Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
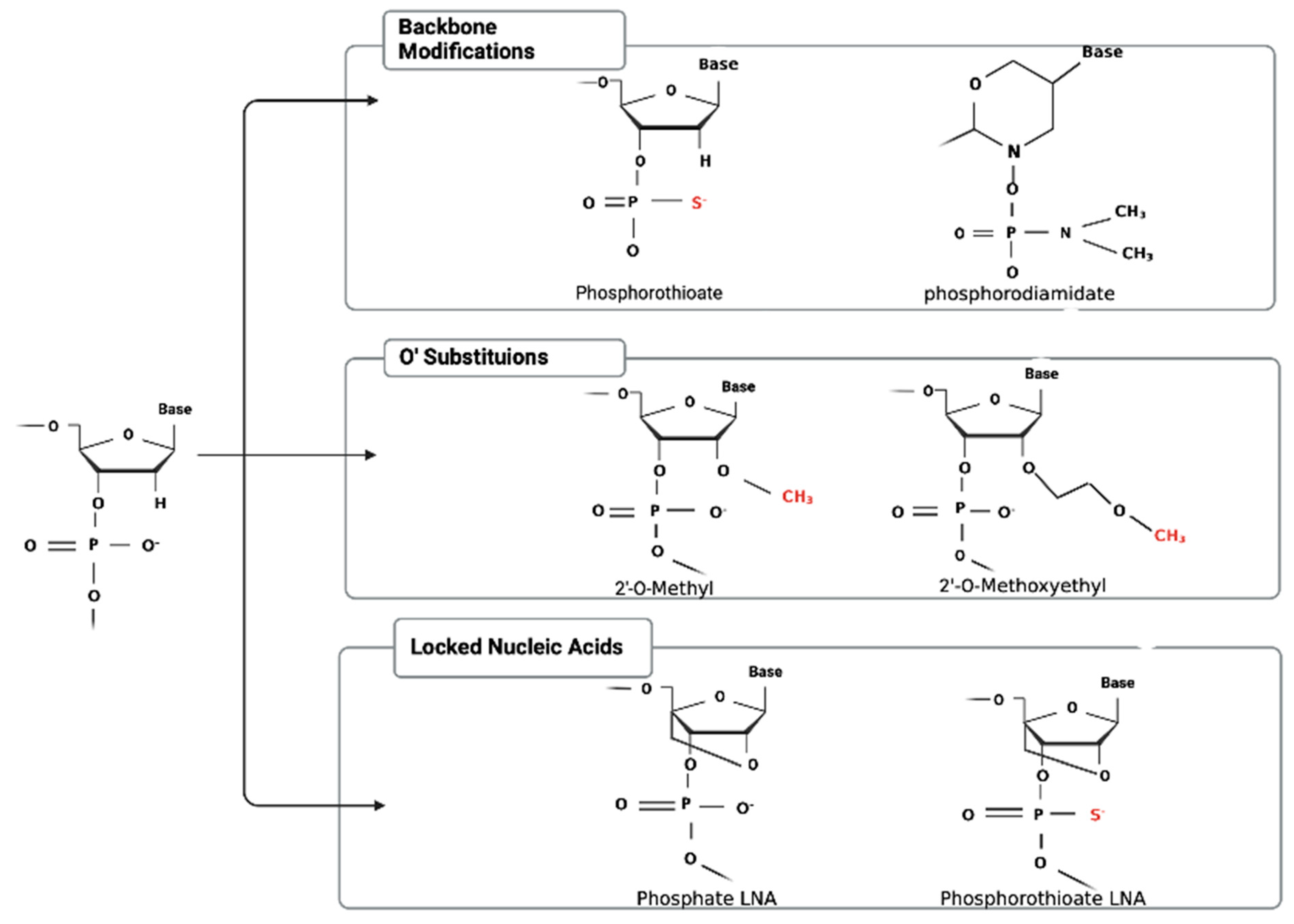
2. Chemical Modifications
3. Delivery Methods
4. Proof-of-Concept Studies
4.1. ASOs In Vitro
4.2. ASOs In Vivo
4.3. ASOs as Adjuvant Therapy
4.4. Clinical Trials
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Anaplastic astrocytoma |
| AS-Ln-4 | Antisense oligonucleotide for the laminin 4 chain |
| ASOs | Antisense oligonucleotides |
| BBB | Blood–brain barrier |
| c-Et | (S)-constrained ethyl |
| CNS | Central nervous system |
| CpG-ODN | Cytosine-guanosine oligonucleotides |
| DIPG | Diffuse intrinsic pontine glioma |
| DVT | Deep vein thrombosis |
| FA-PAMAM | Folate-polyamidoamine dendrimers conjugates |
| FANA | 2′-deoxy-2′-fluoro-beta-D-arabinonucleic acid |
| FGF | Fibroblast growth factor |
| GBM | Glioblastoma |
| IGF-1 | Growth Factor Type 1 |
| LNA | Locked nucleic acid |
| 2′-MOE | 2′-O-methoxyethyl |
| OS | Overall survival |
| PMO | Phosphorodiamidate morpholino oligomer |
| PS | Phosphorothioate |
| PKC | Protein Kinase C |
| TGFB | Transforming Growth Factor-Beta |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar] [PubMed]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Khorkova, O.; Wahlestedt, C. Oligonucleotide therapies for disorders of the nervous system. Nat. Biotechnol. 2017, 35, 249–263. [Google Scholar] [CrossRef]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Papachristodoulou, A.; Silginer, M.; Weller, M.; Schneider, H.; Hasenbach, K.; Janicot, M.; Roth, P. Therapeutic Targeting of TGFbeta Ligands in Glioblastoma Using Novel Antisense Oligonucleotides Reduces the Growth of Experimental Gliomas. Clin. Cancer Res. 2019, 25, 7189–7201. [Google Scholar] [CrossRef] [PubMed]
- Temsamani, J.; Guinot, P. Antisense oligonucleotides: A new therapeutic approach. Biotechnol. Appl. Biochem. 1997, 26, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hyjek-Skladanowska, M.; Vickers, T.A.; Napiorkowska, A.; Anderson, B.A.; Tanowitz, M.; Crooke, S.T.; Liang, X.H.; Seth, P.P.; Nowotny, M. Origins of the Increased Affinity of Phosphorothioate-Modified Therapeutic Nucleic Acids for Proteins. J. Am. Chem. Soc. 2020, 142, 7456–7468. [Google Scholar] [CrossRef]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides: A primer. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Malek-Adamian, E.; Le, P.U.; Meng, A.; Martinez-Montero, S.; Petrecca, K.; Damha, M.J.; Shoichet, M.S. Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, D.; Zhao, S.; Shao, Y.; Xia, Y.; Ni, D.; Qiu, X.; Zhang, J.; Chen, J.; Meng, F.; et al. Immunotherapy of Malignant Glioma by Noninvasive Administration of TLR9 Agonist CpG Nano-Immunoadjuvant. Adv. Sci. 2022, 9, e2103689. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Yuan, X.; Li, F.; Pu, P.; Yu, S.; Shen, C.; Zhang, Z.; Zhang, Y. Evaluation of folate-PAMAM for the delivery of antisense oligonucleotides to rat C6 glioma cells in vitro and in vivo. J. Biomed. Mater. Res. A 2010, 93, 585–594. [Google Scholar] [CrossRef]
