Sex Differences in Anxiety and Depression Conditions among Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Peer Review and Data Extraction
2.3. Records Assessed
2.4. Interventions and Outcomes
2.5. Quality and Bias Assessments
- Level I: Evidence from systematic reviews or meta-analysis of randomized control trials;
- Level II: Evidence from well-designed randomized control trials;
- Level III: Evidence from well-designed control trials that are not randomized;
- Level IV: Evidence from case–control or cohort studies;
- Level V: Evidence from systematic reviews of descriptive or qualitative studies;
- Level VI: Evidence from a single descriptive or qualitative study;
- Level VII: Evidence from expert opinions.
2.6. Main Outcome(s)
2.7. Measures of Effect
2.8. Data Synthesis
3. Results
3.1. Study Characteristics
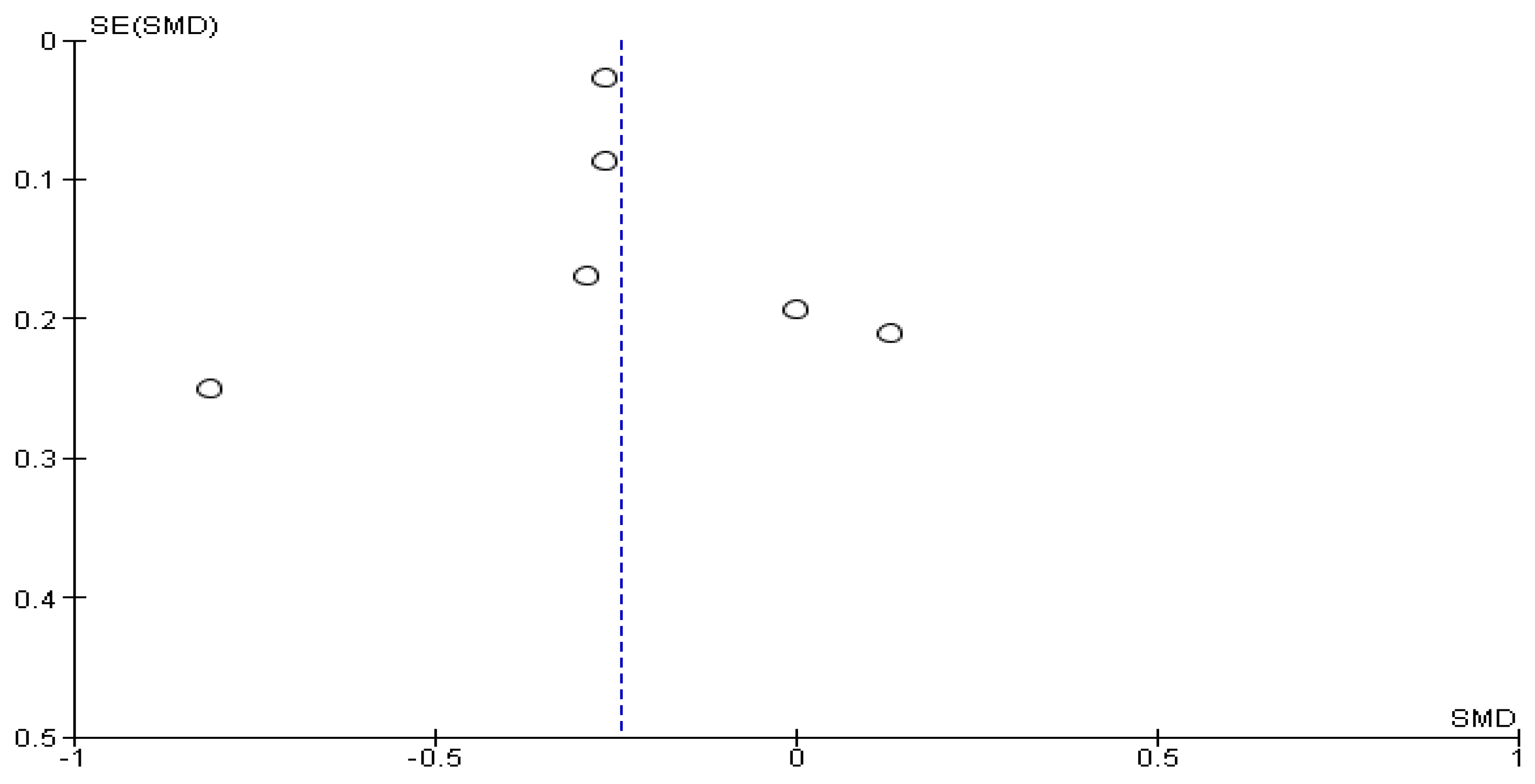
3.2. Bias Risk Assessment
3.3. Meta-Analysis Results for HADS-Anxiety (HADS-A)
3.4. Meta-Analysis Results for HADS-Depression (HADS-D)
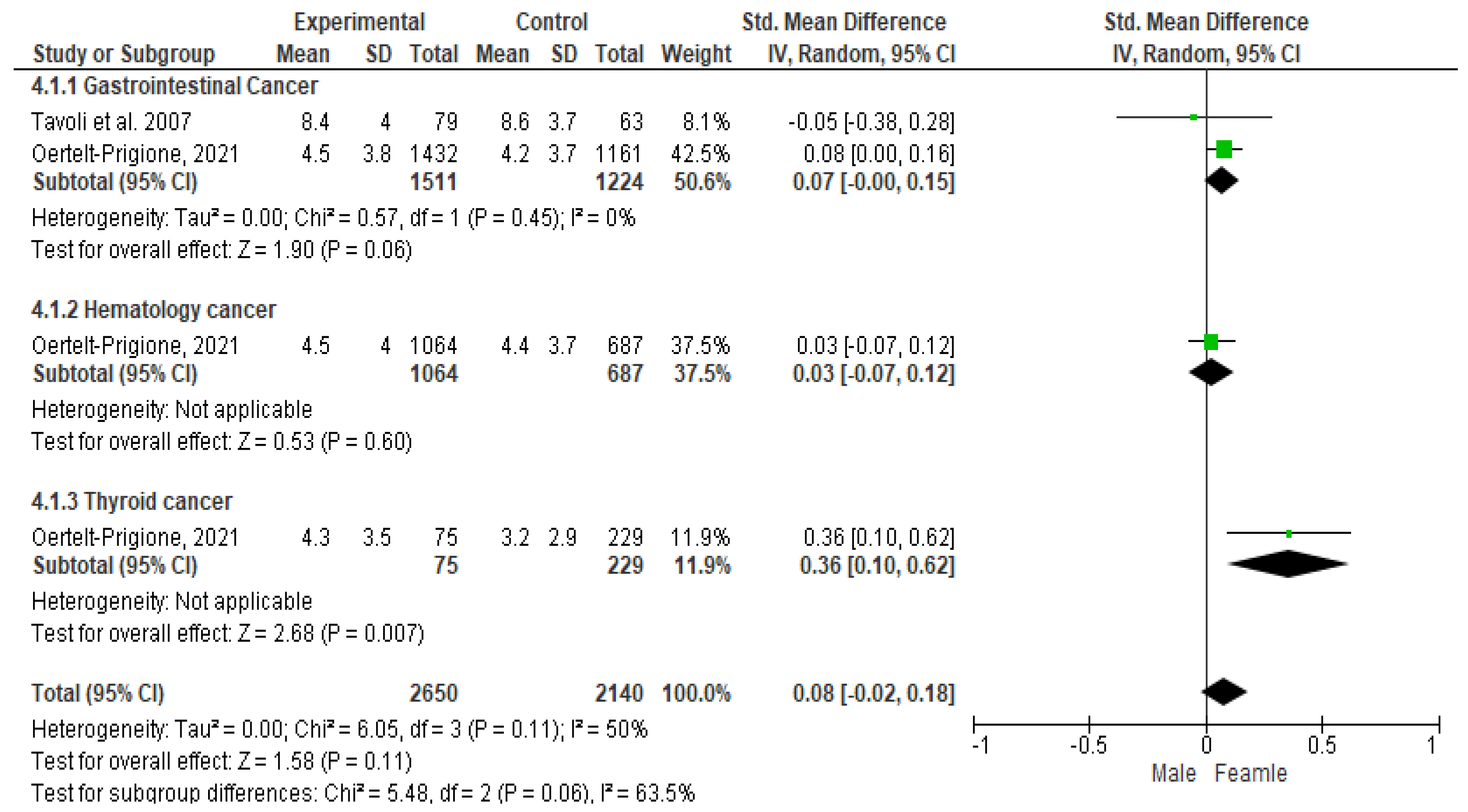
3.5. Meta-Analysis Results for HADS-Anxiety (HADS-A) among Patients Diagnosed with Gastrointestinal, Blood, and Thyroid Cancers
3.6. Meta-Analysis Results for HADS-Depression (HADS-D) among Patients Diagnosed with Gastrointestinal, Blood, and Thyroid Cancers
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Additional Disclosure
References
- World Health Organization (WHO). Cancers. 2020. Available online: https://www.who.int/news (accessed on 12 February 2024).
