Simple Summary
Psilocybin therapy shows promise for reducing anxiety and depression and improving psychological well-being in cancer patients nearing the end of life. However, providing this treatment outside of tightly controlled research studies is extremely challenging. In 2020, Oregon became the first U.S. state to legalize psilocybin therapy for depression related to terminal illnesses such as advanced cancer. This review examines the published evidence on psilocybin’s therapeutic potential as well as the multitude of legal, ethical, and logistical hurdles Oregon has faced while attempting to roll out regulated psilocybin services over the past year. The aim is to shed light on the complex issues involved in responsibly implementing psychedelic-assisted therapy for patient populations with serious psychological distress. By proactively identifying and addressing these challenges, appropriate standards of care and equitable access to psilocybin treatment can be ensured as it transitions from research to real-world clinical practice. Insights from Oregon’s experience as the pioneering state can help guide a rigorous, ethical path forward.
Abstract
Despite the legalization of psilocybin therapy for depression in terminal illnesses such as advanced cancer through Oregon’s Measure 109 in 2020, significant challenges have impeded its implementation. This review synthesizes the empirical data supporting the utilization of psilocybin therapy for addressing cancer-related depression, including an evaluation of its purported benefits and potential adverse effects. It provides a comprehensive examination of therapeutic strategies, dosing regimens, and barriers to ensuring responsible and equitable access. Salient issues explored include the development of ethical protocols, integration within healthcare systems, ensuring statewide availability, resolving legal ambiguities, and defining clinical standards. Oregon’s pioneering role serves as a case study, highlighting the necessity of addressing regulatory, logistical, and ethical obstacles to ensure the establishment of rigorous and equitable psilocybin care models.
1. Introduction
In November 2020, Oregon became the first U.S. state to legalize the therapeutic use of psilocybin, the active compound found in certain species of fungi. This unprecedented legislation paved the way for Oregon Health & Science University’s (OHSU) psilocybin services program, which began offering psilocybin-assisted therapy in January 2023 under the supervision of licensed facilitators for patients with depression and anxiety related to life-threatening illnesses [1,2]. As researchers continue studying psilocybin as a potential treatment for various mental health conditions, Oregon’s legalization has generated substantial interest in the implementation and outcomes of its psilocybin therapy program.
This article provides a comprehensive assessment of the current landscape surrounding psilocybin’s therapeutic potential within the context of cancer-related depression, highlighting both its promises and challenges in light of Oregon’s recent legislation. By synthesizing existing research, clinical trials, and ethical considerations, this review informs healthcare professionals, policymakers, and the public about the evolving role of psilocybin in addressing the profound psychological toll of cancer and the pursuit of enhanced mental well-being. Furthermore, the legal and ethical considerations of expanding access to psilocybin therapy are discussed, along with key questions still needing investigation regarding safety, efficacy, and best practices for structured psilocybin sessions.
2. Depression and Cancer
2.1. Exploring the Burden of Depression in the Cancer Population
Depression is a significant concern among cancer patients in the United States, with prevalence rates varying depending on the type of cancer. Studies have consistently shown that the prevalence of depression is higher among cancer patients than in the general population. According to various estimates, the prevalence of depression ranges from 15% to 25% among cancer patients, depending on the type and stage of cancer as well as other factors [3,4]. Studies have shown that the prevalence of depression can range from 3% in patients with lung cancer to as high as 31% in patients with cancer of the digestive tract [4,5].
Studying the prevalence of depression in cancer patients is important for several reasons. First, understanding the prevalence and risk factors allows for early detection and intervention, enabling healthcare professionals to identify and support patients at a higher risk of depression [6]. Despite the high prevalence of depression among cancer patients, it often goes undiagnosed and undertreated. Healthcare professionals may overlook the psychological aspects of cancer care, focusing primarily on the physical aspects of treatment [7]. While cancer can increase the risk of depression due to the psychological and physical burden of the disease, depression itself may also increase the risk of developing certain types of cancer, potentially due to the physiological effects of chronic stress and inflammation [8]. It can exacerbate physical symptoms, reduce treatment adherence, and negatively affect treatment outcomes [9]. Several studies have suggested that depression in cancer patients is associated with an increased risk of mortality. This may be due to various factors, including reduced treatment adherence, physiological effects of depression on the immune system, and a higher risk of suicide [10]. Additionally, depression in cancer patients is associated with increased healthcare costs and longer hospital stays [11].
Recognizing demoralization as a separate entity from depression and identifying its unique impact on patients’ well-being can lead to more targeted interventions and improved outcomes. Studies have shown that demoralization is prevalent in this population, with rates ranging from 13% to 50% [12]. Demoralization often coexists with depression, anxiety, adjustment disorders, and suicidal ideation in cancer patients [12,13,14]. While demoralization and depression share similarities, they are distinct conditions, with demoralization characterized by feelings of subjective incompetence and a lack of hope [15,16]. Studies have shown that demoralization can impact various aspects of a cancer patient’s life, including quality of life, sleep quality, spiritual interests, and suicide risk [17,18,19]. Furthermore, demoralization has been linked to factors such as existential anxiety, masculine self-esteem, resilience, and perceived stress, all of which play a role in predicting psychological well-being and the development of depression in cancer patients [20,21,22,23]. It is essential to differentiate demoralization from depression, as some patients may experience demoralization without depression, and vice versa [16,24]. Moreover, demoralization has been identified as a risk factor for suicidal behavior, emphasizing the importance of addressing this condition in cancer care [13,25].
The effective management of depression may also lead to better treatment adherence and potentially improved survival rates. Additionally, addressing depression can significantly improve cancer patients’ overall quality of life by reducing their physical and emotional distress and improving their ability to cope with the challenges of treatment [26,27]. Early identification and interventions targeting demoralization, along with hopelessness and depression, are crucial in preventing the development of suicidal ideation in cancer patients [14,28]. Finally, understanding the scope of the problem can inform research efforts and guide the allocation of resources for developing effective screening, treatment, and support strategies for cancer patients with depression.
2.2. Psychiatric Treatment of Depression in Cancer Patients
Psychopharmacological treatment for cancer-related depression is a complex area that requires a tailored approach to meet the individual needs of patients. Research indicates that both psychosocial interventions and pharmacotherapy are effective in treating depression in cancer patients [29]. Antidepressants are frequently prescribed to cancer patients with depression, but studies have shown that they have limited effectiveness in improving depressive symptoms in terminally ill cancer patients [29,30]. While pharmacological treatment is important in managing depression, especially in advanced cancer patients, the characteristics of these patients can influence the choice of pharmacological interventions [31]. Additionally, the pharmacological treatment of depression in cancer patients is complicated by drug–drug interactions between antidepressants and cancer pharmacotherapy, potentially reducing the efficacy of cancer treatment [32,33]. Despite the widespread use of antidepressants in cancer care, their effectiveness remains a subject of debate, with some studies indicating that currently prescribed antidepressants may only be efficacious in a limited subset of patients [34]. Additionally, the use of conventional antidepressants in cancer patients has been linked to decreased compliance with anticancer treatments [35]. Table 1 provides additional details regarding pharmacological interventions for depression in cancer patients.

Table 1.
Targeted psychopharmacological treatment for depression in cancer patients.
Studies have suggested that combined pharmacological and psychological treatments may be more effective than either approach alone in primary depression [38]. However, this combined approach has not been extensively tested in cancer populations, indicating a gap in knowledge that requires further investigation [38]. Additionally, recent literature reviews have provided mixed evidence regarding the effectiveness of antidepressant drugs for depression in cancer patients [39,40].