- Ambady, P.; Wu, Y.J.; Kersch, C.N.; Walker, J.M.; Holland, S.; Muldoon, L.L.; Neuwelt, E.A. Radiation enhances the delivery of antisense oligonucleotides and improves chemo-radiation efficacy in brain tumor xenografts. Cancer Gene. Ther. 2022, 29, 533–542. [Google Scholar] [CrossRef]
- Ambady, P.; Wu, Y.J.; Walker, J.M.; Kersch, C.; Pagel, M.A.; Woltjer, R.L.; Fu, R.; Muldoon, L.L.; Neuwelt, E.A. Enhancing the cytotoxicity of chemoradiation with radiation-guided delivery of anti-MGMT morpholino oligonucleotides in non-methylated solid tumors. Cancer Gene. Ther. 2017, 24, 348–357. [Google Scholar] [CrossRef]
- Adamus, T.; Hung, C.Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucleic Acids 2022, 27, 611–620. [Google Scholar] [CrossRef]
- Ruan, S.; Okcu, M.F.; Pong, R.C.; Andreeff, M.; Levin, V.; Hsieh, J.T.; Zhang, W. Attenuation of WAF1/Cip1 expression by an antisense adenovirus expression vector sensitizes glioblastoma cells to apoptosis induced by chemotherapeutic agents 1,3-bis(2-chloroethyl)-1-nitrosourea and cisplatin. Clin. Cancer Res. 1999, 5, 197–202. [Google Scholar]
- Jafar-Nejad, P.; Powers, B.; Soriano, A.; Zhao, H.; Norris, D.A.; Matson, J.; DeBrosse-Serra, B.; Watson, J.; Narayanan, P.; Chun, S.J.; et al. The atlas of RNase H antisense oligonucleotide distribution and activity in the CNS of rodents and non-human primates following central administration. Nucleic Acids Res. 2021, 49, 657–673. [Google Scholar] [CrossRef]
- Grossman, S.A.; Alavi, J.B.; Supko, J.G.; Carson, K.A.; Priet, R.; Dorr, F.A.; Grundy, J.S.; Holmlund, J.T. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro-Oncology 2005, 7, 32–40. [Google Scholar] [CrossRef]
- Jaschinski, F.; Rothhammer, T.; Jachimczak, P.; Seitz, C.; Schneider, A.; Schlingensiepen, K.H. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-beta2. Curr. Pharm. Biotechnol. 2011, 12, 2203–2213. [Google Scholar] [CrossRef]
- Schlingensiepen, K.H.; Schlingensiepen, R.; Steinbrecher, A.; Hau, P.; Bogdahn, U.; Fischer-Blass, B.; Jachimczak, P. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006, 17, 129–139. [Google Scholar] [CrossRef]
- Andrews, D.W.; Resnicoff, M.; Flanders, A.E.; Kenyon, L.; Curtis, M.; Merli, G.; Baserga, R.; Iliakis, G.; Aiken, R.D. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J. Clin. Oncol. 2001, 19, 2189–2200. [Google Scholar] [CrossRef]
- Baserga, R. The insulin-like growth factor I receptor: A key to tumor growth? Cancer Res. 1995, 55, 249–252. [Google Scholar]