- Powe, B.D.; Finnie, R. Cancer fatalism: The state of the science. Cancer Nurs. 2003, 26, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Naus, M.J.; Ishler, M.D.; Parrott, C.E.; Kovacs, S.A. Cancer survivor adaptation model: Conceptualizing cancer as a chronic illness. J. Clin. Psychol. 2009, 65, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- VanHoose, L.; Black, L.L.; Doty, K.; Sabata, D.; Twumasi-Ankrah, P.; Taylor, S.; Johnson, R. An analysis of the distress thermometer problem list and distress in patients with cancer. Support. Care Cancer 2015, 23, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Aass, N.; Fosså, S.D.; Dahl, A.A.; Moe, T.J. Prevalence of anxiety and depression in cancer patients seen at the Norwegian Radium Hospital. Eur. J. Cancer 1997, 33, 1597–1604. [Google Scholar] [CrossRef]
- Ford, S.; Lewis, S.; Fallowfield, L. Psychological morbidity in newly referred patients with cancer. J. Psychosom. Res. 1995, 39, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bultz, B.D.; Carlson, L.E. Emotional distress: The sixth vital sign in cancer care. J. Clin. Oncol. 2005, 23, 6440–6441. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; DeRubeis, R.J.; Berman, B.S.; Gruman, J.; Champion, V.L.; Massie, M.J.; Holland, J.C.; Partridge, A.H.; Bak, K.; Somerfield, M.R.; et al. American Society of Clinical Oncology. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. J. Clin. Oncol. 2014, 32, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.E.; Waller, A.; Groff, S.L.; Giese-Davis, J.; Bultz, B.D. What goes up does not always come down: Patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psychooncology 2013, 22, 168–176. [Google Scholar] [CrossRef]
- Sanford, S.D.; Beaumont, J.L.; Butt, Z.; Sweet, J.J.; Cella, D.; Wagner, L.I. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J. Pain. Symptom Manag. 2014, 47, 721–730. [Google Scholar] [CrossRef]
- Aarstad, H.J.; Aarstad, A.K.; Heimdal, J.H.; Olofsson, J. Mood, anxiety and sense of humor in head and neck cancer patients in relation to disease stage, prognosis and quality of life. Acta Otolaryngol. 2005, 125, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Wan Ahmad, W.A.; Yusof, M.M.; Ho, G.F.; Krupat, E. Effects of depression and anxiety on mortality in a mixed cancer group: A longitudinal approach using standardised diagnostic interviews. Psychooncology 2015, 24, 718–725. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Semlyen, J.; Tai, S.S.; Killaspy, H.; Osborn, D.; Popelyuk, D.; Nazareth, I. A systematic review of mental disorder, suicide, and deliberate self-harm in lesbian, gay and bisexual people. BMC Psychiatry 2008, 8, 70. [Google Scholar] [CrossRef]
- Mols, F.; Schoormans, D.; de Hingh, I.; Oerlemans, S.; Husson, O. Symptoms of anxiety and depression among colorectal cancer survivors from the population-based, longitudinal PROFILES Registry: Prevalence, predictors, and impact on quality of life. Cancer 2018, 124, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Obispo-Portero, B.; Cruz-Castellanos, P.; Jiménez-Fonseca, P.; Rogado, J.; Hernandez, R.; Castillo-Trujillo, O.A.; Asensio-Martínez, E.; González-Moya, M.; Carmona-Bayonas, A.; Calderon, C. Anxiety and depression in patients with advanced cancer during the COVID-19 pandemic. Support. Care Cancer 2022, 30, 3363–3370. [Google Scholar] [CrossRef]
- Abu-Odah, H.; Molassiotis, A.; Yat Wa Liu, J. Analysis of the unmet needs of Palestinian advanced cancer patients and their relationship to emotional distress: Results from a cross-sectional study. BMC Palliat. Care 2022, 21, 72. [Google Scholar] [CrossRef]
- Nguyen, T.; Tracy, K.; Ullah, A.; Karim, N.A. Effect of Exercise Training on Quality of Life, Symptoms, and Functional Status in Advanced-Stage Lung Cancer Patients: A Systematic Review. Clin. Pract. 2023, 13, 715–730. [Google Scholar] [CrossRef]
