Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Immunohistochemistry and Microscopy
2.3. H-Scoring and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, A.F.; Groenwold, R.H.H.; Amsellem, P.; Bacon, N.; Klungel, O.H.; Hoes, A.W.; de Boer, A.; Kow, K.; Maritato, K.; Kirpensteijn, J.; et al. Which dogs with appendicular osteosarcoma benefit most from chemotherapy after surgery? Results from an individual patient data meta-analysis. Prev. Vet. Med. 2016, 125, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.R.; Lorbach, J.; Husbands, B.D.; Kisseberth, W.C.; Samuels, S.; Silveira, C.; Wustefeld-Janssens, B.G.; Wouda, R.; Keepman, S.; Oblak, M.L.; et al. A retrospective analysis of 11 dogs with surface osteosarcoma. Vet. Comp. Oncol. 2022, 20, 82–90. [Google Scholar] [CrossRef]
- Greene, S. Withrow & MacEwen’s Small Animal Clinical Oncology, 6th edition. Am. J. Vet. Res. 2020, 81, 391. [Google Scholar]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Selvarajah, G.T.; Kirpensteijn, J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet. J. 2010, 185, 28–35. [Google Scholar] [CrossRef]
- Leonardo, L.; Laura, P.; Serena, B.M. miR-1 and miR-133b expression in canine osteosarcoma. Res. Vet. Sci. 2018, 117, 133–137. [Google Scholar] [CrossRef]
- Egenvall, A.; Nodtvedt, A.; von Euler, H. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: Incidence and survival. Can. J. Vet. Res. 2007, 71, 292–299. [Google Scholar] [PubMed]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine osteosarcoma: A naturally occurring disease to inform pediatric oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef]
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Rutland, C.S.; Cockcroft, J.M.; Lothion-Roy, J.; Harris, A.E.; Jeyapalan, J.N.; Simpson, S.; Alibhai, A.; Bailey, C.; Ballard-Reisch, A.C.; Rizvanov, A.A.; et al. Immunohistochemical Characterisation of GLUT1, MMP3 and NRF2 in Osteosarcoma. Front. Vet. Sci. 2021, 8, 704598. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.; de Brot, S.; Alibhai, A.; Bailey, C.; Woodcock, C.L.; Mestas, M.; Akhtar, S.; Jeyapalan, J.N.; Lothion-Roy, J.; et al. Molecular Characterisation of Canine Osteosarcoma in High Risk Breeds. Cancers 2020, 12, 2405. [Google Scholar] [CrossRef] [PubMed]
- Ru, G.; Terracini, B.; Glickman, L.T. Host related risk factors for canine osteosarcoma. Vet. J. 1998, 156, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J.A.; Pablo, N.V.; Crawford, P.C. Prevalence of and intrinsic risk factors for appendicular osteosarcoma in dogs: 179 cases (1996-2005). J. Am. Vet. Med. Assoc. 2007, 231, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Pfeiffer, R.; Murphy, G.; Daw, N.C.; Patino-Garcia, A.; Troisi, R.J.; Hoover, R.N.; Douglass, C.; Schuz, J.; Craft, A.W.; et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control 2011, 22, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef]