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro-Oncology 2011, 13, 132–142. [Google Scholar] [CrossRef]
- Vallieres, L. Trabedersen, a TGFbeta2-specific antisense oligonucleotide for the treatment of malignant gliomas and other tumors overexpressing TGFbeta2. IDrugs 2009, 12, 445–453. [Google Scholar] [PubMed]
- Murphy, P.R.; Sato, Y.; Knee, R.S. Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage-independent growth of a malignant glioblastoma cell line. Mol. Endocrinol. 1992, 6, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Jia, Q.; Ren, Y.; Wang, Y.; Shi, L.; Liu, N.; Wang, G.; Pu, P.; You, Y.; et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol. Rep. 2010, 24, 195–201. [Google Scholar] [CrossRef]
- Pu, P.; Kang, C.; Li, J.; Jiang, H.; Cheng, J. The effects of antisense AKT2 RNA on the inhibition of malignant glioma cell growth in vitro and in vivo. J. Neurooncol. 2006, 76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.; Liu, Y.H.; Wilkinson, J.E.; Krainer, A.R. Antisense oligonucleotide therapy for H3.3K27M diffuse midline glioma. Sci. Transl. Med. 2023, 15, eadd8280. [Google Scholar] [CrossRef]
- Engelhard, H.; Narang, C.; Homer, R.; Duncan, H. Urokinase antisense oligodeoxynucleotides as a novel therapeutic agent for malignant glioma: In vitro and in vivo studies of uptake, effects and toxicity. Biochem. Biophys. Res. Commun. 1996, 227, 400–405. [Google Scholar] [CrossRef]
- D’Cunja, J.; Shalaby, T.; Rivera, P.; von Buren, A.; Patti, R.; Heppner, F.L.; Arcaro, A.; Rorke-Adams, L.B.; Phillips, P.C.; Grotzer, M.A. Antisense treatment of IGF-IR induces apoptosis and enhances chemosensitivity in central nervous system atypical teratoid/rhabdoid tumours cells. Eur. J. Cancer 2007, 43, 1581–1589. [Google Scholar] [CrossRef]
- Liu, S.J.; Malatesta, M.; Lien, B.V.; Saha, P.; Thombare, S.S.; Hong, S.J.; Pedraza, L.; Koontz, M.; Seo, K.; Horlbeck, M.A.; et al. CRISPRi-based radiation modifier screen identifies long non-coding RNA therapeutic targets in glioma. Genome Biol. 2020, 21, 83. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Yuan, Y.; Jin, Z.; Zhai, H.; Liu, B.; Li, Y.; Zhang, C.; Chen, M.; Shi, Y.; et al. LncRNA GSCAR promotes glioma stem cell maintenance via stabilizing SOX2 expression. Int. J. Biol. Sci. 2023, 19, 1681–1697. [Google Scholar] [CrossRef]
- Cho-Chung, Y.S. Antisense oligonucleotide inhibition of serine/threonine kinases: An innovative approach to cancer treatment. Pharmacol. Ther. 1999, 82, 437–449. [Google Scholar] [CrossRef]
- da Rocha, A.B.; Mans, D.R.; Regner, A.; Schwartsmann, G. Targeting protein kinase C: New therapeutic opportunities against high-grade malignant gliomas? Oncologist 2002, 7, 17–33. [Google Scholar] [CrossRef]