- Applebaum, A.J.; Stein, E.M.; Lord-Bessen, J.; Pessin, H.; Rosenfeld, B.; Breitbart, W. Optimism, social support, and mental health outcomes in patients with advanced cancer. Psychooncology 2014, 23, 299–306. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An. updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Vodermaier, A.; Millman, R.D. Accuracy of the Hospital Anxiety and Depression Scale as a screening tool in cancer patients: A systematic review and meta-analysis. Support Care Cancer 2011, 19, 1899–1908. [Google Scholar] [CrossRef]
- Michopoulos, I.; Douzenis, A.; Kalkavoura, C.; Christodoulou, C.; Michalopoulou, P.; Kalemi, G.; Fineti, K.; Patapis, P.; Protopapas, K.; Lykouras, L. Hospital Anxiety and Depression Scale (HADS): Validation in a Greek general hospital sample. Ann. Gen. Psychiatry 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, B.M.; Gallagher-Ford, L.; Long, L.E.; Fineout-Overholt, E. The establishment of evidence-based practice competencies for practicing registered nurses and advanced practice nurses in real-world clinical settings: Proficiencies to improve healthcare quality, reliability, patient outcomes, and costs. Worldviews Evid. Based Nurs. 2014, 11, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2008. [Google Scholar]
- Skarstein, J.; Aass, N.; Fosså, S.D.; Skovlund, E.; Dahl, A.A. Anxiety and depression in cancer patients: Relation between the Hospital Anxiety and Depression Scale and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire. J. Psychosom. Res. 2000, 49, 27–34. [Google Scholar] [CrossRef]
- Tavoli, A.; Mohagheghi, M.A.; Montazeri, A.; Roshan, R.; Tavoli, Z.; Omidvari, S. Anxiety and depression in patients with gastrointestinal cancer: Does knowledge of cancer diagnosis matter? BMC Gastroenterol. 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.T.; Künzler, A.; Nussbeck, F.; Bargetzi, M.; Znoj, H.J. Higher emotional distress in female partners of cancer patients: Prevalence and patient-partner interdependencies in a 3-year cohort. Psychooncology 2013, 22, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Greer, J.A.; El-Jawahri, A.; Traeger, L.; Gallagher, E.R.; Park, E.R.; Jackson, V.A.; Pirl, W.F.; Temel, J.S. Age and Sex Moderate the Impact of Early Palliative Care in Metastatic Non-Small Cell Lung Cancer. Oncologist 2016, 21, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S.; de Rooij, B.H.; Mols, F.; Oerlemans, S.; Husson, O.; Schoormans, D.; Haanen, J.B.; van de Poll-Franse, L.V. Sex-differences in symptoms and functioning in >5000 cancer survivors: Results from the PROFILES registry. Eur. J. Cancer 2021, 156, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Sun, H.; Pokhrel, G.; Wang, B.; Dahal, S.; Yu, S. Performance of distress thermometer and associated factors of psychological distress among Chinese cancer patients. J. Oncol. 2020, 2020, 3293589. [Google Scholar] [CrossRef]
- Hasan, E.M.; Calma, C.L.; Tudor, A.; Oancea, C.; Tudorache, V.; Petrache, I.A.; Tudorache, E.; Papava, I. Coping, Anxiety, and Pain Intensity in Patients Requiring Thoracic Surgery. J. Pers. Med. 2021, 11, 1221. [Google Scholar] [CrossRef]
- Rand, K.L.; Cripe, L.D.; Monahan, P.O.; Tong, Y.; Schmidt, K.; Rawl, S.M. Illness appraisal, religious coping, and psychological responses in men with advanced cancer. Support. Care Cancer 2012, 20, 1719–1728. [Google Scholar] [CrossRef]
- Götze, H.; Friedrich, M.; Taubenheim, S.; Dietz, A.; Lordick, F.; Mehnert, A. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support. Care Cancer 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, Sex, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Van’t Spijker, A.; Trijsburg, R.W.; Duivenvoorden, H.J. Psychological sequelae of cancer diagnosis. Psychosom. Med. 1997, 59, 280–293. [Google Scholar] [CrossRef]
