Insights in Molecular Therapies for Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Risk-Factors and Diagnostic Algorithm of HCC
3. Stage-Dependent Therapeutic Algorithm in HCC
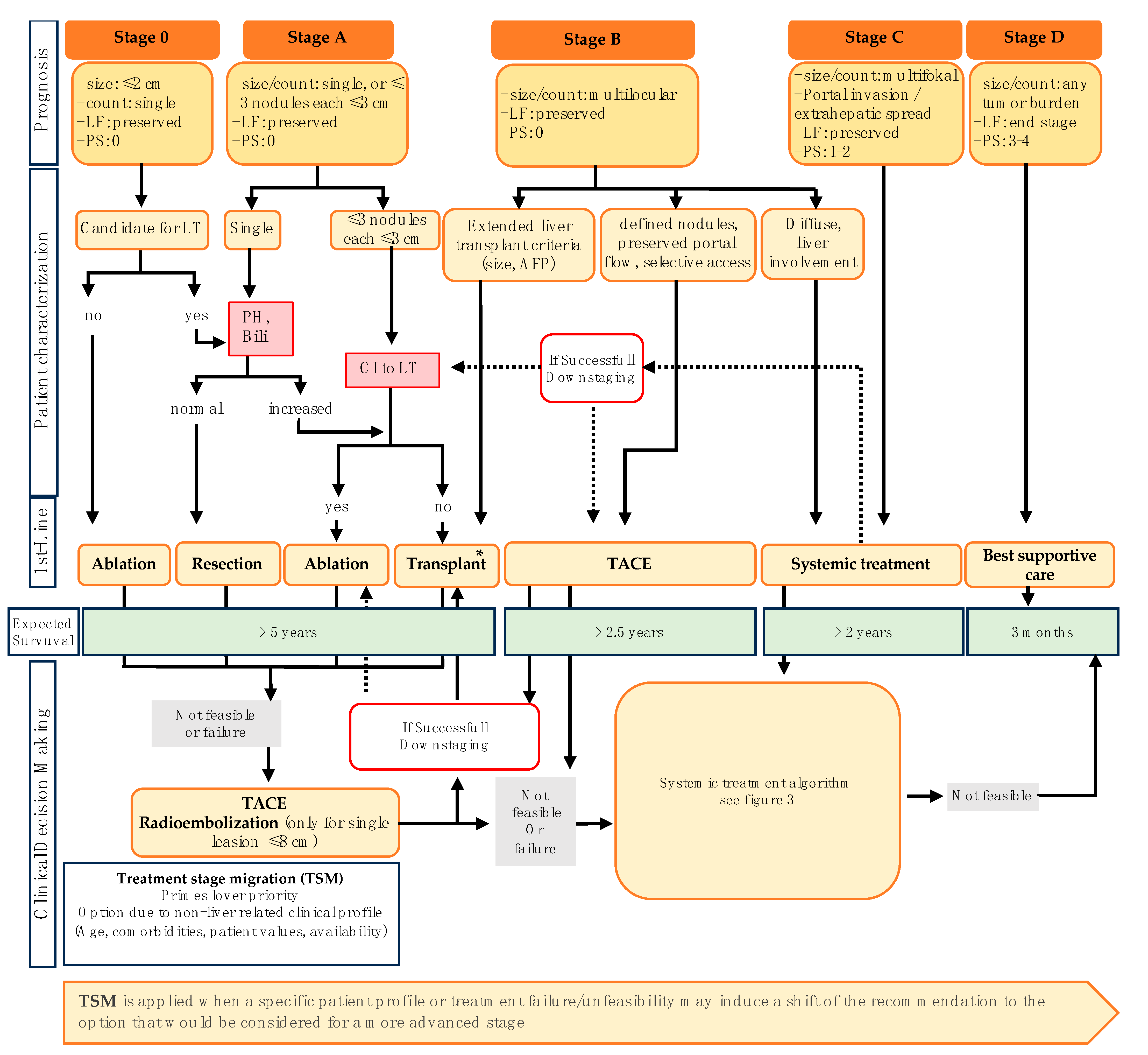
4. Current and Future Molecular Directed Therapeutic Agents in Systemic Treatment of HCC
4.1. Targeting Vascular Endothelial Growth Factor (VEGF) Signaling Pathway
4.2. Immune Checkpoint Inhibitor (CPI) and Immune Checkpoint Inhibitor Combinations
4.3. Combined Inhibition of VEGF Signaling Pathway and CPI
| Study | Phase | Setting | Treatment | ORR | mPFS | mOS | n | NCT |
|---|---|---|---|---|---|---|---|---|
| RESCUE | II | 1L, palliative 2L, palliative | Apa + Cam | 34.4%; 22.5% | 5.7 months; 5.5 months | - | 70 | NCT03463876 |
| - | I | palliative | Nivo + Len | 76.7% | - | - | 30 | NCT03418922 |
| IMMUNIB | II | 1L, palliative | Nivo + Len | 28% | 9 months | 27.1 months | 50 | NCT03841201 |
| KEYNOTE-524 | I | 1L, palliative | Pembro + Len | 36% | 8.6 months | 22.0 months | 104 | NCT03006926 |
| GO30140 | I | 1L, palliative | A + B | 36% | - | - | 104 | NCT02715531 |
| ALTER-H003 | II | 1L, palliative | Anlo + Tori | 32.3% | 11.0 months | 18.2 months | 31 | - |
| KEEP-G04 | II | 1L, palliative | Anlo + Sinti | 55.0% | 12.2 months | - | 20 | NCT04052152 |
| VEGF Liver 100 | I | 1L, palliative | Ave + Axi | 31.8% | - | - | 22 | NCT03289533 |
| KEYNOTE-743 | I | 1L, palliative | Pembro + Rego | 31% | 7.5 months | 26.5 months | 35 | NCT03347292 |
| RENOBATE | II | 1L, palliative | Nivo + Rego | 35.7% | 7.4 months | not reached | 42 | NCT04310709 |
| - | II | 1L, palliative | Tori + B | 46.2% | 9.9 months | not reached | 54 | NCT04605796 |
| JVDJ | I | 2L, palliative | Dur + Ramu | 11% | 4.4 months | 10.7 months | 28 | NCT02572687 |
| CAMILLA | I | 2L, palliative | Cabo + Dur | 66.6% | - | - | 3 | NCT03539822 |
| DEDUCTIVE | I/II | 1L, palliative | Tivo + Dur | 28.6% | - | - | 7 | NCT03970616 |
| - | II | neoadjuvant | Sinti + Len | 36.1% | - | - | 36 | NCT04042805 |
| - | - | 1L, palliative | Len + Cam | 41.7% | 10.3 months | not reached | 92 | [176] |
| - | II | 1L, palliative | Sor + Tori | 35.7% | 4.8 months | - | 28 | NCT04926532 |
4.4. Combination VEGF Signaling Pathway and/or CPI with TACE
4.5. Bispecific Antibodies (BsAbs) as Potential Therapeutic Agents for Systemic Therapy in HCC
4.6. Antibody–Drug Conjugates (ADCs) as Potential Therapeutic Agents for Systemic Therapy in HCC
4.7. Targeting Cyclin-Dependent Kinase 4/6 (CDK4/6) for Systemic Treatment of HCC
