The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture Conditions
2.2. RNA Isolation and Reverse Transcription
2.3. Analysis of mRNA Expression
2.4. Western Blot
2.5. SiRNA and Plasmid Transfection
2.6. Proliferation with the xCELLigence System
2.7. Clonogenic Assay (Proliferation)
2.8. Statistical Analysis
3. Results
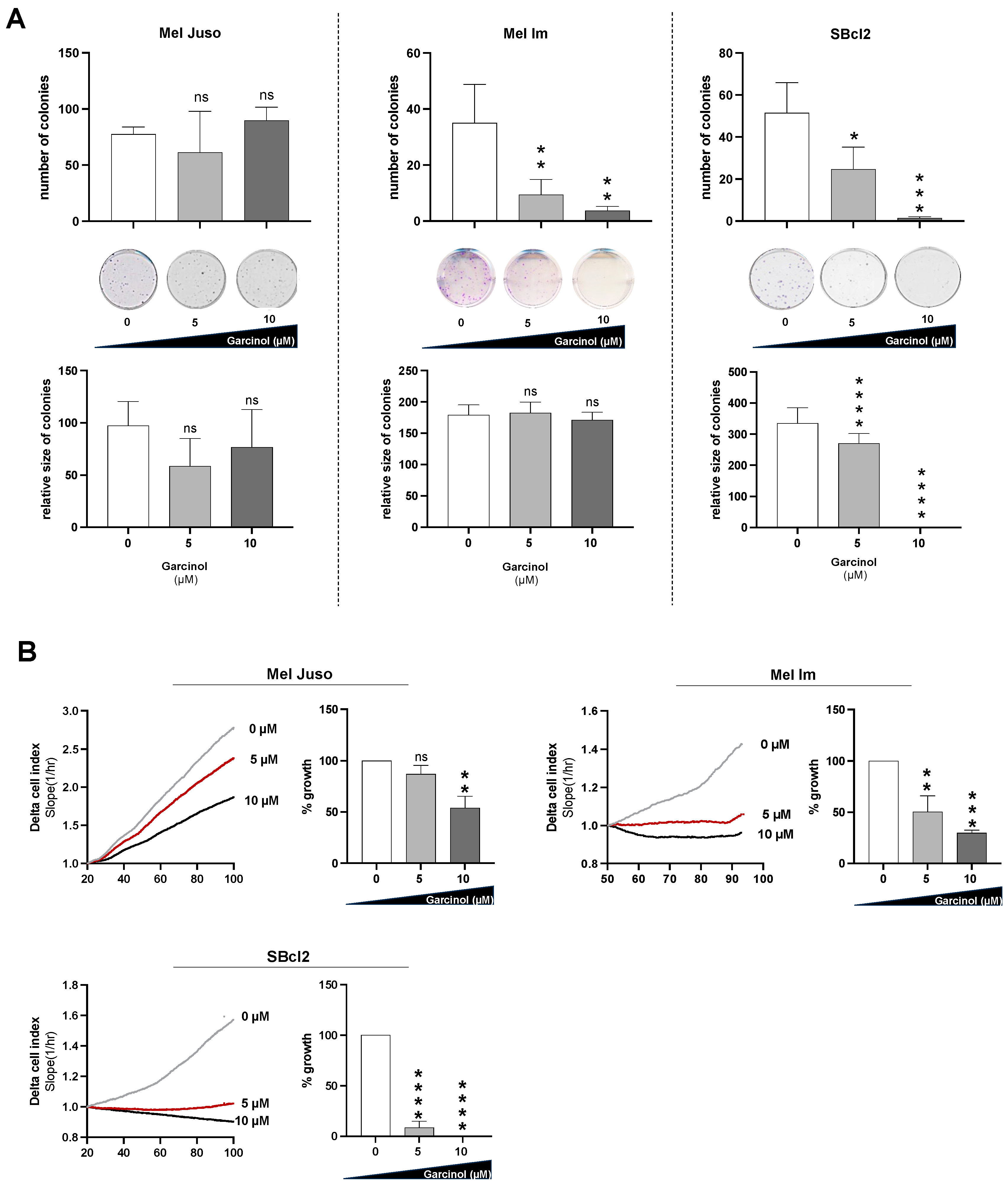
3.1. Garcinol Reduces Proliferation of Different Melanoma Cells
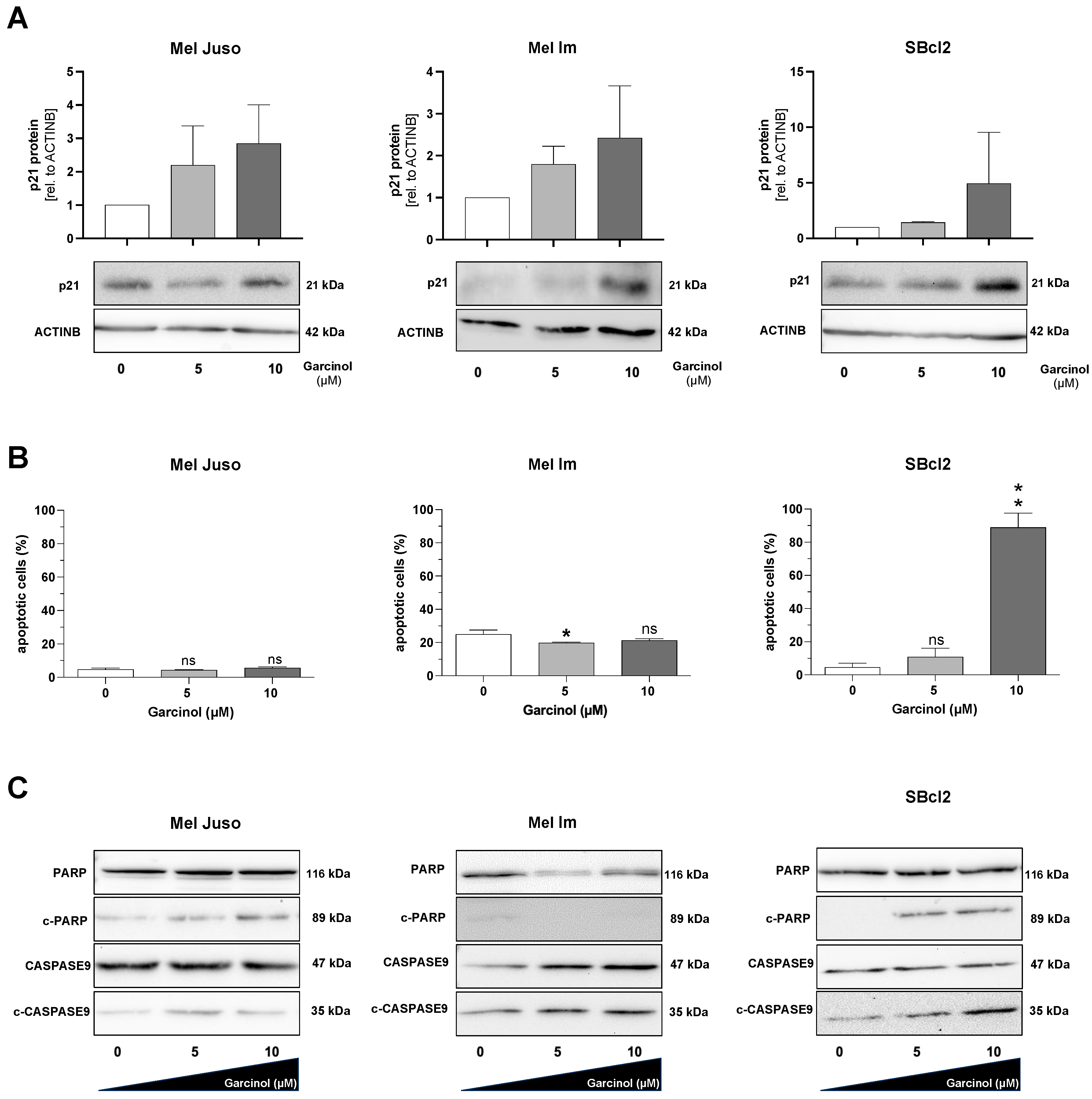
3.2. Garcinol Induces Apoptosis in SBcl2 Cells
3.3. Expression of T-Cadherin (CDH13) Is Not Influenced by Garcinol Treatment
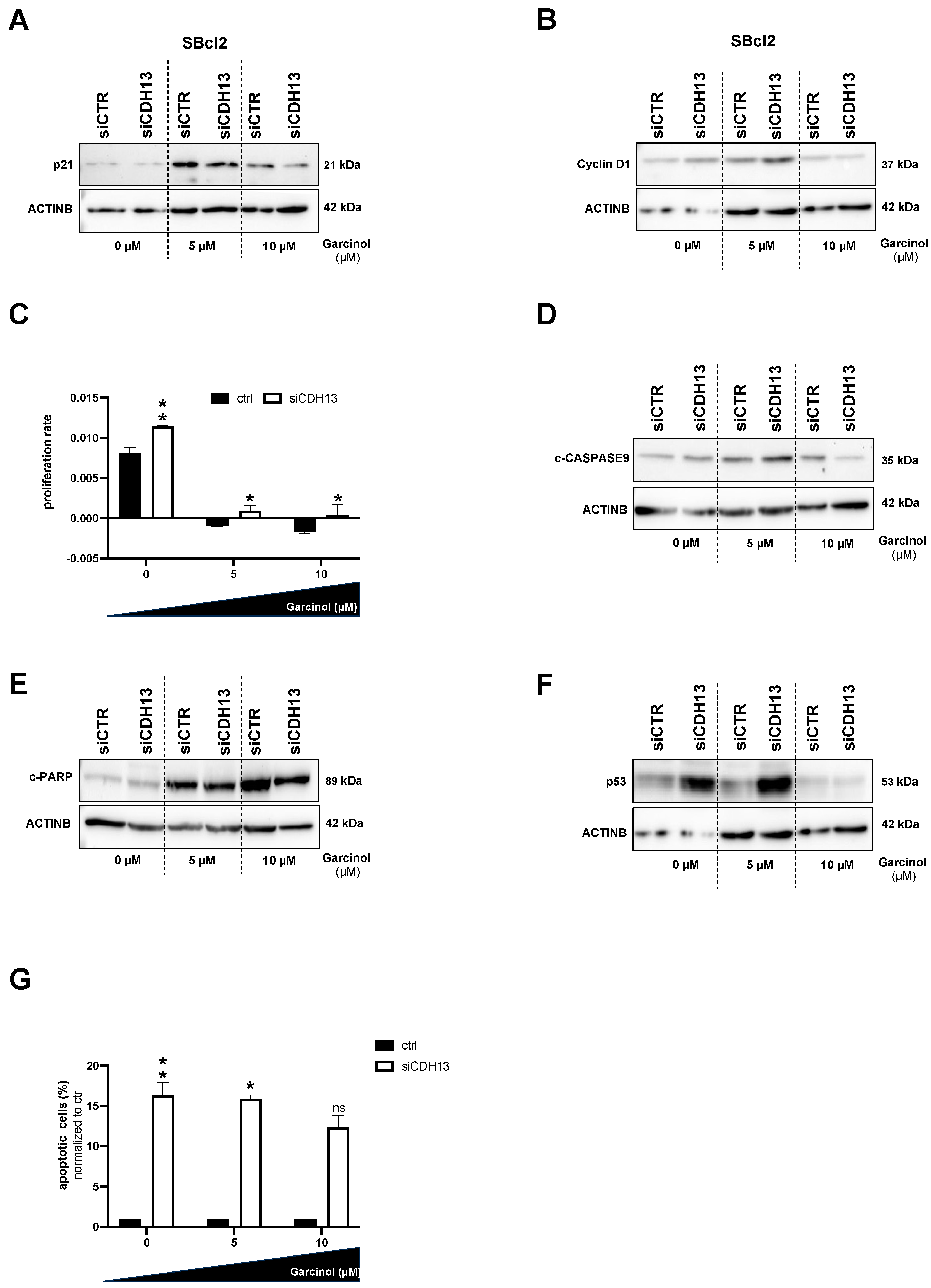
3.4. The Role of T-Cadherin for Garcinol Treatment
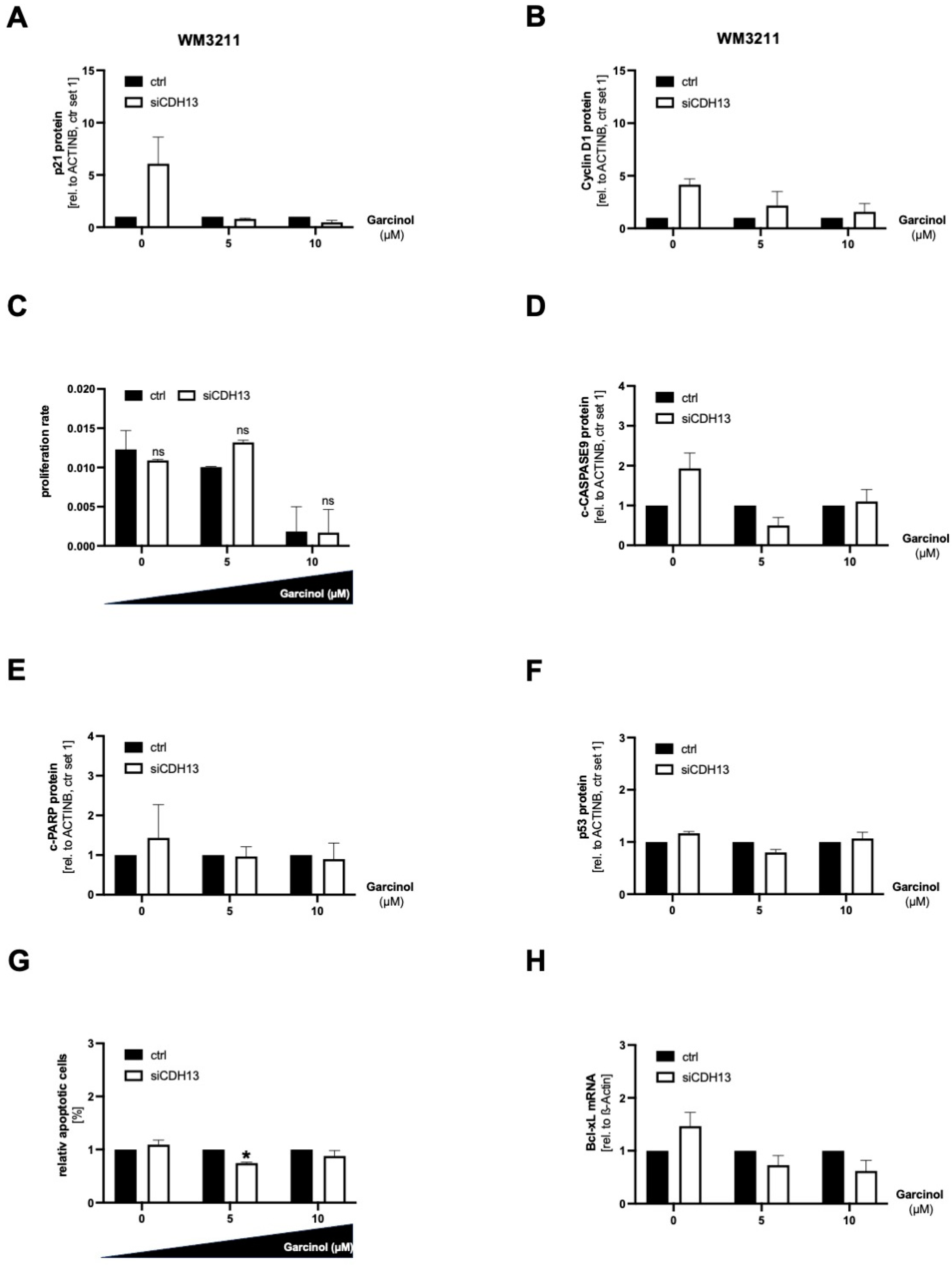
3.5. A Loss of T-Cadherin Influences the Effect of Garcinol Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulikakos, P.I.; Sullivan, R.J.; Yaeger, R. Molecular Pathways and Mechanisms of BRAF in Cancer Therapy. Clin. Cancer Res. 2022, 28, 4618–4628. [Google Scholar] [CrossRef] [PubMed]
- Pastwińska, J.; Karaś, K.; Karwaciak, I.; Ratajewski, M. Targeting EGFR in melanoma—The sea of possibilities to overcome drug resistance. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188754. [Google Scholar] [CrossRef] [PubMed]
- Chantree, P.; Martviset, P.; Thongsepee, N.; Sangpairoj, K.; Sornchuer, P. Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-kappaB Signalling Pathway. Nutrients 2023, 15, 575. [Google Scholar] [CrossRef] [PubMed]