The critical need for a novel drug for depression in cancer patients arises from the limitations of current antidepressants; the unique features and challenges of cancer-related depression; the potential to improve quality of life, treatment adherence, and overall outcomes; and the need for tailored approaches for specific cancer populations [41]. Dysregulated pathways implicated in cancer-induced depression could be addressed by novel therapeutics to alleviate depressive symptoms in these patients [42]. Additionally, drug repositioning approaches focusing on immune alterations and inflammation have demonstrated the potential for novel pharmacological interventions in depression by targeting inflammatory mechanisms [43]. The emergence of new drugs for treating major depression, such as curcumin, provides a hopeful avenue for addressing depression in cancer patients [44]. Novel drug discovery efforts, such as exploring traditional Chinese medicine compounds like oxymatrine, have been suggested as a strategy to develop effective therapies for cancer patients, aiming to enhance treatment efficacy and overcome drug resistance [45].
3. Role of Psilocybin in the Treatment of Depression
3.1. Background on Psilocybin and Its Antidepressant Effects
Psilocybin, a hallucinogenic compound found in certain mushrooms, has garnered attention for its antidepressant effects, particularly in the context of life-threatening diseases such as cancer. Research has shown promising results in using psilocybin to alleviate psychological distress in cancer patients [46,47,48,49,50]. Studies have shown that psilocybin’s antidepressant effects may not necessarily rely on altered perception, suggesting that its mechanism of action in treating depression can be studied independently [51]. Neuroimaging studies in healthy volunteers have revealed that psilocybin produces profound and meaningful alterations in brain function, especially of the default mode network, consistent with an antidepressant effect [52]. Furthermore, investigations into the neural correlates of psilocybin administration have revealed changes in brain activity and connectivity, indicating a putative neural mechanism of action for psilocybin [53,54]. Other studies have also indicated increased global integration in the brain after psilocybin therapy for depression, suggesting potential neuromodulatory effects [55,56]. Psilocybin therapy has also been shown to increase cognitive flexibility for at least four weeks post-treatment [57]. This revival of emotional responsiveness on a neural and psychological level is proposed as a key treatment mechanism for psychedelic therapy.
Psilocin, the active metabolite of psilocybin, has been shown to increase the concentrations of dopamine and serotonin in specific brain pathways [58,59]. These compounds are structurally similar to serotonin, with slight chemical modifications that lead to hallucinogenic effects [60]. Psilocybin is dephosphorylated in vivo to form psilocin, which is responsible for its psychoactive effects [59]. Psilocin is metabolized through oxidative deamination to form 4-hydroxy-3-indoleacetic acid and psilocin-O-glucuronide [61].
The 4-hydroxyl group of psilocin likely contributes to its oral bioavailability and enhanced metabolic stability, aiding in improved central nervous system penetration [62]. The levels of 5-hydroxyindoleacetic acid (5-HIAA) in relation to serotonin were found to be elevated in specific brain regions such as the hypothalamus and pons [63]. Psilocin easily crosses the blood–brain barrier and exerts its psychoactive effects by acting on serotonin receptors [64]. Figure 1 summarizes the pharmacological conversion of psilocybin to psilocin.
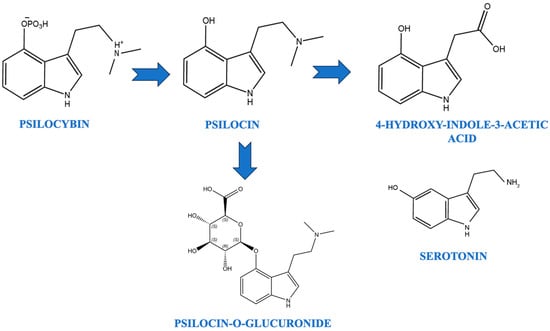
Figure 1.
Pharmacological conversion of psilocybin to psilocin.
The overall psychedelic experience induced by psilocybin results from a complex interplay between various serotonin receptor subtypes, as well as interactions with other neurotransmitter systems and brain regions. Psilocybin exerts its effects primarily through agonist activity at the 5-HT2A receptor [48,65,66,67,68]. The activation of the 5-HT2A receptor by psilocybin has been associated with various outcomes, including visual hallucinations, increased cortical excitability, and alterations in visual-evoked cortical responses [65]. Psilocybin’s stimulation of 5-HT2A/1A receptors has been linked to a reduction in social pain processing and the induction of long-term changes in mindfulness [69,70]. Studies have shown that psilocybin treatment can lead to increased global integration in the brain, with higher-order functional networks becoming more interconnected and flexible [55]. Furthermore, research suggests that the behavioral and synaptic actions of psilocybin may be independent of 5-HT2A receptor activation, as evidenced by studies in which responses to psilocybin were not prevented by a 5-HT2A/2C antagonist [51]. Psilocybin’s effects on brain dynamics have been associated with the distribution of 5-HT2A receptors across the cortex, influencing brain control energy landscapes [71]. Additionally, psilocybin has been shown to activate both 5-HT1A and 5-HT2A receptor subtypes, impacting attention, working memory, and other cognitive functions [72]. Table 2 provides additional data regarding serotonergic receptors affected by psilocybin.

Table 2.
Psilocybin and serotonin receptors.
3.2. Psilocybin for End-of-Life Care and Cancer-Related Depression
Psychedelic therapy, particularly with compounds like psilocybin, has shown promising potential in treating cancer-related depression and psychological distress. Most studies have been conducted in controlled clinical settings, typically involving preparatory sessions, the administration of psilocybin under supervision, and integration sessions afterwards. Participants were generally patients with advanced or terminal cancer diagnoses experiencing anxiety, depression, or existential distress related to their condition [75,76,77,78]. For example, a pilot study at the University of California, Los Angeles, reignited interest in psilocybin treatment for advanced-stage cancer patients, leading to renewed efforts in psilocybin research [58]. Furthermore, the FDA granted the breakthrough therapy designation for psilocybin in the treatment of depression, indicating its potential as a novel therapeutic approach [79,80].
Clinical trials have demonstrated significant reductions in anxiety and depression in cancer patients following psilocybin treatment, with sustained symptom reduction observed [46,47,81,82]. The psychedelic experience, combined with psychological support, appears to help alleviate the mental health burdens often associated with cancer diagnosis and treatment. Psilocybin has been found to produce rapid, notable, and lasting effects, leading to immediate, substantial, and sustained improvements in anxiety and depression; decreases in cancer-related demoralization and hopelessness; and sustained benefits in existential distress [83,84,85,86]. It has also been associated with improvements in feelings of connectedness and acceptance, quality of life, and attitudes toward death [87,88].
Long-term follow-up studies have documented sustained improvements in anxiety and depression, as well as improved quality of life, in cancer patients following psilocybin treatment [82]. These positive effects were often sustained for several months after the psilocybin sessions [89,90,91]. The legalization of psilocybin therapy in certain regions, such as Oregon, in the United States, and Canada, reflects the growing acceptance and recognition of its potential therapeutic benefits [92].