4.8. Targeting Epidermal Growth Factor Receptor (EGFR) for Systemic Treatment of HCC
4.9. Targeting ROS1/ALK/MET Alterations for Systemic Treatment of HCC
4.10. Targeting the PI3K/Akt/mTOR Signaling Pathway for Systemic Treatment of HCC
4.11. Targeting the RAS/RAF/MEK/ERK Signaling Pathway for Systemic Treatment of HCC
4.12. Targeting the Wnt/β-Catenin Signaling Pathway for Systemic Treatment of HCC
4.13. Targeting Dickkopf-1 (DKK-1) for Systemic Treatment of HCC
4.14. Targeting Neurotrophe Tyrosine Receptor Kinase (NTRK) for Systemic Treatment of HCC
4.15. Adoptive Cell Transfer (ACT) as Potential Treatment Option in HCC
4.16. Therapeutic Vaccination Strategies in the Setting of HCC
4.17. Cytokine-Directed Therapeutic Regimens in the Setting of HCC
5. Adjuvant Treatment of HCC
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Kefas, J.; Bridgewater, J.; Vogel, A.; Stein, A.; Primrose, J. Adjuvant therapy of biliary tract cancers. Ther. Adv. Med. Oncol. 2023, 15, 17588359231163785. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Omino, R.; Mittal, S.; Kramer, J.R.; Richardson, P.; Thrift, A.P.; El-Serag, H.B.; Kanwal, F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J. Hepatol. 2017, 66, 355–362. [Google Scholar] [CrossRef]
- Schütte, K.; Schulz, C.; Poranzke, J.; Antweiler, K.; Bornschein, J.; Bretschneider, T.; Arend, J.; Ricke, J.; Malfertheiner, P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014, 14, 117. [Google Scholar] [CrossRef]
- Rovesti, G.; Orsi, G.; Kalliopi, A.; Vivaldi, C.; Marisi, G.; Faloppi, L.; Foschi, F.G.; Silvestris, N.; Pecora, I.; Aprile, G.; et al. Impact of Baseline Characteristics on the Overall Survival of HCC Patients Treated with Sorafenib: Ten Years of Experience. Gastrointest. Tumors 2019, 6, 92–107. [Google Scholar] [CrossRef]
- Le, D.C.; Nguyen, T.M.; Nguyen, D.H.; Nguyen, D.T.; Nguyen, L.T.M. Survival Outcome and Prognostic Factors among Patients with Hepatocellular Carcinoma: A Hospital-Based Study. Clin. Med. Insights Oncol. 2023, 17, 11795549231178171. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Mamtani, R.; Gimotty, P.A.; Karasic, T.B.; Yang, Y.X. Outcomes of hepatocellular carcinoma by etiology with first-line atezolizumab and bevacizumab: A real-world analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Cao, K.; Wang, Z.; Lin, D. Clinical efficacy and safety of atezolizumab plus bevacizumab versus lenvatinib in the treatment of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Medicine 2023, 102, e33852. [Google Scholar] [CrossRef] [PubMed]