- Sadykova, L.R.; Ntekim, A.I.; Muyangwa-Semenova, M.; Rutland, C.S.; Jeyapalan, J.N.; Blatt, N.; Rizvanov, A.A. Epidemiology and Risk Factors of Osteosarcoma. Cancer Investig. 2020, 38, 259–269. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, G.L.; Smalley, M.J.; Beck, S.; Errington, R.J.; Gould, S.; Winter, H.; Brodbelt, D.C.; O’Neill, D.G. Dog breeds and body conformations with predisposition to osteosarcoma in the UK: A case-control study. Canine Med. Genet. 2021, 8, 2. [Google Scholar] [CrossRef]
- Selvarajah, G.T.; Kirpensteijn, J.; van Wolferen, M.E.; Rao, N.A.; Fieten, H.; Mol, J.A. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol. Cancer 2009, 8, 72. [Google Scholar] [CrossRef]
- Szewczyk, M.; Lechowski, R.; Zabielska, K. What do we know about canine osteosarcoma treatment? Review. Vet. Res. Commun. 2015, 39, 61–67. [Google Scholar] [CrossRef]
- Rubin, J.A.; Suran, J.N.; Brown, D.C.; Agnello, K.A. Factors associated with pathological fractures in dogs with appendicular primary bone neoplasia: 84 cases (2007-2013). J. Am. Vet. Med. Assoc. 2015, 247, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Paoloni, M.; Davis, S.; Lana, S.; Withrow, S.; Sangiorgi, L.; Picci, P.; Hewitt, S.; Triche, T.; Meltzer, P.; Khanna, C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genom. 2009, 10, 625. [Google Scholar] [CrossRef]
- Kehl, A.; Aupperle-Lellbach, H.; de Brot, S.; van der Weyden, L. Review of Molecular Technologies for Investigating Canine Cancer. Animals 2024, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Ogawa, Y. Forkhead box class O family member proteins: The biology and pathophysiological roles in diabetes. J. Diabetes Investig. 2017, 8, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Luo, B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell. Mol. Life Sci. 2020, 77, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Rached, M.T.; Kode, A.; Xu, L.; Yoshikawa, Y.; Paik, J.H.; Depinho, R.A.; Kousteni, S. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Kops, G.J.; de Ruiter, N.D.; De Vries-Smits, A.M.; Powell, D.R.; Bos, J.L.; Burgering, B.M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 1999, 398, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005, 24, 7410–7425. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e116. [Google Scholar] [CrossRef]
- Sergi, C.; Shen, F.; Liu, S.M. Insulin/IGF-1R, SIRT1, and FOXOs Pathways-An Intriguing Interaction Platform for Bone and Osteosarcoma. Front. Endocrinol. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bao, Y.; Chen, X.; Yi, J.; Liu, S.; Fang, Z.; Zheng, S.; Chen, J. Mir-664 promotes osteosarcoma cells proliferation via downregulating of FOXO4. Biomed. Pharmacother. 2015, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, L.; Xu, X.; Han, P.; Wu, J.; Tian, X.; Li, M. FOXO4 Inhibits the Migration and Metastasis of Colorectal Cancer by Regulating the APC2/beta-Catenin Axis. Front. Cell Dev. Biol. 2021, 9, 659731. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, X.; Chai, N.; Lv, L.; Wang, R.; Li, X.; Nie, Y.; Shi, Y.; Fan, D. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer 2014, 14, 378. [Google Scholar] [CrossRef]