- Monia, B.P.; Holmlund, J.; Dorr, F.A. Antisense approaches for the treatment of cancer. Cancer Investig. 2000, 18, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Way, K.J.; Chou, E.; King, G.L. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol. Sci. 2000, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Cripps, M.C.; Figueredo, A.T.; Oza, A.M.; Taylor, M.J.; Fields, A.L.; Holmlund, J.T.; McIntosh, L.W.; Geary, R.S.; Eisenhauer, E.A. Phase II randomized study of ISIS 3521 and ISIS 5132 in patients with locally advanced or metastatic colorectal cancer: A National Cancer Institute of Canada clinical trials group study. Clin. Cancer Res. 2002, 8, 2188–2192. [Google Scholar]
- Advani, R.; Lum, B.L.; Fisher, G.A.; Halsey, J.; Geary, R.S.; Holmlund, J.T.; Kwoh, T.J.; Dorr, F.A.; Sikic, B.I. A phase I trial of aprinocarsen (ISIS 3521/LY900003), an antisense inhibitor of protein kinase C-alpha administered as a 24-hour weekly infusion schedule in patients with advanced cancer. Investig. New Drugs 2005, 23, 467–477. [Google Scholar] [CrossRef]
- Besson, A.; Yong, V.W. Involvement of p21(Waf1/Cip1) in protein kinase C alpha-induced cell cycle progression. Mol. Cell Biol. 2000, 20, 4580–4590. [Google Scholar] [CrossRef][Green Version]
- Baltuch, G.H.; Dooley, N.P.; Rostworowski, K.M.; Villemure, J.G.; Yong, V.W. Protein kinase C isoform alpha overexpression in C6 glioma cells and its role in cell proliferation. J. Neurooncol. 1995, 24, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Nagato, S.; Nakagawa, K.; Harada, H.; Kohno, S.; Fujiwara, H.; Sekiguchi, K.; Ohue, S.; Iwata, S.; Ohnishi, T. Downregulation of laminin alpha4 chain expression inhibits glioma invasion in vitro and in vivo. Int. J. Cancer 2005, 117, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kondo, S.; Tanaka, Y.; Haqqi, T.; Barna, B.P.; Cowell, J.K. Inhibition of telomerase increases the susceptibility of human malignant glioblastoma cells to cisplatin-induced apoptosis. Oncogene 1998, 16, 2243–2248. [Google Scholar] [CrossRef]
- Guensberg, P.; Wacheck, V.; Lucas, T.; Monia, B.; Pehamberger, H.; Eichler, H.G.; Jansen, B. Bcl-xL antisense oligonucleotides chemosensitize human glioblastoma cells. Chemotherapy 2002, 48, 189–195. [Google Scholar] [CrossRef]
- Belenkov, A.I.; Paiement, J.P.; Panasci, L.C.; Monia, B.P.; Chow, T.Y. An antisense oligonucleotide targeted to human Ku86 messenger RNA sensitizes M059K malignant glioma cells to ionizing radiation, bleomycin, and etoposide but not DNA cross-linking agents. Cancer Res. 2002, 62, 5888–5896. [Google Scholar] [PubMed]