There is increasing interest in understanding the negative outcomes associated with psilocybin use [93]. Research indicates that psilocybin administration can lead to effects such as silliness, laughter, and playfulness, which may be perceived as unconventional but can be modulated to align with the desired clinical outcomes [94]. It is important to note that psilocybin has potent psychedelic properties and is illegal in most countries, with reports of serious negative health-related outcomes in non-clinical settings due to poorly regulated behavior [95,96,97]. Studies have found that younger individuals are more likely to require emergency medical treatment after using psilocybin than older individuals. The most frequently reported adverse reactions are psychological in nature, such as severe anxiety, panic attacks, paranoia, and psychotic symptoms [98]. Physiological side effects such as headaches, dizziness, gastrointestinal distress, and changes in blood pressure are also possible when taking psilocybin [99]. The primary reasons cited for adverse incidents are an unprepared or negative psychological state prior to the psilocybin experience, an unsuitable or unsupportive physical environment or setting, and the mixing of psilocybin with other substances or drugs [98].
3.3. Risk of Combining Psilocybin and Traditional Antidepressants in Cancer Patients
Combining psilocybin with traditional antidepressants in cancer patients raises concerns due to potential interactions and safety issues. While psilocybin has shown promise in enhancing the well-being of cancer patients [100], its combination with antidepressants may have varying effects. The main concern with combining psilocybin and antidepressant medications is the risk of developing serotonin syndrome. Additionally, serotonergic antidepressant use has been associated with weaker psilocybin effects and potential adverse reactions [89]. Certain antidepressants like MAOIs are generally contraindicated with psilocybin due to the risk of hazardous interactions [101]. Moreover, abruptly stopping antidepressants before psilocybin use can trigger discontinuation symptoms [102]. Table 3 summarizes these drug–drug interactions.

Table 3.
Interaction between psilocybin and antidepressants.
4. Psilocybin in Oregon
The state of Oregon has taken a progressive stance on psilocybin therapy by legalizing it through Oregon Measure 109, the Psilocybin Mushroom Services Program Initiative [92]. This initiative allows adults aged 21 years and older to take psilocybin under supervision in state-licensed service centers, making Oregon the first state in the United States to decriminalize the use of psilocybin-containing mushrooms [113]. Psilocybin-assisted therapy has demonstrated rapid-acting and persisting antidepressant effects from just one or two doses, as evidenced by early phase studies [86]. This suggests that Oregon’s Measure 109 aligns with the growing body of research supporting the potential therapeutic benefits of psilocybin in the treatment of psychiatric disorders. Despite the passage of the law, psilocybin continues to be prohibited under federal law unless it is rescheduled. Until such rescheduling takes place, there is a possibility of a form of “cooperative federalism” emerging with regard to psilocybin use in Oregon [82].
The Oregon Psilocybin Services Act mandates that the Oregon Health Authority (OHA) oversee the licensing, manufacturing, transportation, delivery, sale, and purchase of psilocybin products, creating a structured framework for the provision of psilocybin services within medical care settings [53]. The Act aims to establish a system of licensing, training, and tracking to ensure the safe and responsible delivery of psilocybin services [114]. It specifies that advertising psilocybin products and services is generally prohibited, along with importing, transporting, distributing, or possessing psilocybin products without the proper licensing. It does not allow for retail sale. Additionally, the Act requires psychological support throughout psilocybin therapy to ensure the safety and well-being of participants [115]. Table 4 outlines some key aspects of the regulatory oversight established under this Act.

Table 4.
Aspects of comprehensive regulatory framework for psilocybin products and psilocybin services in Oregon.
Oregon’s Measure 109 reflects a shift toward embracing the potential of psilocybin as a therapeutic agent, drawing from the historical use of psilocybin and modern medical research [116]. The crux is the regulatory and ethical complexities of implementing a treatment modality involving an illegal substance in a non-medicalized framework while still ensuring appropriate oversight, training, and access. Individuals with licenses are immune to Oregon’s criminal statutes concerning the possession, distribution, or production of psilocybin, as well as aiding and abetting others in these activities, or any other criminal act where psilocybin possession, distribution, or production is involved [117]. The proposed measure enables terminally ill patients suffering from anxiety and depression to avail themselves of psilocybin therapy. However, an Oregon law provision also enabled counties and cities to vote against permitting psilocybin services within their boundaries after the statewide measure was approved [118]. Numerous counties and municipalities across Oregon exercised this option to prohibit psilocybin services, resulting in substantial areas of the state opting out of the voter-approved program [119]. This severely limits access to psilocybin therapy for cancer patients in those opt-out areas, depriving them of a potential treatment option to help cope with the psychological distress and anxiety often experienced during cancer treatment [120]. Moreover, practical implementation for terminally ill individuals also faces regulatory hurdles, systemic barriers, clinical reservations, and a lack of specialized training and oversight protocols [121,122,123]. Table 5 outlines some potential issues that could limit the use of psilocybin for terminally ill individuals, despite initial state approval.

Table 5.
Barriers to psilocybin access for terminally ill patients despite state legalization.
Although the approval of psilocybin presents promising therapeutic opportunities, it is beset by challenges related to inadequate supply and availability, while terminally ill patients cannot afford to delay access [143]. This psilocybin program allows the use of Psilocybe cubensis, despite there being over 100 other types of psilocybin-producing fungi [144]. Drawing a parallel with the cannabis industry, which offers various strains catering to different needs [145], the researchers argue that different strains of psilocybin-producing fungi may offer different benefits [146,147,148]. Unfortunately, Oregon’s current rules may overlook the potential benefits of other psilocybin varieties [81,147]. According to the Right to Try (RTT) framework, the therapeutic use of psilocybin for patients with life-threatening illnesses or conditions is protected, as psilocybin qualifies as an “eligible investigational drug”, having completed Phase I clinical trials while still under investigation [149]. Prior to the implementation of Oregon’s Psilocybin Services Act (PSA), terminally ill individuals in Oregon struggling with anxiety and/or depression were legally able to access psilocybin therapy, although supporting data on this pathway are unavailable [150,151].
This narrative review’s scope is restricted by the quality and features of the currently available published research examining the use of psilocybin for alleviating psychological distress in individuals with cancer. While randomized trials were included, sample sizes were often small, and the study populations lacked diversity across racial/ethnic, gender, and socioeconomic factors. There are minimal data on psilocybin therapy outside of life-threatening cancer diagnoses or comparing its efficacy to existing interventions. Significant uncertainty remains regarding optimal dosing strategies, long-term safety, delivery methods beyond oral administration, and the neurobiological mechanisms underlying therapeutic effects. Furthermore, the logistical and systemic challenges surrounding the equitable implementation and healthcare integration of psilocybin therapy in real-world settings have not been well characterized. Despite the comprehensive nature of this review, the interpretability and generalizability of the findings are constrained by the limitations of the primary evidence base. Continued rigorous research involving larger, more diverse clinical trials is needed to address these limitations.
5. Conclusions
The emerging body of evidence supports the potential therapeutic benefits of psilocybin for cancer-related depression and anxiety. Multiple clinical trials have demonstrated rapid and sustained reductions in depression, anxiety, demoralization, and existential distress among cancer patients treated with psilocybin, along with improved quality of life and attitudes toward death. Neural imaging studies point to putative mechanisms involving increased brain network integration and connectivity following psilocybin therapy.