- Lu, Y.; Shen, Y.; Li, L.; Zhang, M.; Wang, M.; Ge, L.; Yang, J.; Tang, X. Clinicopathological Significance of FOXO4 Expression and Correlation with Prx1 in Head and Neck Squamous Cell Carcinoma. Anal. Cell. Pathol. 2021, 2021, 5510753. [Google Scholar] [CrossRef]
- Paik, J.-H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FoxOs Are Lineage-Restricted Redundant Tumor Suppressors and Regulate Endothelial Cell Homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.M.; Lee, M.K.; Jameson, J.L. Splice variants of the forkhead box protein AFX exhibit dominant negative activity and inhibit AFXalpha-mediated tumor cell apoptosis. PLoS ONE 2008, 3, e2743. [Google Scholar] [CrossRef]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008, 26, 535–584. [Google Scholar] [CrossRef]
- Moorman, H.R.; Reategui, Y.; Poschel, D.B.; Liu, K. IRF8: Mechanism of Action and Health Implications. Cells 2022, 11, 2630. [Google Scholar] [CrossRef]
- Cytlak, U.; Resteu, A.; Pagan, S.; Green, K.; Milne, P.; Maisuria, S.; McDonald, D.; Hulme, G.; Filby, A.; Carpenter, B.; et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity 2020, 53, 353–370.e358. [Google Scholar] [CrossRef]
- Nixon, B.G.; Kuo, F.; Ji, L.; Liu, M.; Capistrano, K.; Do, M.; Franklin, R.A.; Wu, X.; Kansler, E.R.; Srivastava, R.M.; et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity 2022, 55, 2044–2058.e2045. [Google Scholar] [CrossRef] [PubMed]
- Durmowicz, M.C.; Cui, C.Y.; Schlessinger, D. The EDA gene is a target of, but does not regulate Wnt signaling. Gene 2002, 285, 203–211. [Google Scholar] [CrossRef]
- Eastman, Q.; Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 1999, 11, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Steinke, F.C.; Xue, H.H. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol. Res. 2014, 59, 45–55. [Google Scholar] [CrossRef]
- Reya, T.; O’Riordan, M.; Okamura, R.; Devaney, E.; Willert, K.; Nusse, R.; Grosschedl, R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000, 13, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Held, W.; Clevers, H.; Grosschedl, R. Redundant functions of TCF-1 and LEF-1 during T and NK cell development, but unique role of TCF-1 for Ly49 NK cell receptor acquisition. Eur. J. Immunol. 2003, 33, 1393–1398. [Google Scholar] [CrossRef]
- Phan, Q.M.; Fine, G.M.; Salz, L.; Herrera, G.G.; Wildman, B.; Driskell, I.M.; Driskell, R.R. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 2020, 9, e60066. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, Z.; Xi, Q.; Zhao, F.; Hu, J.; Wang, J.; Liu, X.; Zhao, Z.; Li, M.; Luo, Y.; et al. Lef1 and Dlx3 May Facilitate the Maturation of Secondary Hair Follicles in the Skin of Gansu Alpine Merino. Genes 2022, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.M.; Azevedo, A.L.K.; Giner, I.S.; Gomig, T.H.B.; Ribeiro, E.; Cavalli, I.J. Biomarker potential of the LEF1/TCF family members in breast cancer: Bioinformatic investigation on expression and clinical significance. Genet. Mol. Biol. 2023, 46, e20220346. [Google Scholar] [CrossRef]
- Cordray, P.; Satterwhite, D.J. TGF-beta induces novel Lef-1 splice variants through a Smad-independent signaling pathway. Dev. Dyn. 2005, 232, 969–978. [Google Scholar] [CrossRef]