- Piccinelli, M.; Wilkinson, G. Sex differences in depression. Critical review. Br. J. Psychiatry 2000, 177, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Goldzweig, G.; Andritsch, E.; Hubert, A.; Walach, N.; Perry, S.; Brenner, B.; Baider, L. How relevant is marital status and Sex variables in coping with colorectal cancer? A sample of middle-aged and older cancer survivors. Psychooncology 2009, 18, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Lawson, J.M.; Schumacher, M.M.; Hughes, T.; Arnold, S. Sex differences in psychosocial responses to lung cancer. Gend. Med. 2010, 7, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hasan, E.M.; Calma, C.L.; Tudor, A.; Vernic, C.; Palade, E.; Tudorache, E.; Oancea, C.; Papava, I. Sex Differences in Coping, Depression, and Anxiety in Patients with Non-Metastatic Lung Cancer. Cancer Manag. Res. 2022, 14, 2041–2052. [Google Scholar] [CrossRef]
- Zhang, L. Anxiety and depression in recurrent gastric cancer: Their prevalence and independent risk factors analyses. Medicine 2021, 100, e28358. [Google Scholar] [CrossRef] [PubMed]
- Nordin, K.; Glimelius, B. Reactions to gastrointestinal cancer--variation in mental adjustment and emotional well-being over time in patients with different prognoses. Psychooncology 1998, 7, 413–423. [Google Scholar] [CrossRef]
- Nordin, K.; Glimelius, B.; Påhlman, L.; Sjödén, P.O. Anxiety, depression and worry in gastrointestinal cancer patients attending medical follow-up control visits. Acta Oncol. 1996, 35, 411–416. [Google Scholar] [CrossRef]
- Nordin, K.; Glimelius, B. Psychological reactions in newly diagnosed gastrointestinal cancer patients. Acta Oncol. 1997, 36, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Esser, P.; Mehnert, A.; Hinz, A.; Johansen, C.; Lordick, F.; Götze, H. Risk for depression and anxiety in long-term survivors of hematologic cancer. Health Psychol. 2019, 38, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, L.; Ashby, S.; Cannon, R.B.; Hunt, J.P. Psychosocial distress in patients with thyroid cancer. Otolaryngol. Head. Neck Surg. 2015, 152, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Stordal, E.; Mykletun, A.; Dahl, A.A. The association between age and depression in the general population: A multivariate examination. Acta Psychiatr. Scand. 2003, 107, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Seedat, S.; Scott, K.M.; Angermeyer, M.C.; Berglund, P.; Bromet, E.J.; Brugha, T.S.; Demyttenaere, K.; de Girolamo, G.; Haro, J.M.; Jin, R.; et al. Cross-national associations between Sex and mental disorders in the World Health Organization World Mental Health Surveys. Arch. Gen. Psychiatry 2009, 66, 785–795. [Google Scholar] [CrossRef]
- Noto, B.; Asmus, I.; Schäfers, M.; Görlich, D.; Riemann, B. Predictors of Anxiety and Depression in Differentiated Thyroid Cancer Survivors: Results of a Cross-Sectional Study. Thyroid 2022, 32, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, A.; Konecny, L.M. Quality of life, social support, and uncertainty among Latina breast cancer survivors. Oncol. Nurs. Forum. 2008, 35, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Rassoulian, A.; Gaiger, A.; Loeffler-Stastka, H. Sex differences in psychosocial, religious, and spiritual aspects in coping: A cross-sectional study with cancer patients. Women’s Health Rep. 2021, 2, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Henrich, M.K.G. Illness-related distress: Does it mean the same for men and women?: Sex aspects in cancer patients’ distress and adjustment. Acta Oncol. 1999, 38, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ryu, E. Effects of symptom clusters and depression on the quality of life in patients with advanced lung cancer. Eur. J. Cancer Care 2018, 27, e12508. [Google Scholar] [CrossRef] [PubMed]
- Obispo, B.; Cruz-Castellanos, P.; Hernandez, R.; Gil-Raga, M.; González-Moya, M.; Rogado, J.; López-Ceballos, H.; García-Carrasco, M.; Jiménez-Fonseca, P.; Calderon, C. Perceived Dignity of Advanced Cancer Patients and Its Relationship to Sociodemographic, Clinical, and Psychological Factors. Front. Psychol. 2022, 13, 855704. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.W.; Soong, I.; Yau, T.K.; Wong, K.Y.; Tsang, J.; Yeo, W.; Suen, J.; Ho, W.M.; Sze, W.K.; Ng, A.W.; et al. The evolution of psychological distress trajectories in women diagnosed with advanced breast cancer: A longitudinal study. Psychooncology 2013, 22, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