- Zhang, C.; Kang, C.; Wang, P.; Cao, Y.; Lv, Z.; Yu, S.; Wang, G.; Zhang, A.; Jia, Z.; Han, L.; et al. MicroRNA-221 and -222 regulate radiation sensitivity by targeting the PTEN pathway. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Cotter, F.E. Antisense therapy of hematologic malignancies. Semin. Hematol. 1999, 36, 9–14. [Google Scholar]
- Waters, J.S.; Webb, A.; Cunningham, D.; Clarke, P.A.; Raynaud, F.; di Stefano, F.; Cotter, F.E. Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. 2000, 18, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.N.; Zhang, M.; Datto, M.B.; Bigner, D.D.; Wang, X.F. Transforming growth factor-beta-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J. Biol. Chem. 1999, 274, 35053–35058. [Google Scholar] [CrossRef]
- Vega, E.A.; Graner, M.W.; Sampson, J.H. Combating immunosuppression in glioma. Future Oncol. 2008, 4, 433–442. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Kleinschmidt-DeMasters, B.K.; Franzusoff, A.; Ng, K.Y.; Lillehei, K.O. TGF-beta2 inhibition augments the effect of tumor vaccine and improves the survival of animals with pre-established brain tumors. J. Neurooncol. 2007, 81, 149–162. [Google Scholar] [CrossRef]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef]
- Ursu, R.; Carpentier, A.; Metellus, P.; Lubrano, V.; Laigle-Donadey, F.; Capelle, L.; Guyotat, J.; Langlois, O.; Bauchet, L.; Desseaux, K.; et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur. J. Cancer 2017, 73, 30–37. [Google Scholar] [CrossRef]
- Andrews, D.W.; Judy, K.D.; Scott, C.B.; Garcia, S.; Harshyne, L.A.; Kenyon, L.; Talekar, K.; Flanders, A.; Atsina, K.B.; Kim, L.; et al. Phase Ib Clinical Trial of IGV-001 for Patients with Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2021, 27, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.Y.; Hanft, S.; Schulder, M.; Judy, K.D.; Wong, E.T.; Elder, J.B.; Evans, L.T.; Zuccarello, M.; Wu, J.; Aulakh, S.; et al. Autologous cell immunotherapy (IGV-001) with IGF-1R antisense oligonucleotide in newly diagnosed glioblastoma patients. Future Oncol. 2024, 20, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Perez-Olle, R.; Diwanji, M.; Pennock, G.; Scott, C.; Andrews, D. Tips-18 trial in progress: A randomized, multicenter, double-blind, placebo-controlled, phase 2b study to assess the safety and efficacy of igv-001, an autologous cell immunotherapy with antisense oligonucleotide (imv-001) targeting igf-1r, in newly diagnosed patients with glioblastoma. Neurooncol. Adv. 2023, 5, iii38. [Google Scholar]