- Voesch, S.; Bitzer, M.; Blödt, S.; Follmann, M.; Freudenberger, P.; Langer, T.; Lorenz, P.; Jansen, P.L.; Steubesand, N.; Galle, P.; et al. S3-Leitlinie: Diagnostik und Therapie des hepatozellulären Karzinoms und biliärer Karzinome—Version 2.0—Juni 2021, AWMF-Registernummer: 032-053OL. Z. Gastroenterol. 2022, 60, e131–e185. [Google Scholar] [CrossRef]
- Ramai, D.; Deliwala, S.S.; Chandan, S.; Lester, J.; Singh, J.; Samanta, J.; di Nunzio, S.; Perversi, F.; Cappellini, F.; Shah, A.; et al. Risk of Hepatocellular Carcinoma in Patients with Porphyria: A Systematic Review. Cancers 2022, 14, 2947. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bonkovsky, H.L.; Lim, J.K.; Balwani, M. AGA Clinical Practice Update on Diagnosis and Management of Acute Hepatic Porphyrias: Expert Review. Gastroenterology 2023, 164, 484–491. [Google Scholar] [CrossRef]
- Lissing, M.; Vassiliou, D.; Floderus, Y.; Harper, P.; Bottai, M.; Kotopouli, M.; Hagström, H.; Sardh, E.; Wahlin, S. Risk of primary liver cancer in acute hepatic porphyria patients: A matched cohort study of 1244 individuals. J. Intern. Med. 2022, 291, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.B.; Al Sbihi, A.; Chaudhary, A.J.; Haider, S.M.; Edhi, A.I. Hereditary hemochromatosis: Temporal trends, sociodemographic characteristics, and independent risk factor of hepatocellular cancer—Nationwide population-based study. World J. Hepatol. 2022, 14, 1804–1816. [Google Scholar] [CrossRef]
- Groopman, J.D.; Scholl, P.; Wang, J.S. Epidemiology of human aflatoxin exposures and their relationship to liver cancer. Prog. Clin. Biol. Res. 1996, 395, 211–222. [Google Scholar]
- Qian, G.-S.; Ross, R.K.; Yu, M.C.; Yuan, J.-M.; Gao, Y.-T.; Henderson, B.E.; Wogan, G.N.; Groopman, J.D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 1994, 3, 3–10. [Google Scholar]
- Kew, M.C. Hepatocellular carcinoma: Epidemiology and risk factors. J. Hepatocell. Carcinoma 2014, 1, 115–125. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Mason, A.C. Risk factors for the rising rates of primary liver cancer in the United States. Arch. Intern. Med. 2000, 160, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Makarova-Rusher, O.V.; Altekruse, S.F.; McNeel, T.S.; Ulahannan, S.; Duffy, A.G.; Graubard, B.I.; Greten, T.F.; McGlynn, K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016, 122, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Teckman, J.; Di Bisceglie, A.M.; Brenner, D.A. Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency. Clin. Gastroenterol. Hepatol. 2012, 10, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.G. Liver disease in alpha 1-antitrypsin deficiency. Aspects of incidence and prognosis. Scand. J. Gastroenterol. 1985, 20, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Dragani, T.A. Risk of HCC: Genetic heterogeneity and complex genetics. J. Hepatol. 2010, 52, 252–257. [Google Scholar] [CrossRef]