- Chen, W.Y.; Liu, S.Y.; Chang, Y.S.; Yin, J.J.; Yeh, H.L.; Mouhieddine, T.H.; Hadadeh, O.; Abou-Kheir, W.; Liu, Y.N. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget 2015, 6, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, R.; Rietkotter, E.; Wenske, B.; Wlochowitz, D.; Sparrer, D.; Vollmer, E.; Muller, G.; Seegerer, J.; Sun, X.; Dettmer, K.; et al. LEF1 supports metastatic brain colonization by regulating glutathione metabolism and increasing ROS resistance in breast cancer. Int. J. Cancer 2020, 146, 3170–3183. [Google Scholar] [CrossRef]
- Chen, C.L.; Tsai, Y.S.; Huang, Y.H.; Liang, Y.J.; Sun, Y.Y.; Su, C.W.; Chau, G.Y.; Yeh, Y.C.; Chang, Y.S.; Hu, J.T.; et al. Lymphoid Enhancer Factor 1 Contributes to Hepatocellular Carcinoma Progression Through Transcriptional Regulation of Epithelial-Mesenchymal Transition Regulators and Stemness Genes. Hepatol. Commun. 2018, 2, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.J.; Moon, R.T.; Chien, A.J. Wnt and related signaling pathways in melanomagenesis. Cancers 2010, 2, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Yao, Y.; Jiang, L.L.; Hu, T.H.; Ma, J.Q.; Liao, Z.J.; Yao, J.T.; Li, D.F.; Wang, S.H.; Nan, K.J. Knockdown of lymphoid enhancer factor 1 inhibits colon cancer progression in vitro and in vivo. PLoS ONE 2013, 8, e76596. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhu, H.; Fu, Y.; Shen, W.; Miao, K.; Hong, M.; Xu, W.; Fan, L.; Young, K.H.; Liu, P.; et al. High LEF1 expression predicts adverse prognosis in chronic lymphocytic leukemia and may be targeted by ethacrynic acid. Oncotarget 2016, 7, 21631–21643. [Google Scholar] [CrossRef]
- Schmeel, L.C.; Schmeel, F.C.; Kim, Y.; Endo, T.; Lu, D.; Schmidt-Wolf, I.G. Targeting the Wnt/beta-catenin pathway in multiple myeloma. Anticancer Res. 2013, 33, 4719–4726. [Google Scholar] [PubMed]
- Nguyen, A.; Rosner, A.; Milovanovic, T.; Hope, C.; Planutis, K.; Saha, B.; Chaiwun, B.; Lin, F.; Imam, S.A.; Marsh, J.L.; et al. Wnt pathway component LEF1 mediates tumor cell invasion and is expressed in human and murine breast cancers lacking ErbB2 (her-2/neu) overexpression. Int. J. Oncol. 2005, 27, 949–956. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Staib, P.A.; Minke, K.; Gehrke, I.; Plickert, G.; Schlosser, A.; Schmitt, E.K.; Hallek, M.; Kreuzer, K.A. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia 2010, 12, 326–335. [Google Scholar] [CrossRef]
- Gatti, G.; Betts, C.; Rocha, D.; Nicola, M.; Grupe, V.; Ditada, C.; Nuñez, N.G.; Roselli, E.; Araya, P.; Dutto, J.; et al. High IRF8 expression correlates with CD8 T cell infiltration and is a predictive biomarker of therapy response in ER-negative breast cancer. Breast Cancer Res. 2021, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Saleem, A.; Wang, J.Y.; Rieger, K.E.; Brown, R.A.; Novoa, R.A. Diagnostic Utility of LEF1 Immunohistochemistry in Differentiating Deep Penetrating Nevi From Histologic Mimics. Am. J. Surg. Pathol. 2020, 44, 1413–1418. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- McCarty, K.S., Jr.; Miller, L.S.; Cox, E.B.; Konrath, J.; McCarty, K.S., Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar] [PubMed]