- Thompson, P.A.; Drissi, R.; Muscal, J.A.; Panditharatna, E.; Fouladi, M.; Ingle, A.M.; Ahern, C.H.; Reid, J.M.; Lin, T.; Weigel, B.J.; et al. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: A Children’s Oncology Group Phase I Consortium Study (ADVL1112). Clin. Cancer Res. 2013, 19, 6578–6584. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; Hummel, T.R.; Kumar, S.S.; Dorris, K.; Li, S.; Lin, T.; Daryani, V.M.; Stewart, C.F.; Miles, L.; Poussaint, T.Y.; et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: A pediatric brain tumor consortium study. J. Neurooncol. 2016, 129, 443–451. [Google Scholar] [CrossRef]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J. Pediatr. Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef]

| Drug Name | Chemical Modifications | Mechanism | Route of Administration | Safety Profile | Outcomes |
|---|---|---|---|---|---|
| Aprinocarsen (ISIS 3521) | Phosphorothioate backbone | RNase H degradation | Intravenous infusion | Increased intracranial pressure, tumor progression, and neural deficit | No clinical benefit |
| Trabedersen (AP12009) | Phosphorothioate backbone | mRNA degradation | Intratumoral injection | Fever, headache, and GI discomfort | Increased time to recurrence |
| CpG-ODN | Cytosine triphosphate linked to guanine triphosphate | Activation of TLR-9 | Intratumoral injection | Fever and post-op hematoma | Increased OS |
| IMV-001 | Single-stranded 18-mer oligonucleotide | Steric hindrance of translation | Abdominal bio-diffusion chamber | Hematomas and wound complications | Increased PFS and OS |
| Imetelstat | Thiophosphoramidate oligonucleotide | Competitive inhibition of Telomerase Enzyme | Intravenous injection | Thrombocytopenia, lymphopenia, neutropenia, and intratumoral hemorrhage | Persistent knockdown of telomerase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desgraves, J.F.; Mendez Valdez, M.J.; Chandar, J.; Gurses, M.E.; Henderson, L.; Castro, J.R.; Seetheram, D.; Ivan, M.E.; Komotar, R.J.; Shah, A.H. Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma. Cancers 2024, 16, 1944. https://doi.org/10.3390/cancers16101944
Desgraves JF, Mendez Valdez MJ, Chandar J, Gurses ME, Henderson L, Castro JR, Seetheram D, Ivan ME, Komotar RJ, Shah AH. Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma. Cancers. 2024; 16(10):1944. https://doi.org/10.3390/cancers16101944
Chicago/Turabian StyleDesgraves, Jelisah F., Mynor J. Mendez Valdez, Jay Chandar, Muhammet Enes Gurses, Lisa Henderson, Jesus R. Castro, Deepa Seetheram, Michael E. Ivan, Ricardo J. Komotar, and Ashish H. Shah. 2024. "Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma" Cancers 16, no. 10: 1944. https://doi.org/10.3390/cancers16101944
APA StyleDesgraves, J. F., Mendez Valdez, M. J., Chandar, J., Gurses, M. E., Henderson, L., Castro, J. R., Seetheram, D., Ivan, M. E., Komotar, R. J., & Shah, A. H. (2024). Antisense Oligonucleotides for Rapid Translation of Gene Therapy in Glioblastoma. Cancers, 16(10), 1944. https://doi.org/10.3390/cancers16101944