As the first state to legalize psilocybin services, Oregon’s initiatives reflect this promise but have also exposed challenges in practically implementing psilocybin therapy outside of tightly controlled research settings. Legal ambiguities, social stigma, lack of professional integration pathways, and limited regulatory guidance could hinder appropriate patient access and standards of care. Key considerations include training requirements for facilitators, ethical administration protocols, equitable availability regardless of geography, and avenues for rigorous data collection to assess safety and outcomes. Further research is still needed on optimal therapeutic approaches, coordination with existing cancer care, dosing strategies, long-term effects, and suitability for specific patient populations. Addressing knowledge gaps through careful study and open dialogue among stakeholders will be critical as psilocybin therapy transitions from empirical investigation to approved clinical practice. Despite the hurdles ahead, Oregon’s pioneering framework offers invaluable lessons for establishing comprehensive psychedelic medicine programs focused on patient well-being.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Smith, W.R.; Sisti, D.A.; Appelbaum, P.S. The safety of supported psilocybin use in Oregon. Nat. Med. 2024, 30, 17–18. [Google Scholar] [CrossRef]
- Sheppard, B. A Trip Through Employment Law: Protecting Therapeutic Psilocybin Users in the Workplace. J. Law Health 2021, 35, 146–180. [Google Scholar]
- Mejareh, Z.N.; Abdollahi, B.; Hoseinipalangi, Z.; Jeze, M.S.; Hosseinifard, H.; Rafiei, S.; Aghajani, F.; Dehnad, A.; Ardakani, M.F.; Ahmadi, S.; et al. Global, regional, and national prevalence of depression among cancer patients: A systematic review and meta-analysis. Indian J. Psychiatry 2021, 63, 527–535. [Google Scholar] [CrossRef]
- Krebber, A.; Buffart, L.; Kleijn, G.; Riepma, I.; Bree, R.; Leemans, C.; Becker, A.; Brug, J.; Van Straten, A.; Cuijpers, P. Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology 2013, 23, 121–130. [Google Scholar] [CrossRef]
- Kouhestani, M.; Gharaei, H.; Fararouei, M.; Ghahremanloo, H.; Ghaiasvand, R.; Dianatinasab, M. Global and regional geographical prevalence of depression in gastric cancer: A systematic review and meta-analysis. BMJ Support. Palliat. Care 2020, 12, e526–e536. [Google Scholar] [CrossRef]
- Colizzi, M.; Lasalvia, A.; Ruggeri, M. Prevention and early intervention in youth mental health: Is it time for a multidisciplinary and trans-diagnostic model for care? Int. J. Ment. Health Syst. 2020, 14, 23. [Google Scholar] [CrossRef]
- Alwhaibi, M.; AlRuthia, Y.; Sales, I. The impact of depression and anxiety on adult cancer patients’ health-related quality of life. J. Clin. Med. 2023, 12, 2196. [Google Scholar] [CrossRef]
- Nakhlband, A.; Farahzadi, R.; Saeedi, N.; Barzegar, H.; Montazersaheb, S.; Soofiyani, S.R. Bidirectional relations between anxiety, depression, and cancer: A review. Curr. Drug Targets 2023, 24, 118–130. [Google Scholar] [CrossRef]
- Naser, A.Y.; Hameed, A.N.; Mustafa, N.; Alwafi, H.; Dahmash, E.Z.; Alyami, H.S.; Khalil, H. Depression and Anxiety in Patients with Cancer: A Cross-Sectional Study. Front. Psychol. 2021, 12, 585534. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Archer, J.; Hutchison, I.; Korszun, A. Mood and malignancy: Head and neck cancer and depression. J. Oral Pathol. Med. 2008, 37, 255–270. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ji, Q.; Wu, Q.; Wei, J.; Zhu, P. Prevalence, associated factors and adverse outcomes of demoralization in cancer patients: A decade of systematic review. Am. J. Hosp. Palliat. Med. 2023, 40, 1216–1230. [Google Scholar] [CrossRef]
- Tang, P.; Wang, H.; Chou, F. A systematic review and meta-analysis of demoralization and depression in patients with cancer. Psychosomatics 2015, 56, 634–643. [Google Scholar] [CrossRef]
- Liu, S.T.; Wu, X.; Wang, N.; Zhao, Q.Q.; Xiao, L.; Fang, C.K.; Yu, Y.; Lin, D.M.; Zhang, L.L. Serial multiple mediation of demoralization and depression in the relationship between hopelessness and suicidal ideation. Psycho-Oncology 2020, 29, 1321–1328. [Google Scholar] [CrossRef]
- Figueiredo, J.; Zhu, B.; Patel, A.; Kohn, R.; Koo, B.; Louis, E. From perceived stress to demoralization in Parkinson disease: A path analysis. Front. Psychiatry 2022, 13, 876445. [Google Scholar] [CrossRef]
- Fang, C.K.; Chang, M.C.; Chen, P.J.; Lin, C.C.; Chen, G.S.; Lin, J.; Hsieh, R.K.; Chang, Y.F.; Chen, H.W.; Wu, C.L.; et al. A correlational study of suicidal ideation with psychological distress, depression, and demoralization in patients with cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2014, 22, 3165–3174. [Google Scholar] [CrossRef]
- Chang, T.; Hung, C.; Huang, P.; Hsu, C.; Yen, T. Demoralization and its association with quality of life, sleep quality, spiritual interests, and suicide risk in breast cancer inpatients: A cross-sectional study. Int. J. Environ. Res. Public Health 2022, 19, 12815. [Google Scholar] [CrossRef]
- Nanni, M.G.; Caruso, R.; Travado, L.; Ventura, C.; Palma, A.; Berardi, A.M.; Meggiolaro, E.; Ruffilli, F.; Martins, C.; Kissane, D.; et al. Relationship of demoralization with anxiety, depression, and quality of life: A Southern European study of Italian and Portuguese cancer patients. Psycho-Oncology 2018, 27, 2616–2622. [Google Scholar] [CrossRef]
- Bovero, A.; Opezzo, M.; Tesio, V. Relationship between demoralization and quality of life in end-of-life cancer patients. Psycho-Oncology 2023, 32, 429–437. [Google Scholar] [CrossRef]
- Kouhpas, E.; Karimi, Z.; Rahmani, B.; Shoaee, F. The relationship between existential anxiety and demoralization syndrome in predicting psychological well-being of patient with cancer. Pract. Clin. Psychol. 2020, 8, 175–182. [Google Scholar] [CrossRef]
- Scandurra, C.; Mangiapia, F.; La Rocca, R.; Di Bello, F.; De Lucia, N.; Muzii, B.; Cantone, M.; Zampi, R.; Califano, G.; Maldonato, N.M.; et al. A cross-sectional study on demoralization in prostate cancer patients: The role of masculine self-esteem, depression, and resilience. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 7021–7030. [Google Scholar] [CrossRef]
- Hong, Y.T.; Lin, Y.A.; Pan, Y.X.; Lin, J.L.; Lin, X.J.; Zhang, J.; Huang, F.F. Understanding factors influencing demoralization among cancer patients based on the bio-psycho-social model: A systematic review. Psycho-Oncology 2022, 31, 2036–2049. [Google Scholar] [CrossRef]
- Koo, B.B.; Chow, C.A.; Shah, D.R.; Khan, F.H.; Steinberg, B.; Derlein, D.; Nalamada, K.; Para, K.S.; Kakade, V.M.; Patel, A.S.; et al. Demoralization in Parkinson disease. Neurology 2018, 90, e1613–e1617. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Yu, C.; Kwok, D.; Wan, J. Prevalence and factors associated with demoralization in palliative care patients: A cross-sectional study from Hong Kong. Palliat. Support. Care 2022, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.; Baertschi, M.; Richard-Lepouriel, H.; Weber, K.; Berardelli, I.; Pompili, M.; Canuto, A. Demoralization and Its Relationship with Depression and Hopelessness in Suicidal Patients Attending an Emergency Department. Int. J. Environ. Res. Public Health 2020, 17, 2232. [Google Scholar] [CrossRef]