- Neo, J.; Fettes, L.; Gao, W.; Higginson, I.J.; Maddocks, M. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 61, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.L.; McDonald, C.F.; Irving, L.; Clark, R.A.; Gough, K.; Murnane, A.; Mileshkin, L.; Krishnasamy, M.; Denehy, L. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014, 83, 292–299. [Google Scholar] [CrossRef] [PubMed]





| Population | Cancer patients |
| Intervention | Anxiety and depression assessments |
| Outcome | Anxiety and depression between females and males |
| Name of Author, Year and Country | Design Level of Evidence | Sample Size | Age (min.–max. Years) | Eligible Criteria | Instruments | Findings | |
|---|---|---|---|---|---|---|---|
| Inclusion | Exclusion | ||||||
| Skarstein et al., 2000 Norway [28] | Comparative study III level | F: 215 M: 353 T: 568 | 15–92 (median: 55) | Any type of cancer | NR | HADS-A HADS-D | Older male patients reported less distress than younger patients. Anxiety and depression directly impact on QoL. |
| Tavoli et al., 2007 Iran [29] | Cross-sectional study III level | F: 63 M: 79 T: 142 | 39–69 | GI | Patients with cognitive problems | HADS-A HADS-D | Patients who knew their diagnosis reported higher distress. Family background and kind of information improve the psychological outcome. |
| Moser et al., 2013 Switzerland [30] | Cohort study IV level | F: 53 M: 69 T: 122 | F: 30–84 M: 26–89 | Newly diagnosed cancer patient | NR | HADS-A HADS-D QOL SCL-K9 | Males experienced greater psychology problems than females. |
| Nipp et al., 2016 USA [31] | RCT II level | F: 50 M: 57 T: 107 | >65 <65 | NSCLC metastatic patient English language knowledge | Patients addressed to palliative care | FACT-L HADS-A PHQ-9 | Male and younger patients had better QoL and anxiety when they received early palliative care. |
| Oertelt-Prigione et al., 2021 The Netherlands [32] | Cohort study IV level | F: 2413 M: 2926 | 40–70 | Colorectal cancer Blood basal cell/squamous cell Thyroid cancer Dutch language knowledge | Impaired cognitive functions | EORTC HADS-A HADS-D SCQ. | Male patients reported more severe symptoms than female patients. |
| Author(s) Publication Year | Country | Reporting (Total Score: 10) | External Validity (Total Score: 3) | Internal Validity-Confounding (Selection Bias) | Total Quality Score |
|---|---|---|---|---|---|
| Skarstein et al., 2000 [27] | Norway | 8 | 3 | 12 | 23 |
| Tavoli et al., 2007 [28] | Iran | 7 | 3 | 11 | 21 |
| Moser et al., 2013 [29] | Switzerland | 8 | 2 | 10 | 20 |
| Nipp et al., 2016 [30] | USA | 8 | 2 | 12 | 22 |
| Oertelt-Prigione et al., 2021 [31] | The Netherlands | 7 | 3 | 11 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, E.; Halemani, K.; Shetty, A.; Chang, Y.-C.; Hu, W.-Y.; Massafra, R.; Moretti, A. Sex Differences in Anxiety and Depression Conditions among Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 1969. https://doi.org/10.3390/cancers16111969
Vitale E, Halemani K, Shetty A, Chang Y-C, Hu W-Y, Massafra R, Moretti A. Sex Differences in Anxiety and Depression Conditions among Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(11):1969. https://doi.org/10.3390/cancers16111969
Chicago/Turabian StyleVitale, Elsa, Kurvatteppa Halemani, Asha Shetty, Yun-Chen Chang, Wen-Yu Hu, Raffaella Massafra, and Annamaria Moretti. 2024. "Sex Differences in Anxiety and Depression Conditions among Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 16, no. 11: 1969. https://doi.org/10.3390/cancers16111969
APA StyleVitale, E., Halemani, K., Shetty, A., Chang, Y.-C., Hu, W.-Y., Massafra, R., & Moretti, A. (2024). Sex Differences in Anxiety and Depression Conditions among Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 16(11), 1969. https://doi.org/10.3390/cancers16111969










