |
| AC-TH | 5 | 6.1 |
| AC alone | 4 | 4.9 |
| Other | 4 | 4.9 |
| Surgery of the primary tumor | 76 | 92.6 |
| Quadrantectomy + sentinel lymph node * | 10 | 12.2 |
| Quadrantectomy + axillary lymph node dissection * | 18 | 22 |
| Modified radical mastectomy | 38 | 46.3 |
| Simple mastectomy + sentinel lymph node | 8 | 9.8 |
| Other | 2 | 2.4 |
| Adjuvant chemotherapy | 25 | 30.4 |
| AC-T | 4 | 4.9 |
| AC-TH | 2 | 2.4 |
| AC alone | 4 | 4.9 |
| Adjuvant capecitabine | 8 | 9.8 |
| Adjuvant trastuzumab | 5 | 6.1 |
| Other | 2 | 2.4 |
| Adjuvant radiotherapy | 58 | 70.7 |
| 3DCRT | 41 | 50 |
| IMRT | 17 | 20.7 |
| Adjuvant hormone therapy | 39 | 47.5 |
| Tamoxifen | 26 | 31.7 |
| Tamoxifen + aromatase inhibitor | 9 | 11 |
| Aromatase inhibitor | 2 | 2.4 |
| GNRH analogues + tamoxifen | 2 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria-Salas, M.C.; Pedroza-Duran, A.; Díaz-Casas, S.E.; Nuñez Lemus, M.; Grillo-Ardila, C.F.; Briceño-Morales, X.; García-Mora, M.; Ángel-Aristizábal, J.; Mariño Lozano, I.F.; Suarez Rodríguez, R.A.; et al. Management and Clinical Outcomes of Breast Cancer in Women Diagnosed with Hereditary Cancer Syndromes in a Clinic-Based Sample from Colombia. Cancers 2024, 16, 2020. https://doi.org/10.3390/cancers16112020
Sanabria-Salas MC, Pedroza-Duran A, Díaz-Casas SE, Nuñez Lemus M, Grillo-Ardila CF, Briceño-Morales X, García-Mora M, Ángel-Aristizábal J, Mariño Lozano IF, Suarez Rodríguez RA, et al. Management and Clinical Outcomes of Breast Cancer in Women Diagnosed with Hereditary Cancer Syndromes in a Clinic-Based Sample from Colombia. Cancers. 2024; 16(11):2020. https://doi.org/10.3390/cancers16112020
Chicago/Turabian StyleSanabria-Salas, María Carolina, Ana Pedroza-Duran, Sandra E. Díaz-Casas, Marcela Nuñez Lemus, Carlos F. Grillo-Ardila, Ximena Briceño-Morales, Mauricio García-Mora, Javier Ángel-Aristizábal, Iván Fernando Mariño Lozano, Raúl Alexis Suarez Rodríguez, and et al. 2024. "Management and Clinical Outcomes of Breast Cancer in Women Diagnosed with Hereditary Cancer Syndromes in a Clinic-Based Sample from Colombia" Cancers 16, no. 11: 2020. https://doi.org/10.3390/cancers16112020
APA StyleSanabria-Salas, M. C., Pedroza-Duran, A., Díaz-Casas, S. E., Nuñez Lemus, M., Grillo-Ardila, C. F., Briceño-Morales, X., García-Mora, M., Ángel-Aristizábal, J., Mariño Lozano, I. F., Suarez Rodríguez, R. A., & Guzmán Abisaab, L. H. (2024). Management and Clinical Outcomes of Breast Cancer in Women Diagnosed with Hereditary Cancer Syndromes in a Clinic-Based Sample from Colombia. Cancers, 16(11), 2020. https://doi.org/10.3390/cancers16112020