- Kopytko, P.; Piotrowska, K.; Janisiak, J.; Tarnowski, M. Garcinol—A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021, 22, 2828. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Chaturvedi Parashar, N.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumour Cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free Radical Scavenging Activity and Antiulcer Activity of Garcinol from Garcinia indica Fruit Rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar] [CrossRef]
- Liao, C.; Sang, S.; Liang, Y.; Ho, C.; Lin, J. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 in downregulating nuclear factor-kappa B pathway by Garcinol. Mol. Carcinog. 2004, 41, 140–149. [Google Scholar] [CrossRef]
- Paul, B.; Gaonkar, R.H.; Mukhopadhyay, R.; Ganguly, S.; Debnath, M.C.; Mukherjee, B. Garcinol-loaded novel cationic nanoliposomes: In vitro and in vivo study against B16F10 melanoma tumour model. Nanomedicine 2019, 14, 2045–2065. [Google Scholar] [CrossRef]
- Ahmad, A.; Sarkar, S.H.; Bitar, B.; Ali, S.; Aboukameel, A.; Sethi, S.; Li, Y.; Bao, B.; Kong, D.; Banerjee, S.; et al. Garcinol regulates EMT and Wnt signalling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol. Cancer Ther. 2012, 11, 2193–2201. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Ji, J.; Li, C.; Li, Z.; Li, L. Garcinol exerts anti-cancer effect in human cervical cancer cells through upregulation of T-cadherin. Biomed. Pharmacother. 2018, 107, 957–966. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, J.; Lu, X.; Zhang, T.; Zhao, K.; Han, W.; Yang, W.; Qian, Y. Garcinol suppresses RANKL-induced osteoclastogenesis and its underlying mechanism. J. Cell. Physiol. 2019, 234, 7498–7509. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.K.; Ellmann, L.; Quast, A.S.; Eberle, J.; Boyle, G.M.; Kuphal, S. Loss of T-cadherin (CDH-13) regulates AKT signaling and desensitizes cells to apoptosis in melanoma. Mol. Carcinog. 2013, 53, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Ellmann, L.; Joshi, M.B.; Resink, T.J.; Bosserhoff, A.K.; Kuphal, S. BRN2 is a transcriptional repressor of CDH13 (T-cadherin) in melanoma cells. Lab. Investig. 2012, 92, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Kuphal, S.; Martyn, A.C.; Pedley, J.; Crowther, L.M.; Bonazzi, V.F.; Parsons, P.G.; Bosserhoff, A.K.; Hayward, N.K.; Boyle, G.M. H-Cadherin expression reduces invasion of malignant melanoma. Pigment. Cell Melanoma Res. 2009, 22, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.V.; Kutuzov, M.A. Cadherin 13 in cancer. Genes. Chromosomes Cancer 2010, 49, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Philippova, M.; Ivanov, D.; Tkachuk, V.; Erne, P.; Resink, T.J. Polarisation of T-cadherin to the leading edge of migrating vascular cells in vitro: A function in vascular cell motility? Histochem. Cell Biol. 2003, 120, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, C.; Bahna, F.; Zampieri, N.; VanSteenhouse, H.C.; Katsamba, P.S.; Ahlsen, G.; Harrison, O.J.; Brasch, J.; Jin, X.; Posy, S.; et