- Detre, S.; Saclani Jotti, G.; Dowsett, M. A “quickscore” method for immunohistochemical semiquantitation: Validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- Bryan, J.N. Updates in Osteosarcoma. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 523–539. [Google Scholar] [CrossRef]
- Ferracini, R.; Angelini, P.; Cagliero, E.; Linari, A.; Martano, M.; Wunder, J.; Buracco, P. MET oncogene aberrant expression in canine osteosarcoma. J. Orthop. Res. 2000, 18, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Engleberg, A.I.; Yuzbasiyan-Gurkan, V. Establishment and Characterization of Cell Lines from Canine Metastatic Osteosarcoma. Cells 2024, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.F.; Grenho, I.; Martel, P.J.; Ferreira, B.I.; Link, W. FOXO family isoforms. Cell Death Dis. 2023, 14, 702. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chauhan, A.S.; Zhuang, L.; Gan, B. FoxO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018, 50, 65–76. [Google Scholar] [CrossRef]
- Dansen, T.B.; Burgering, B.M. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008, 18, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Minderman, H.; Maguire, O.; O’Loughlin, K.L.; Muhitch, J.; Wallace, P.K.; Abrams, S.I. Total cellular protein presence of the transcription factor IRF8 does not necessarily correlate with its nuclear presence. Methods 2017, 112, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liss, F.; Frech, M.; Wang, Y.; Giel, G.; Fischer, S.; Simon, C.; Weber, L.M.; Nist, A.; Stiewe, T.; Neubauer, A.; et al. IRF8 Is an AML-Specific Susceptibility Factor That Regulates Signaling Pathways and Proliferation of AML Cells. Cancers 2021, 13, 764. [Google Scholar] [CrossRef] [PubMed]
- Muhitch, J.B.; Hoffend, N.C.; Azabdaftari, G.; Miller, A.; Bshara, W.; Morrison, C.D.; Schwaab, T.; Abrams, S.I. Tumor-associated macrophage expression of interferon regulatory Factor-8 (IRF8) is a predictor of progression and patient survival in renal cell carcinoma. J. Immunother. Cancer 2019, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Park, S.Y.; Kim, J.H.; Kang, H.G.; Yoon, J.H.; Na, Y.S.; Kim, Y.N.; Park, B.K. Interferon consensus sequence-binding protein (ICSBP) promotes epithelial-to-mesenchymal transition (EMT)-like phenomena, cell-motility, and invasion via TGF-β signaling in U2OS cells. Cell Death Dis. 2014, 5, e1224. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Kim, J.H.; Kang, H.G.; Park, J.W.; Park, S.Y.; Park, B.K.; Kim, Y.N. ICSBP-induced PD-L1 enhances osteosarcoma cell growth. Front. Oncol. 2022, 12, 918216. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.F.; Barros, J.S.; Costa, S.S.D.; Carmo, G.B.D.; Scliar, M.O.; Lengert, A.V.H.; Boldrini, É.; Silva, S.; Vidal, D.O.; Maschietto, M.; et al. Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development. Int. J. Mol. Sci. 2023, 24, 10463. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, Y.; Han, E.Q.; Li, H.; Chen, D.; Jacobs, J.J.; Gitelis, S.; O’Keefe, R.J.; Konttinen, Y.T.; Yin, G.; et al. Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem. Biophys. Res. Commun. 2013, 431, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qiao, L.; Liu, Y. Long noncoding RNA LEF1-AS1 binds with HNRNPL to boost the proliferation, migration, and invasion in osteosarcoma by enhancing the mRNA stability of LEF1. J. Cell. Biochem. 2020, 121, 4064–4073. [Google Scholar] [CrossRef]