- Andersen, B.L.; Lacchetti, C.; Ashing, K.; Berek, J.S.; Berman, B.S.; Bolte, S.; Dizon, D.S.; Given, B.; Nekhlyudov, L.; Pirl, W.; et al. Management of Anxiety and Depression in Adult Survivors of Cancer: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3426–3453. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, A.; Vehling, S.; Höcker, A.; Lehmann, C.; Koch, U. Demoralization and depression in patients with advanced cancer: Validation of the German version of the demoralization scale. J. Pain Symptom Manag. 2011, 42, 768–776. [Google Scholar] [CrossRef]
- Smith, H. Depression in cancer patients: Pathogenesis, implications and treatment (review). Oncol. Lett. 2015, 9, 1509–1514. [Google Scholar] [CrossRef]
- Williams, S.; Dale, J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: A systematic review. Br. J. Cancer 2006, 94, 372–390. [Google Scholar] [CrossRef]
- Okamura, M.; Akizuki, N.; Nakano, T.; Shimizu, K.; Ito, T.; Akechi, T.; Uchitomi, Y. Clinical experience of the use of a pharmacological treatment algorithm for major depressive disorder in patients with advanced cancer. Psycho-Oncology 2008, 17, 154–160. [Google Scholar] [CrossRef]
- Henry, N.; Stearns, V.; Flockhart, D.; Hayes, D.; Riba, M. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am. J. Psychiatry 2008, 165, 1251–1255. [Google Scholar] [CrossRef]
- Wedret, J.; Tu, T.; Paul, D.; Rousseau, C.; Bonta, A.; Bota, R. Interactions between antidepressants, sleep aids and selected breast cancer therapy. Ment. Illn. 2019, 11, 36–38. [Google Scholar] [CrossRef][Green Version]
- Schmauss, C. An hdac-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci. Rep. 2015, 5, 8171. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kim, E.J.; Nyiramana, M.M.; Shin, E.J.; Jin, H.; Ryu, J.H.; Kang, K.R.; Lee, G.W.; Kim, H.J.; Han, J.; et al. Paroxetine Induces Apoptosis of Human Breast Cancer MCF-7 Cells through Ca2+-and p38 MAP Kinase-Dependent ROS Generation. Cancers 2019, 11, 64. [Google Scholar] [CrossRef]
- Mehta, R.D.; Roth, A.J. Psychiatric considerations in the oncology setting. CA A Cancer J. Clin. 2015, 65, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Voican, C.S.; Corruble, E.; Naveau, S.; Perlemuter, G. Antidepressant-induced liver injury: A review for clinicians. Am. J. Psychiatry 2014, 171, 404–415. [Google Scholar] [CrossRef]
- Li, M.; Kennedy, E.; Byrne, N.; Gérin-Lajoie, C.; Katz, M.; Keshavarz, H.; Sellick, S.; Green, E. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psycho-Oncology 2016, 26, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Shoval, G.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Eger, G.; Weizman, A.; Zalsman, G.; Stubbs, B.; Golubchik, P.; Gordon, B.; et al. Adherence to antidepressant medications is associated with reduced premature mortality in patients with cancer: A nationwide cohort study. Depress. Anxiety 2019, 36, 921–929. [Google Scholar] [CrossRef]
- Park, S.C.; Oh, H.S.; Oh, D.H.; Jung, S.A.; Na, K.S.; Lee, H.Y.; Kang, R.H.; Choi, Y.K.; Lee, M.S.; Park, Y.C. Evidence-based, non-pharmacological treatment guideline for depression in Korea. J. Korean Med. Sci. 2014, 29, 12–22. [Google Scholar] [CrossRef]
- Pu, B.; Wang, N.; Wang, C.; Sun, B. Clinical observation on the benefits of antidepressant intervention in advanced cancer patients. Medicine 2022, 101, e29771. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Singh, G. Biological mechanisms of cancer-induced depression. Front. Psychiatry 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Zhu, X.; Hasegawa, Y.; Karma, S.; Obayashi, M.; Alway, E.; Kamiya, A. Inflamed brain: Targeting immune changes and inflammation for treatment of depression. Psychiatry Clin. Neurosci. 2021, 75, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Dhir, A. Current investigational drugs for major depression. Expert Opin. Investig. Drugs 2009, 18, 767–788. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, Y.; Li, M.; Zhang, Y.; Li, H.; Tan, Z. Oxymatrine promotes S-phase arrest and inhibits cell proliferation of human breast cancer cells in vitro through mitochondrial-mediated apoptosis. Biol. Pharm. Bull. 2017, 40, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Johnson, M.; Griffiths, R.; Hendricks, P.; Henningfield, J. The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Thrul, J. Innovations in group-based psilocybin-assisted therapy of major depression in patients with cancer. Cancer 2023, 130, 1028–1030. [Google Scholar] [CrossRef]
- Swift, T.C.; Belser, A.B.; Agin-Liebes, G.; Devenot, N.; Terrana, S.; Friedman, H.L.; Guss, J.; Bossis, A.P.; Ross, S. Cancer at the Dinner Table: Experiences of Psilocybin-Assisted Psychotherapy for the Treatment of Cancer-Related Distress. J. Humanist. Psychol. 2017, 57, 488–519. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.; Wulff, A.; Cole, A.; Thompson, S. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-ht2r activation in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Nutt, D.; Carhart-Harris, R. The current status of psychedelics in psychiatry. JAMA Psychiatry 2021, 78, 121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Psilocybin history, action and reaction: A narrative clinical review. J. Psychopharmacol. 2023, 37, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.; Chadderton, P. Psilocybin reduces low frequency oscillatory power and neuronal phase-locking in the anterior cingulate cortex of awake rodents. Sci. Rep. 2022, 12, 12702. [Google Scholar] [CrossRef] [PubMed]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.; Wall, M.; Roseman, L.; Demetriou, L.; Nutt, D.; Carhart-Harris, R. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J. Psychopharmacol. 2020, 34, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; Považan, M.; Rosenberg, M.; Sepeda, N.; Davis, A.; Finan, P.; Smith, G.S.; Pekar, J.J.; Barker, P.B.; Griffiths, R.R.; et al. Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl. Psychiatry 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The therapeutic potential of psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. In vivo validation of psilacetin as a prodrug yielding modestly lower peripheral psilocin exposure than psilocybin. Front. Psychiatry 2024, 14, 1303365. [Google Scholar] [CrossRef]
- Gomonit, M. Quantification of psilocin in human whole blood using liquid chromatography–tandem mass spectrometry (lc–ms/ms). J. Forensic Sci. 2023, 69, 678–687. [Google Scholar] [CrossRef]