al. T-cadherin structures reveal a novel adhesive binding mechanism. Nat. Struct. Mol. Biol. 2010, 17, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Seefried, F.; Haller, L.; Fukuda, S.; Thongmao, A.; Schneider, N.; Utikal, J.; Higashiyama, S.; Bosserhoff, A.K.; Kuphal, S. Nuclear AREG affects a low-proliferative phenotype and contributes to drug resistance of melanoma. Int. J. Cancer 2022, 151, 2244–2264. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Schulte, U.; Raghunath, M.; Luger, T.A.; Schwarz, T.; Funk, J.O.; Behrmann, I.; Kortylewski, M.; Heinrich, P.C.; Kues, T. Interleukin-6-resistant melanoma cells exhibit reduced activation of STAT3 and lack of inhibition of cyclin e-associated kinase activity. J. Investig. Dermatol. 2001, 117, 132–140. [Google Scholar] [CrossRef][Green Version]
- Hannus, M.; Beitzinger, M.; Engelmann, J.C.; Weickert, M.-T.; Spang, R.; Hannus, S.; Meister, G. siPools: Highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res. 2014, 42, 8049–8061. [Google Scholar] [CrossRef]
- Pommer, M.; Kuphal, S.; Bosserhoff, A.K. Amphiregulin Regulates Melanocytic Senescence. Cells 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Chen, Y.; Jiang, J.; Song, X.; Zhang, L.; He, Q.; Ye, B.; Wu, L.; Wu, R.; et al. Aberrant promoter methylation of T-cadherin in sera is associated with a poor prognosis in oral squamous cell carcinoma. Neoplasma 2021, 68, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, P.; Janisiak, J.; Rogińska, D.; Perużyńska, M.; Machaliński, B.; Tarnowski, M. Garcinol and Anacardic Acid, Natural Inhibitors of Histone Acetyltransferases, Inhibit Rhabdomyosarcoma Growth and Proliferation. Molecules 2023, 28, 5292. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.M.; Amouzgar, M.; Pfeiffer, S.M.; Howes, T.R.; Medina, E.; Travers, M.; Steiner, G.; Weber, J.S.; Wolchok, J.D.; Larkin, J.; et al. Prior anti-CTLA-4 therapy impacts molecular characteristics associated with anti-PD-1 response in advanced melanoma. Cancer Cell 2023, 41, 791–806.e4. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shklovskaya, E.; Lee, J.H.; Pedersen, B.; Stewart, A.; Ming, Z.; Irvine, M.; Shivalingam, B.; Saw, R.P.M.; Menzies, A.M.; et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2023, 14, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yuan, L.; Zhang, L.; Zhao, J.; Zhang, C.M.; Deng, H.Y. Garcinol, an acetyltransferase inhibitor, suppresses proliferation of breast cancer cell line MCF-7 promoted by 17beta-estradiol. Asian Pac. J. Cancer Prev. 2014, 15, 5001–5007. [Google Scholar] [CrossRef]
- Aggarwal, S.; Das, S.N. Garcinol inhibits tumour cell proliferation, angiogenesis, cell cycle progression and induces apoptosis via NF-kappaB inhibition in oral cancer. Tumour Biol. 2016, 37, 7175–7184. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Chang, W.-L.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J. Agric. Food Chem. 2001, 49, 1464–1474. [Google Scholar] [CrossRef]