- Han, G.; Guo, Q.; Li, N.; Bi, W.; Xu, M.; Jia, J. Screening and Analysis of Biomarkers in the miRNA-mRNA Regulatory Network of Osteosarcoma. J. Healthc. Eng. 2022, 2022, 8055052. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Shi, Y.; Yang, F. Circular RNA circDOCK1 contributes to osteosarcoma progression by acting as a ceRNA for miR-936 to regulate LEF1. J. Bone Oncol. 2022, 36, 100453. [Google Scholar] [CrossRef] [PubMed]
- Pongsuchart, M.; Kuchimaru, T.; Yonezawa, S.; Tran, D.T.P.; Kha, N.T.; Hoang, N.T.H.; Kadonosono, T.; Kizaka-Kondoh, S. Novel lymphoid enhancer-binding factor 1-cytoglobin axis promotes extravasation of osteosarcoma cells into the lungs. Cancer Sci. 2018, 109, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, H.; Xu, C.X.; Bi, W.Z.; Wang, Y. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med. Oncol. 2014, 31, 972. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ikeda, K.; Shiraishi, K.; Eguchi, A.; Mori, T.; Yoshimoto, K.; Shibata, H.; Ito, T.; Baba, Y.; Baba, H. Aberrant methylation and silencing of IRF8 expression in non-small cell lung cancer. Oncol. Lett. 2014, 8, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiong, X.; Shao, Q.; Xiang, T.; Li, L.; Yin, X.; Li, X.; Tao, Q.; Ren, G. The tumor suppressor interferon regulatory factor 8 inhibits β-catenin signaling in breast cancers, but is frequently silenced by promoter methylation. Oncotarget 2017, 8, 48875–48888. [Google Scholar] [CrossRef] [PubMed]
- Rangan, A.; Reinig, E.; McPhail, E.D.; Rech, K.L. Immunohistochemistry for LEF1 and SOX11 adds diagnostic specificity in small B-cell lymphomas. Hum. Pathol. 2022, 121, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, G.; An, J. Discovery of small molecule inhibitors of the Wnt/beta-catenin signaling pathway by targeting beta-catenin/Tcf4 interactions. Exp. Biol. Med. 2017, 242, 1185–1197. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Erdfelder, F.; Hertweck, M.; Filipovich, A.; Uhrmacher, S.; Kreuzer, K.A. High lymphoid enhancer-binding factor-1 expression is associated with disease progression and poor prognosis in chronic lymphocytic leukemia. Hematol. Rep. 2010, 2, e3. [Google Scholar] [CrossRef]
- Erbilgin, Y.; Hatirnaz Ng, O.; Can, I.; Firtina, S.; Kucukcankurt, F.; Karaman, S.; Karakas, Z.; Celkan, T.T.; Zengin, E.; Aylan Gelen, S.; et al. Prognostic evidence of LEF1 isoforms in childhood acute lymphoblastic leukemia. Int. J. Lab. Hematol. 2021, 43, 1093–1103. [Google Scholar] [CrossRef]
- Kuhnl, A.; Gokbuget, N.; Kaiser, M.; Schlee, C.; Stroux, A.; Burmeister, T.; Mochmann, L.H.; Hoelzer, D.; Hofmann, W.K.; Thiel, E.; et al. Overexpression of LEF1 predicts unfavorable outcome in adult patients with B-precursor acute lymphoblastic leukemia. Blood 2011, 118, 6362–6367. [Google Scholar] [CrossRef]