- Cumming, P.; Scheidegger, M.; Dornbierer, D.; Palner, M.; Quednow, B.; Martin-Soelch, C. Molecular and functional imaging studies of psychedelic drug action in animals and humans. Molecules 2021, 26, 2451. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A. Psychedelic-like activity of norpsilocin analogues. ACS Chem. Neurosci. 2024, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.; Smith, T.; MacDonald, E.; Boermans, H.; Squires, E. Effects of feeding a blend of grains naturally contaminated with fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent1. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Shang, Q.; Qian, H.; An, R.; Liu, H.; Shao, G.; Li, T.; Liu, X. Psilocin suppresses methamphetamine-induced hyperlocomotion and acquisition of conditioned place preference via D2R-mediated ERK signaling. CNS Neurosci. Ther. 2023, 29, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Kometer, M.; Schmidt, A.; Jäncke, L.; Vollenweider, F. Activation of serotonin 2a receptors underlies the psilocybin-induced effects on oscillations, n170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.K.; Chatha, M.; Laskowski, L.J.; Anderson, E.I.; Brandt, S.D.; Chapman, S.J.; McCorvy, J.D.; Halberstadt, A.L. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2020, 4, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Glatfelter, G.C.; Pottie, E.; Partilla, J.S.; Sherwood, A.M.; Kaylo, K.; Pham, D.N.K.; Naeem, M.; Sammeta, V.R.; DeBoer, S.; Golen, J.A.; et al. Structure-Activity Relationships for Psilocybin, Baeocystin, Aeruginascin, and Related Analogues to Produce Pharmacological Effects in Mice. ACS Pharmacol. Transl. Sci. 2022, 5, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases-A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Pokorny, T.; Hock, A.; Kraehenmann, R.; Stämpfli, P.; Seifritz, E.; Scheidegger, M.; Vollenweider, F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA 2016, 113, 5119–5124. [Google Scholar] [CrossRef]
- Madsen, M.K.; Fisher, P.M.; Stenbæk, D.S.; Kristiansen, S.; Burmester, D.; Lehel, S.; Páleníček, T.; Kuchař, M.; Svarer, C.; Ozenne, B.; et al. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2020, 33, 71–80. [Google Scholar] [CrossRef]
- Singleton, S.P.; Luppi, A.I.; Carhart-Harris, R.L.; Cruzat, J.; Roseman, L.; Nutt, D.J.; Deco, G.; Kringelbach, M.L.; Stamatakis, E.A.; Kuceyeski, A. Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain’s control energy landscape. Nat. Commun. 2022, 13, 5812. [Google Scholar] [CrossRef] [PubMed]
- Carter, O.; Burr, D.; Pettigrew, J.; Wallis, G.; Hasler, F.; Vollenweider, F. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1a and 2a receptors. J. Cogn. Neurosci. 2005, 17, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Erkizia-Santamaría, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. = Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar] [CrossRef]
- Odland, A.U.; Kristensen, J.L.; Andreasen, J.T. Investigating the role of 5-HT2A and 5-HT2C receptor activation in the effects of psilocybin, DOI, and citalopram on marble burying in mice. Behav. Brain Res. 2021, 401, 3093. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Muthukumaraswamy, A.P.S.; Morunga, E.; Evans, W.; Cavadino, A.; Bansal, M.; Lawrence, N.J.; Ashley, A.; Hoeh, N.R.; Sundram, F.; et al. PAM trial protocol: A randomised feasibility study of psychedelic microdosing-assisted meaning-centred psychotherapy in advanced stage cancer patients. Pilot Feasibility Stud. 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Ziff, S.; Stern, B.; Lewis, G.; Majeed, M.; Gorantla, V.R. Analysis of psilocybin-assisted therapy in medicine: A narrative review. Cureus 2022, 14, e21944. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research Psychedelics and psychedelic-assisted psychotherapy. Focus (Am. Psychiatr. Publ.) 2021, 19, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.O.; Beaussant, Y.; Garcia, A.C.M. The therapeutic potential of psychedelic-assisted therapies for symptom control in patients diagnosed with serious illness: A systematic review. J. Pain Symptom Manag. 2022, 63, e725–e738. [Google Scholar] [CrossRef]
- Corrigan, K.; Haran, M.; McCandliss, C.; McManus, R.; Cleary, S.; Trant, R.; Kelly, Y.; Ledden, K.; Rush, G.; O’Keane, V.; et al. Psychedelic perceptions: Mental health service user attitudes to psilocybin therapy. Ir. J. Med. Sci. 2022, 191, 1385–1397. [Google Scholar] [CrossRef]
- DellaCrosse, M.; Pleet, M.; Morton, E.; Ashtari, A.; Sakai, K.; Woolley, J.; Michalak, E. "A sense of the bigger picture:" A qualitative analysis of follow-up interviews with people with bipolar disorder who self-reported psilocybin use. PLoS ONE 2022, 17, e0279073. [Google Scholar] [CrossRef]
- Garakani, A. Psychedelics, with a focus on psilocybin: Issues for the clinician. J. Psychiatr. Pract. 2023, 29, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L. Oregon’s pioneering effort to enact state law to allow access to psilocybin. Willamette Law Rev. 2020, 57, 12. Available online: https://ssrn.com/abstract=3783484 (accessed on 1 April 2024).
- Geiger, H.; Wurst, M.; Daniels, R. Dark classics in chemical neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Heilman, J. The history, legalization and potentials of psilocybin-assisted psychotherapy. J. Sci. Explor. 2023, 36, 623–640. [Google Scholar] [CrossRef]
- Husain, M. Psilocybin for treatment-resistant depression without psychedelic effects: Study protocol for a 4-week, double-blind, proof-of-concept randomised controlled trial. Bjpsych Open 2023, 9, e134. [Google Scholar] [CrossRef] [PubMed]
- Sloshower, J.; Skosnik, P.D.; Safi-Aghdam, H.; Pathania, S.; Syed, S.; Pittman, B.; D’Souza, D.C. Psilocybin-assisted therapy for major depressive disorder: An exploratory placebo-controlled, fixed-order trial. J. Psychopharmacol. 2023, 37, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Day, C.; Krzanowski, J.; Nutt, D.; Carhart-Harris, R. Patients’ accounts of increased “connectedness” and “acceptance” after psilocybin for treatment-resistant depression. J. Humanist. Psychol. 2017, 57, 520–564. [Google Scholar] [CrossRef]
- Whelan, A.; Johnson, M. Lysergic Acid Diethylamide Psilocybin Manag. Patients Persistent Pain: A Potential Role? Pain Manag. 2018, 8, 217–229. [Google Scholar] [CrossRef]
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J. Psychopharmacol. 2022, 36, 151–158. [Google Scholar] [CrossRef]
- Barber, G.S.; Aaronson, S.T. The emerging field of psychedelic psychotherapy. Curr. Psychiatry Rep. 2022, 24, 583–590. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Chiruta, V.; Zemla, P.K.; Miller, P.; Santarossa, N.; Hannan, J.A. Critique of the Royal Australian and New Zealand College of Psychiatrists Psychedelic Therapy Clinical Memorandum, Dated May 2020. J. Med. Health Stud. 2021, 2, 145–160. [Google Scholar] [CrossRef]
- Bienemann, B.; Ruschel, N.S.; Campos, M.L.; Negreiros, M.A.; Mograbi, D.C. Self-reported negative outcomes of psilocybin users: A quantitative textual analysis. PLoS ONE 2020, 15, e0229067. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R. Psilocybin therapeutic research: The present and future paradigm. ACS Med. Chem. Lett. 2020, 11, 399–402. [Google Scholar] [CrossRef]