- Ahmad, A.; Sarkar, S.H.; Aboukameel, A.; Ali, S.; Biersack, B.; Seibt, S.; Li, Y.; Bin Bao, B.; Kong, D.; Banerjee, S.; et al. Anticancer action of garcinol in vitro and in vivo is in part mediated through inhibition of STAT-3 signaling. Carcinogenesis 2012, 33, 2450–2456. [Google Scholar] [CrossRef]
- Farhan, M.; Malik, A.; Ullah, M.F.; Afaq, S.; Faisal, M.; Farooqi, A.A.; Biersack, B.; Schobert, R.; Ahmad, A. Garcinol Sensitizes NSCLC Cells to Standard Therapies by Regulating EMT-Modulating miRNAs. Int. J. Mol. Sci. 2019, 20, 800. [Google Scholar] [CrossRef] [PubMed]
- Vandyck, H.H.; Hillen, L.M.; Bosisio, F.M.; Oord, J.v.D.; Hausen, A.Z.; Winnepenninckx, V. Rethinking the biology of metastatic melanoma: A holistic approach. Cancer Metastasis Rev. 2021, 40, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [PubMed]
- Bustos, B.d.U.; Estal, R.M.; Simó, G.P.; Jimenez, I.d.J.; Muñoz, B.E.; Serna, M.R.; de Miquel, V.A.; Ros, M.L.; Sánchez, R.B.; Enguídanos, E.N.; et al. Towards Personalized Medicine in Melanoma: Implementation of a Clinical Next-Generation Sequencing Panel. Sci. Rep. 2017, 7, 495. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| hβ-Actin | CTACGTCGCCCTGGACTTCGAGC | GATGGAGCCGCCGATCCACACGG |
| hCDH13 | GGCAATTGACAGTGGCAACC | TGCAGGAGCACACTTGTACC |
| hCDH-2 | TGGATGAAGATGGCATGG | AGGTGGCCACTGTGCTTAC |
| hBcl-xL | TGACCACCTAGAGCCTTGGA | TGAACAGGATACTTTTGTGGAACT |
| Antibody | Company | Reference | Dilution |