- Lu, H.; Allende, D.; Liu, X.; Zhang, Y. Lymphoid Enhancer Binding Factor 1 (LEF1) and Paired Box Gene 8 (PAX8): A Limited Immunohistochemistry Panel to Distinguish Solid Pseudopapillary Neoplasms and Pancreatic Neuroendocrine Tumors. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, C.; Huang, L.; Niu, S.; Lu, Q.; Gong, D.; Huang, S.; Yuan, Y.; Chen, H. Prognostic value of association of OCT4 with LEF1 expression in esophageal squamous cell carcinoma and their impact on epithelial-mesenchymal transition, invasion, and migration. Cancer Med. 2018, 7, 3977–3987. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Feng, J.; Lu, J.; Xu, L.; Wang, W.; Fan, S. Expression of LEF1 and TCF1 (TCF7) proteins associates with clinical progression of nasopharyngeal carcinoma. J. Clin. Pathol. 2019, 72, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Mahjoubi, F.; Motalebzadeh, J. An integrated study on TFs and miRNAs in colorectal cancer metastasis and evaluation of three co-regulated candidate genes as prognostic markers. Gene 2018, 679, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chang, J.; Ruan, H.; Zhi, W.; Wang, X.; Zhao, F.; Ma, X.; Sun, X.; Liang, Q.; Xu, H.; et al. Cantharidin inhibits osteosarcoma proliferation and metastasis by directly targeting miR-214-3p/DKK3 axis to inactivate β-catenin nuclear translocation and LEF1 translation. Int. J. Biol. Sci. 2021, 17, 2504–2522. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.J.; Diefenderfer, D.L.; Goldschmidt, M.H.; Newton, C.D. Canine axial skeletal osteosarcoma. A retrospective study of 116 cases (1986 to 1989). Vet. Surg. 1992, 21, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Boerman, I.; Selvarajah, G.T.; Nielen, M.; Kirpensteijn, J. Prognostic factors in canine appendicular osteosarcoma—A meta-analysis. BMC Vet. Res. 2012, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, J.L.; Shaevitz, M.H.; Garrett, L.D.; Ruple, A.; Selmic, L.E. Demographic characteristics, site and phylogenetic distribution of dogs with appendicular osteosarcoma: 744 dogs (2000–2015). PLoS ONE 2019, 14, e0223243. [Google Scholar] [CrossRef]
- Williams, K.; Parker, S.; MacDonald-Dickinson, V. Risk factors for appendicular osteosarcoma occurrence in large and giant breed dogs in western Canada. Can. Vet. J. 2023, 64, 167–173. [Google Scholar] [PubMed]
- Phillips, J.C.; Stephenson, B.; Hauck, M.; Dillberger, J. Heritability and segregation analysis of osteosarcoma in the Scottish deerhound. Genomics 2007, 90, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Saam, D.E.; Liptak, J.M.; Stalker, M.J.; Chun, R. Predictors of outcome in dogs treated with adjuvant carboplatin for appendicular osteosarcoma: 65 cases (1996–2006). J. Am. Vet. Med. Assoc. 2011, 238, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Nielen, M.; Klungel, O.H.; Hoes, A.W.; de Boer, A.; Groenwold, R.H.; Kirpensteijn, J.; Investigators, V.S.S.O. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: An individual patient data meta-analysis. Prev. Vet. Med. 2013, 112, 414–422. [Google Scholar] [CrossRef] [PubMed]