- Madsen, M. CCH attack frequency reduction after psilocybin correlates with hypothalamic functional connectivity. Headache J. Head Face Pain 2024, 64, 55–67. [Google Scholar] [CrossRef]
- Madsen, M.K.; Petersen, A.S.; Stenbaek, D.S.; Sorensen, I.M.; Schionning, H.; Fjeld, T.; Nykjaer, C.; Larsen, S.M.U.; Grzywacz, M.; Mathiesen, T.; et al. Psilocybin-induced reduction in chronic cluster headache attack frequency correlates with changes in hypothalamic functional connectivity. medRxiv 2022. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Nava, B.; Sampiere, L.; Jacobs, R. Predictors of medical students’ perceptions of psilocybin-assisted therapy for use in medical practice. Cureus 2023, 15, e37450. [Google Scholar] [CrossRef] [PubMed]
- Kopra, E.I.; Ferris, J.A.; Winstock, A.R.; Young, A.H.; Rucker, J.J. Adverse experiences resulting in emergency medical treatment seeking following the use of magic mushrooms. J. Psychopharmacol. 2022, 36, 965–973. [Google Scholar] [CrossRef]
- Yerubandi, A.; Thomas, J.E.; Bhuiya, N.M.M.A.; Harrington, C.; Villa Zapata, L.; Caballero, J. Acute adverse effects of therapeutic doses of psilocybin: A systematic review and meta-analysis. JAMA Netw. Open 2024, 7, e245960. [Google Scholar] [CrossRef]
- Agin-Liebes, G.I.; Malone, T.; Yalch, M.M.; Mennenga, S.E.; Ponté, K.L.; Guss, J.; Bossis, A.P.; Grigsby, J.; Fischer, S.; Ross, S. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J. Psychopharmacol. 2020, 34, 155–166. [Google Scholar] [CrossRef]
- Blei, F.; Dörner, S.; Fricke, J.; Baldeweg, F.; Trottmann, F.; Komor, A.; Meyer, F.; Hertweck, C.; Hoffmeister, D. Simultaneous Production of Psilocybin and a Cocktail of β-Carboline Monoamine Oxidase Inhibitors in "Magic" Mushrooms. Chemistry 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Erritzoe, D.; Barba, T.; Spriggs, M.J.; Rosas, F.E.; Nutt, D.J.; Carhart-Harris, R. Effects of discontinuation of serotonergic antidepressants prior to psilocybin therapy versus escitalopram for major depression. J. Psychopharmacol. 2024, 02698811241237870. [Google Scholar] [CrossRef]
- Pędzich, B.D.; Medrano, M.; Buckinx, A.; Smolders, I.; De Bundel, D. Psychedelic-Induced Serotonin 2A Receptor Downregulation Does Not Predict Swim Stress Coping in Mice. Int. J. Mol. Sci. 2022, 23, 15284. [Google Scholar] [CrossRef]
- Bonson, K.R.; Buckholtz, J.W.; Murphy, D.L. Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1996, 14, 425–436. [Google Scholar] [CrossRef]
- Sarparast, A.; Thomas, K.; Malcolm, B.; Stauffer, C.S. Drug-drug interactions between psychiatric medications and MDMA or psilocybin: A systematic review. Psychopharmacology 2022, 239, 1945–1976. [Google Scholar] [CrossRef]
- Strumila, R.; Nobile, B.; Korsakova, L.; Lengvenyte, A.; Olie, E.; Lopez-Castroman, J.; Guillaume, S.; Courtet, P. Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals 2021, 14, 1213. [Google Scholar] [CrossRef]
- Halman, A.; Kong, G.; Sarris, J.; Perkins, D. Drug-drug interactions involving classic psychedelics: A systematic review. J. Psychopharmacol. 2024, 38, 3–18. [Google Scholar] [CrossRef]
- Cuomo, A.; Ballerini, A.; Bruni, A.C.; Decina, P.; Di Sciascio, G.; Fiorentini, A.; Scaglione, F.; Vampini, C.; Fagiolini, A. Clinical guidance for the use of trazodone in major depressive disorder and concomitant conditions: Pharmacology and clinical practice. Riv. Psichiatr. 2019, 54, 137–149. [Google Scholar] [CrossRef]
- Jaffer, K.Y.; Chang, T.; Vanle, B.; Dang, J.; Steiner, A.J.; Loera, N.; Abdelmesseh, M.; Danovitch, I.; Ishak, W.W. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci. 2017, 14, 24–34. [Google Scholar]
- Pokorny, T.; Preller, K.H.; Kraehenmann, R.; Vollenweider, F.X. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2016, 26, 756–766. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N,N-dimethyltryptamine. Pharmacol. Biochem. Behav. 2016, 143, 1–10. [Google Scholar] [CrossRef]
- Van den Eynde, V.; Abdelmoemin, W.R.; Abraham, M.M.; Amsterdam, J.D.; Anderson, I.M.; Andrade, C.; Baker, G.B.; Beekman, A.T.F.; Berk, M.; Birkenhäger, T.K.; et al. The prescriber’s guide to classic MAO inhibitors (phenelzine, tranylcypromine, isocarboxazid) for treatment-resistant depression. CNS Spectr. 2022, 1–14. [Google Scholar] [CrossRef]
- Boehnke, K.; Davis, A.; McAfee, J. Applying lessons from cannabis to the psychedelic highway. JAMA Health Forum 2022, 3, e221618. [Google Scholar] [CrossRef]
- Wexler, A.; Sisti, D. Brain wellness “spas”—Anticipating the off-label promotion of psychedelics. JAMA Psychiatry 2022, 79, 748. [Google Scholar] [CrossRef]
- Tai, S.J.; Nielson, E.M.; Lennard-Jones, M.; Johanna Ajantaival, R.L.; Winzer, R.; Richards, W.A.; Reinholdt, F.; Richards, B.D.; Gasser, P.; Malievskaia, E. Development and Evaluation of a Therapist Training Program for Psilocybin Therapy for Treatment-Resistant Depression in Clinical Research. Front. Psychiatry 2021, 12, 586682. [Google Scholar] [CrossRef]
- Nichols, D. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686. [Google Scholar] [CrossRef]
- Sandbrink, J.D.; Johnson, K.; Gill, M.; Yaden, D.B.; Savulescu, J.; Hannikainen, I.R.; Earp, B.D. Strong Bipartisan Support for Controlled Psilocybin Use as Treatment or Enhancement in a Representative Sample of US Americans: Need for Caution in Public Policy Persists. AJOB Neurosci. 2024, 15, 82–89. [Google Scholar] [CrossRef]
- DiCarlo, G. Majority of Oregon Counties Vote against Psilocybin Therapy. Oregon Public Broadcasting (“OPB”). Available online: https://www.opb.org/article/2022/11/13/think-out-loud-majority-of-oregon-counties-vote-against-psilocybin-therapy/ (accessed on 6 March 2024).
- McInally, M. Thousands of Oregonians Vote against Psilocybin Centers. Oregon Capital Chronicle. Available online: https://oregoncapitalchronicle.com/2022/11/14/thousands-of-oregonians-vote-against-psilocybin-centers/ (accessed on 6 March 2024).
- Belouin, S.J.; Averill, L.A.; Henningfield, J.E.; Xenakis, S.N.; Donato, I.; Grob, C.S.; Berger, A.; Magar, V.; Danforth, A.L.; Anderson, B.T. Policy considerations that support equitable access to responsible, accountable, safe, and ethical uses of psychedelic medicines. Neuropharmacology 2022, 219, 109214. [Google Scholar] [CrossRef]
- Villiger, D. Giving consent to the ineffable. Neuroethics 2024, 17, 11. [Google Scholar] [CrossRef]
- Beaussant, Y.; Tulsky, J.; Guérin, B.; Schwarz-Plaschg, C.; Sanders, J.J. Radcliffe Institute for Advanced Study Working Group on Psychedelic Research in Serious Illness Mapping an agenda for psychedelic-assisted therapy research in patients with serious illness. J. Palliat. Med. 2021, 24, 1657–1666. [Google Scholar] [CrossRef]
- Barber, G.S.; Dike, C.C. Ethical and practical considerations for the use of psychedelics in psychiatry. Psychiatr. Serv. 2023, 74, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Marks, M. The varieties of Psychedelic Law. Neuropharmacology, NIH Special Issue on Psilocybin (2023), FSU College of Law, Public Law Research Paper. Available online: https://ssrn.com/abstract=4286450 (accessed on 25 November 2022).