|---|---|---|---|
| ACTINB | Sigma Aldrich, Steinheim, Germany | A5541 | 1:5000 |
| CASPASE9 | Cell Signalling Technology, Frankfurt a. M. Germany | #9502 | 1:1000 |
| CDH13 | Abcam, Berlin, Germany | ab36905 | 1:1000 |
| Cyclin D1 | Santa Cruz Biotechnology, Heidelberg, Germany | sc-8396 | 1:500 |
| p53 | Santa Cruz Biotechnology, Heidelberg, Germany | sc-126 | 1:2000 |
| p21 | Abcam, Berlin, Germany | ab109199 | 1:1000 |
| PARP | Cell Signalling Technology, Frankfurt a. M. Germany | #9542 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staebler, S.; Hoechst, S.; Thongmao, A.; Schneider, N.; Bosserhoff, A.-K.; Kuphal, S. The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma. Cancers 2024, 16, 1853. https://doi.org/10.3390/cancers16101853
Staebler S, Hoechst S, Thongmao A, Schneider N, Bosserhoff A-K, Kuphal S. The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma. Cancers. 2024; 16(10):1853. https://doi.org/10.3390/cancers16101853
Chicago/Turabian StyleStaebler, Sebastian, Sebastian Hoechst, Aranya Thongmao, Nadja Schneider, Anja-Katrin Bosserhoff, and Silke Kuphal. 2024. "The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma" Cancers 16, no. 10: 1853. https://doi.org/10.3390/cancers16101853
APA StyleStaebler, S., Hoechst, S., Thongmao, A., Schneider, N., Bosserhoff, A.-K., & Kuphal, S. (2024). The Role of T-Cadherin (CDH13) in Treatment Options with Garcinol in Melanoma. Cancers, 16(10), 1853. https://doi.org/10.3390/cancers16101853