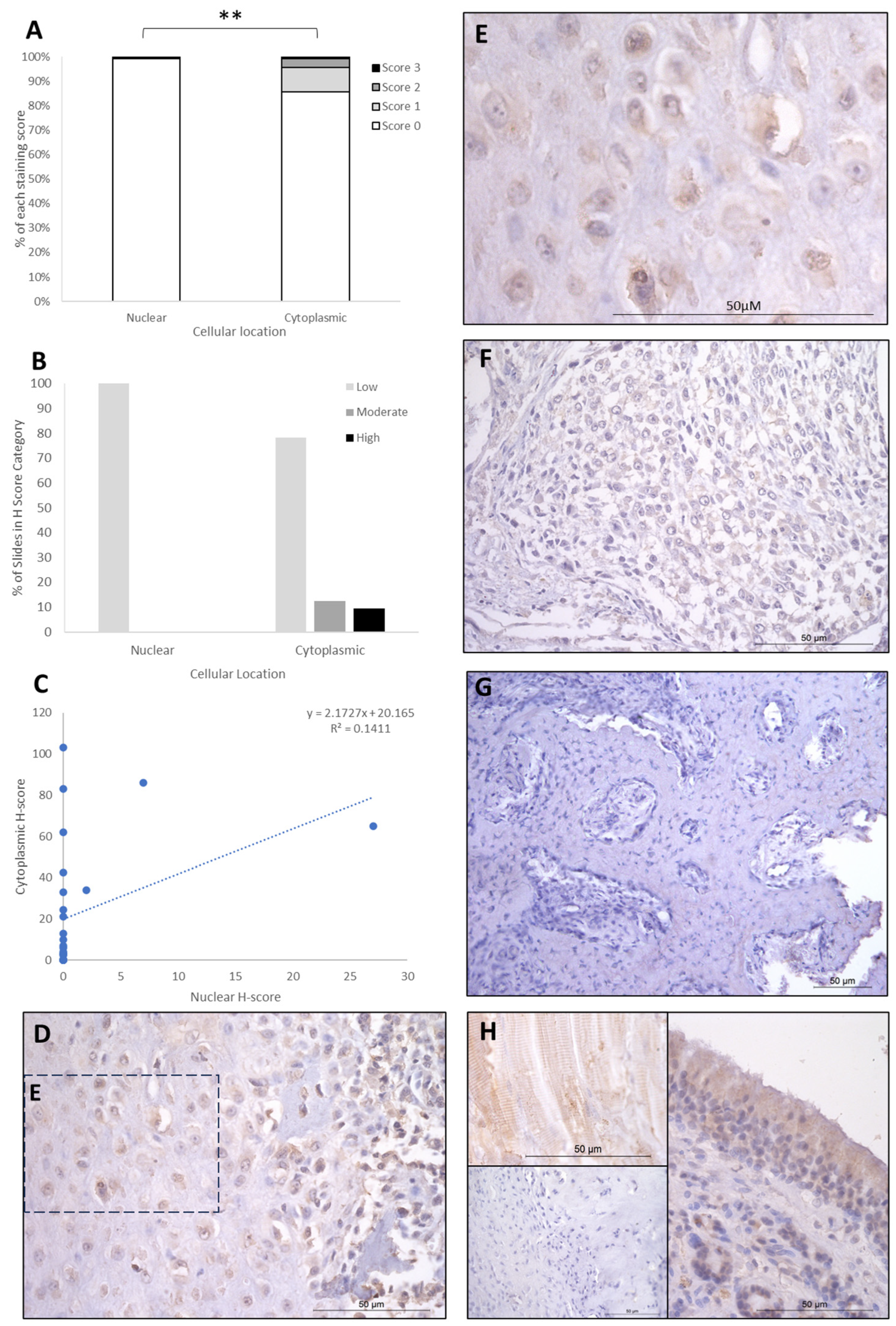
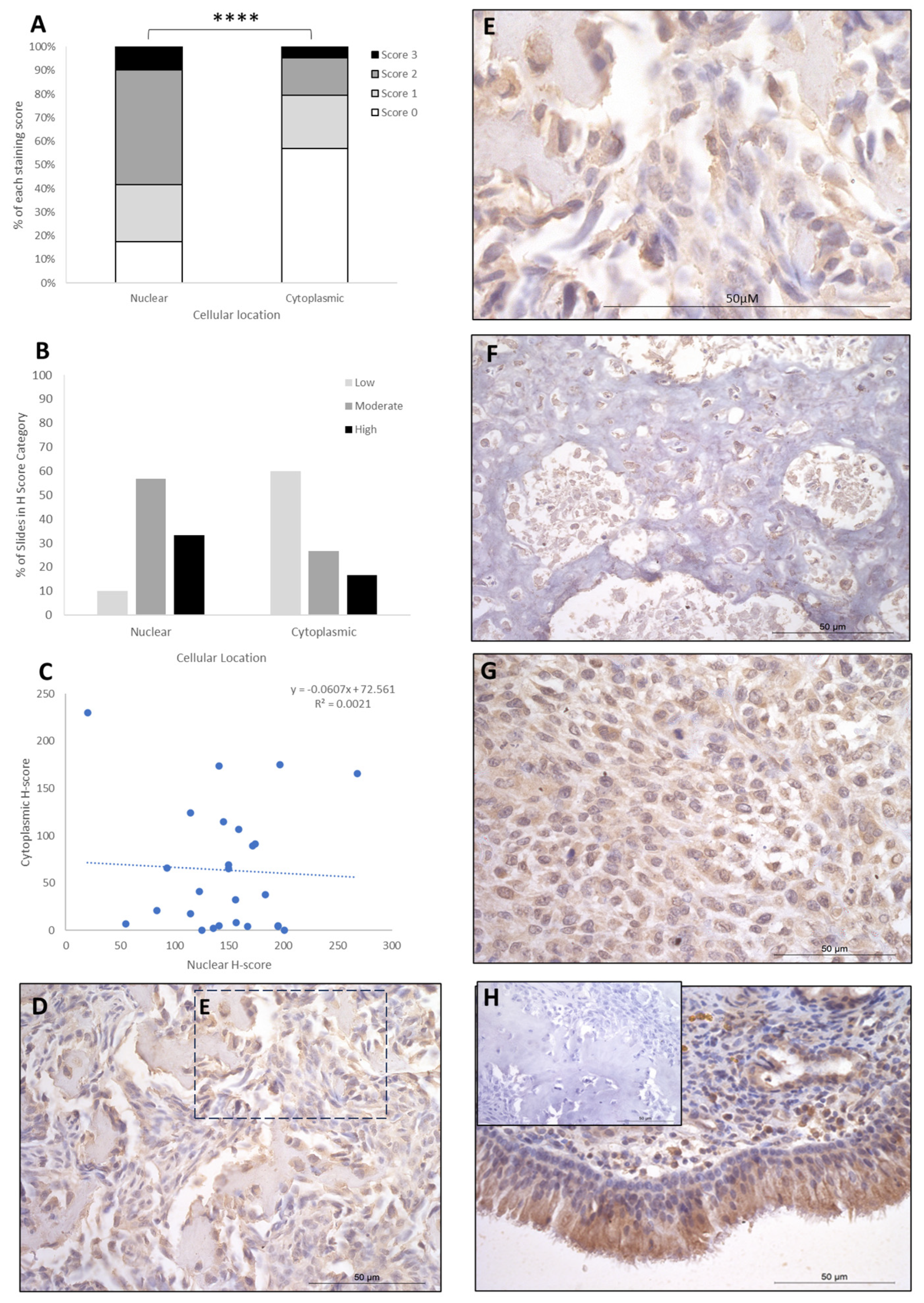
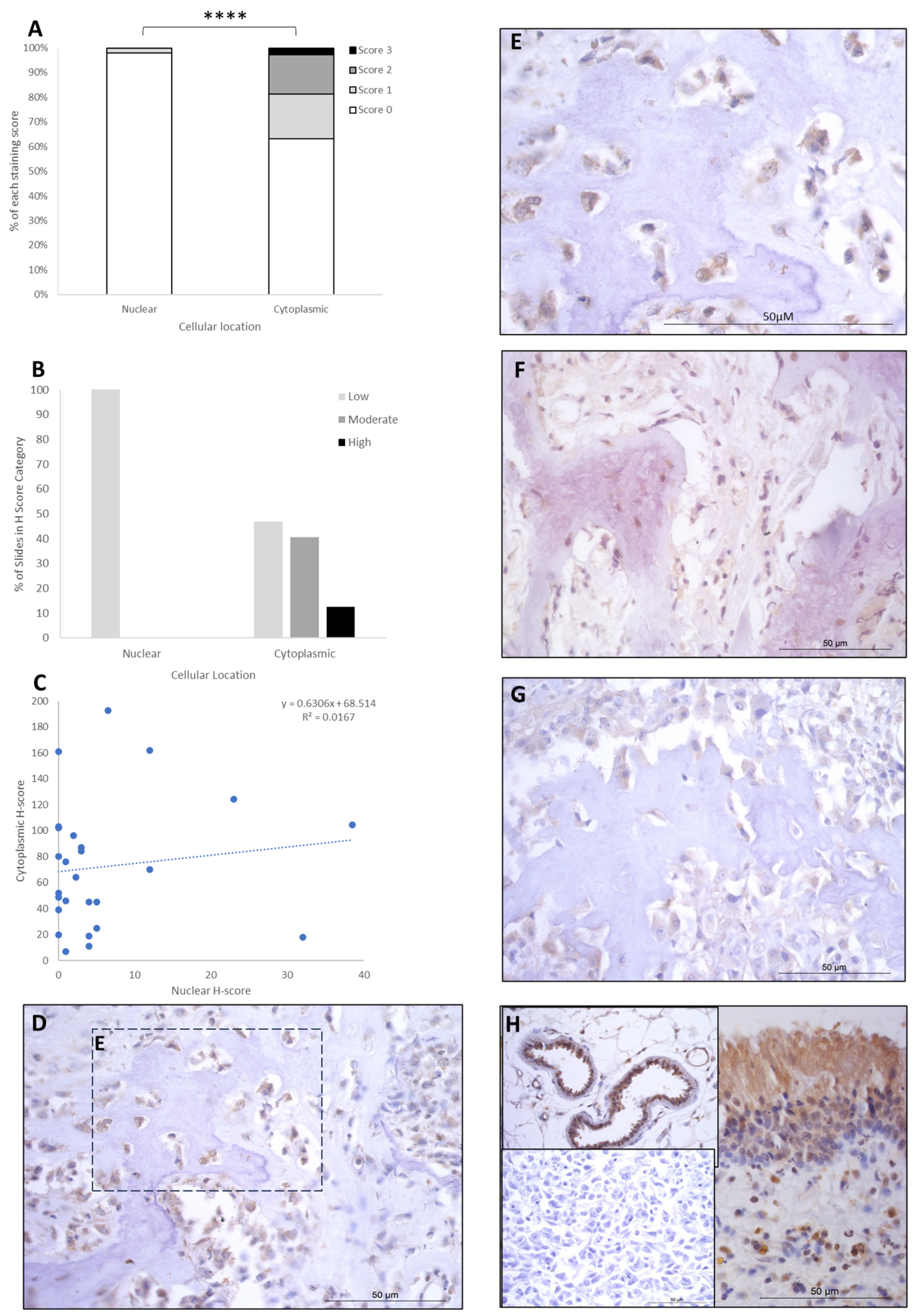
| Cytoplasmic | FOXO4 | IRF8 | LEF1 |
|---|---|---|---|
| Absent | - | 2 (8%) | 8 (31%) |
| Low | 20 (77%) | - | 4 (15.5%) |
| Moderate | 3 (11.5%) | 15 (58%) | 10 (38%) |
| High | 3 (11.5%) | 9 (34%) | 4 (15.5%) |
| Nuclear | |||
| Absent | 23 (88.5%) | - | - |
| Low | 3 (11.5%) | 17 (65.5%) | 26 (100%) |
| Moderate | - | 6 (23%) | - |
| High | - | 3 (11.5%) | - |
| Cytoplasmic Score | |||||
|---|---|---|---|---|---|
| FOXO4 | Absent | Low | Moderate | High | |
| Nuclear score | Absent | - | - | - | - |
| Low | - | 20 (77%) | 3 (11.5%) | 3 (11.5%) | |
| Moderate | - | - | - | - | |
| High | - | - | - | - | |
| IRF8 | Absent | Low | Moderate | High | |
| Absent | - | - | - | - | |
| Low | - | 1 (4%) | - | 1 (4%) | |
| Moderate | - | 11 (42%) | 3 (11.5%) | 1 (4%) | |
| High | - | 5 (19%) | 3 (11.5%) | 1 (4%) | |
| LEF1 | Absent | Low | Moderate | High | |
| Absent | - | - | - | - | |
| Low | 8 (31%) | 4 (15.5%) | 10 (38%) | 4 (15.5%) | |
| Moderate | - | - | - | - | |
| High | - | - | - | - | |
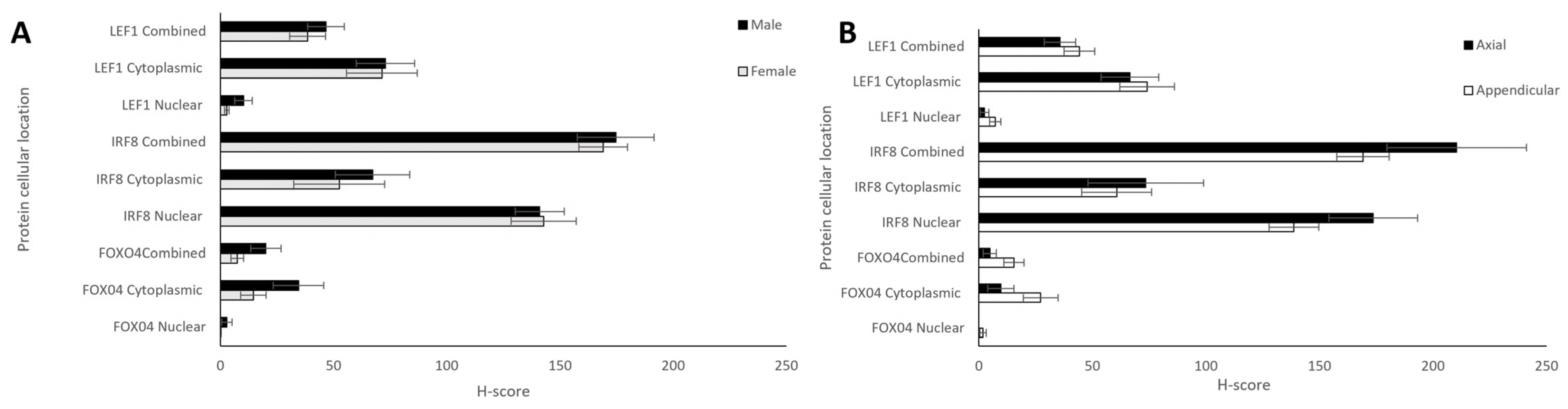
| H-Score | ||||
|---|---|---|---|---|
| Protein (n = 26) | Cellular Location | Mean ± SEM | p-Value (Cytoplasmic vs. Nuclear) | Range (Min-Max) |
| FOXO4 | Cytoplasmic | 23.17 ± 5.53 | 0.002 | 103 (0–103) |
| Nuclear | 1.38 ± 0.96 | 27 (0–27) | ||
| IRF8 | Cytoplasmic | 63.65 ± 12.15 | 0.0001 | 230 (0–230) |
| Nuclear | 146.81 ± 9.16 | 48 (20–68) | ||
| LEF1 | Cytoplasmic | 70.66 ± 8.64 | 0.0001 | 185.5 (7–192.5) |
| Nuclear | 6.13 ± 1.77 | 38.5 (0–38.5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brot, S.d.; Cobb, J.; Alibhai, A.A.; Jackson-Oxley, J.; Haque, M.; Patke, R.; Harris, A.E.; Woodcock, C.L.; Lothion-Roy, J.; Varun, D.; et al. Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma. Cancers 2024, 16, 1945. https://doi.org/10.3390/cancers16101945
Brot Sd, Cobb J, Alibhai AA, Jackson-Oxley J, Haque M, Patke R, Harris AE, Woodcock CL, Lothion-Roy J, Varun D, et al. Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma. Cancers. 2024; 16(10):1945. https://doi.org/10.3390/cancers16101945
Chicago/Turabian StyleBrot, Simone de, Jack Cobb, Aziza A. Alibhai, Jorja Jackson-Oxley, Maria Haque, Rodhan Patke, Anna E. Harris, Corinne L. Woodcock, Jennifer Lothion-Roy, Dhruvika Varun, and et al. 2024. "Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma" Cancers 16, no. 10: 1945. https://doi.org/10.3390/cancers16101945
APA StyleBrot, S. d., Cobb, J., Alibhai, A. A., Jackson-Oxley, J., Haque, M., Patke, R., Harris, A. E., Woodcock, C. L., Lothion-Roy, J., Varun, D., Thompson, R., Gomes, C., Kubale, V., Dunning, M. D., Jeyapalan, J. N., Mongan, N. P., & Rutland, C. S. (2024). Immunohistochemical Investigation into Protein Expression Patterns of FOXO4, IRF8 and LEF1 in Canine Osteosarcoma. Cancers, 16(10), 1945. https://doi.org/10.3390/cancers16101945