- Licensed Premises Location Requirements. In Public Health Division—Chapter 333 (No. 333-333–4300). Oregon Health Authority. Available online: https://secure.sos.state.or.us/oard/viewSingleRule.action?ruleVrsnRsn=309262 (accessed on 8 March 2024).
- Bathje, G.J.; Majeski, E.; Kudowor, M. Psychedelic integration: An analysis of the concept and its practice. Front. Psychol. 2022, 13, 824077. [Google Scholar] [CrossRef] [PubMed]
- Pilecki, B.; Luoma, J.B.; Bathje, G.J.; Rhea, J.; Narloch, V.F. Ethical and legal issues in psychedelic harm reduction and integration therapy. Harm Reduct. J. 2021, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Facilitator Scope of Practice. Oregon Health Authority. Public Health Division—Chapter 333. Available online: https://secure.sos.state.or.us/oard/viewSingleRule.action;JSESSIONID_OARD=xbMMYQBXykOhsAtpk6H6d209c41BdGdaQkC4jVvEOFCnbpSV37WM!1131481227?ruleVrsnRsn=297867 (accessed on 27 December 2022).
- Kopilak, D. Oregon Psilocybin Services Act: It’s Non-Medical, but Not Anti-Medical. Emerge Law Group. Available online: https://emergelawgroup.com/blog/oregon-psilocybin-services-act-its-non-medical-but-not-anti-medical/ (accessed on 8 March 2024).
- Chesak, J. Will Health Insurance Providers Cover Psychedelic-Assisted Therapy? Verywell Health. Available online: https://www.verywellhealth.com/psychedelic-therapy-will-insurance-cover-it-7564887 (accessed on 27 July 2023).
- Holoyda, B.J. Malpractice and other civil liability in psychedelic psychiatry. Psychiatr. Serv. 2023, 74, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Cohen, I.G. Psychedelic therapy: A roadmap for wider acceptance and utilization. Nat. Med. 2021, 27, 1669–1671. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.S.; Daily, J.E.; Perry, D.A.; Nicol, G.E. Psychedelic drug legislative reform and legalization in the US. JAMA Psychiatry 2023, 80, 77–83. [Google Scholar] [CrossRef]
- Mocanu, V.; Mackay, L.; Christie, D.; Argento, E. Safety considerations in the evolving legal landscape of psychedelic-assisted psychotherapy. Subst. Abus. Treat. Prev. Policy 2022, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Goldhill, O. ‘It’s Not Medical’: Oregon Wrestles with How to Offer Psychedelics outside the Health Care System. STAT. Available online: https://www.statnews.com/2022/03/10/oregon-wrestles-with-offering-psychedelic-therapy-outside-health-care-system/ (accessed on 8 March 2024).
- Yaden, D.B.; Earp, B.D.; Griffiths, R.R. Ethical issues regarding nonsubjective psychedelics as standard of care. Camb. Q. Healthc. Ethics CQ Int. J. Healthc. Ethics Comm. 2022, 31, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, N.; Oliveira Da Silva, M.; Madeira, L. Ethics of psychedelic use in psychiatry and beyond—Drawing upon legal, social and clinical challenges. Philosophies 2023, 8, 76. [Google Scholar] [CrossRef]
- Harrison, T.R. Altered stakes: Identifying gaps in the informed consent process for psychedelic-assisted therapy trials. J. Psychedelic Stud. 2023, 7, 48–60. [Google Scholar] [CrossRef]
- Division 333 Psilocybin Informed Consent. In Oregon Health Authority Public Health Division—Chapter 333 (No. 333-333–5040). Oregon Health Authority. Available online: https://secure.sos.state.or.us/oard/viewSingleRule.action?ruleVrsnRsna=309282 (accessed on 8 March 2024).
- OHA Public Health Division—Chapter 333 Psilocybin. (N.D.). In Oregon Health Authority. Available online: https://secure.sos.state.or.us/oard/displayDivisionRules.action?selectedDivision=7102 (accessed on 5 March 2024).
- Belser, A.B.; Agin-Liebes, G.; Swift, T.C.; Terrana, S.; Devenot, N.; Friedman, H.L.; Guss, J.; Bossis, A.; Ross, S. Patient experiences of psilocybin-assisted psychotherapy: An interpretative phenomenological analysis. J. Humanist. Psychol. 2017, 57, 354–388. [Google Scholar] [CrossRef]
- Notice of Proposed Rulemaking. Including Statement of Need Fiscal Impact. Chapter 333 Oregon Health Authority Public Health Division. In Office of the Secretary of State. Archives Division. Oregon Health Authority. Oregon Psilocybin Services. Available online: https://www.oregon.gov/oha/PH/PREVENTIONWELLNESS/Documents/333-333-Notice-of-Proposed-Rulemaking-11.1.2023.pdf (accessed on 6 March 2024).
- Keridwen, C. Cancer Patients Struggle to Access Psilocybin before They Die. Medscape. Available online: https://www.medscape.com/viewarticle/985501 (accessed on 5 March 2024).
- Abbas, A.I.; Carter, A.; Jeanne, T.; Knox, R.; Korthuis, P.T.; Hamade, A.; Stauffer, C.; Uehling, J. Oregon Psilocybin Advisory Board Rapid Evidence Review and Recommendations; Oregon Psilocybin Advisory Board: Salem, OR, USA, 2021. [Google Scholar]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Strauss, D.; Ghosh, S.; Murray, Z.; Gryzenhout, M. Global species diversity and distribution of the psychedelic fungal genus Panaeolus. Heliyon 2023, 9, e16338. [Google Scholar] [CrossRef] [PubMed]
- Van Court, R.C.; Wiseman, M.S.; Meyer, K.W.; Ballhorn, D.J.; Amses, K.R.; Slot, J.C.; Dentinger, B.T.M.; Garibay-Orijel, R.; Uehling, J.K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319. [Google Scholar] [CrossRef]
- Basky, G. Policy in focus: Is psilocybin the next cannabis? CMAJ Can. Med. Assoc. J. = J. De L’association Medicale Can. 2021, 193, E1741–E1742. [Google Scholar] [CrossRef]
- Evans, J. Waiting for a Miracle: Medical Psilocybin and Mdma under ’Right to Try’. 2021. Available online: https://ssrn.com/abstract=3762134 (accessed on 23 April 2024).
- Whinkin, E.; Opalka, M.; Watters, C.; Jaffe, A.; Aggarwal, S. Psilocybin in Palliative Care: An Update. Curr. Geriatr. Rep. 2023, 12, 50–59. [Google Scholar] [CrossRef]
- Kurtz, J.S.; Patel, N.A.; Gendreau, J.L.; Yang, C.; Brown, N.; Bui, N.; Picton, B.; Harris, M.; Hatter, M.; Beyer, R.; et al. The use of psychedelics in the treatment of medical conditions: An analysis of currently registered psychedelics studies in the American Drug Trial Registry. Cureus 2022, 14, e29167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).