- Elzouki, A.N.; Eriksson, S. Risk of hepatobiliary disease in adults with severe alpha 1-antitrypsin deficiency (PiZZ): Is chronic viral hepatitis B or C an additional risk factor for cirrhosis and hepatocellular carcinoma? Eur. J. Gastroenterol. Hepatol. 1996, 8, 989–994. [Google Scholar] [CrossRef]
- Michielsen, P.P.; Francque, S.M.; van Dongen, J.L. Viral hepatitis and hepatocellular carcinoma. World J. Surg. Oncol. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.P.; Hwang, L.Y.; Lin, C.C.; Chien, C.S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981, 2, 1129–1133. [Google Scholar] [CrossRef]
- Fattovich, G.; Pantalena, M.; Zagni, I.; Realdi, G.; Schalm, S.W.; Christensen, E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: A cohort study of 297 patients. Am. J. Gastroenterol. 2002, 97, 2886–2895. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1261. [Google Scholar] [CrossRef]
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Risch, H.; Lu, L.; Ma, X.; Irwin, M.L.; Lim, J.K.; Taddei, T.; Pawlish, K.; Stroup, A.; Brown, R.; et al. Risk factors for hepatocellular carcinoma (HCC) in the northeast of the United States: Results of a case-control study. Cancer Causes Control. 2020, 31, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Montalto, G.; Cervello, M.; Giannitrapani, L.; Dantona, F.; Terranova, A.; Castagnetta, L.A. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 2002, 963, 13–20. [Google Scholar] [CrossRef]
- Gomaa, A.I.; Khan, S.A.; Toledano, M.B.; Waked, I.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008, 14, 4300–4308. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut 2005, 54, 533–539. [Google Scholar] [CrossRef]
- Yang, J.D.; Mohamed, H.A.; Cvinar, J.L.; Gores, G.J.; Roberts, L.R.; Kim, W.R. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients with Hepatitis C Cirrhosis. Am. J. Gastroenterol. 2016, 111, 1573–1580. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef]
- Barth, B.M.; Shanmugavelandy, S.S.; Tacelosky, D.M.; Kester, M.; Morad, S.A.; Cabot, M.C. Gaucher’s disease and cancer: A sphingolipid perspective. Crit. Rev. Oncog. 2013, 18, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Regenboog, M.; van Dussen, L.; Verheij, J.; Weinreb, N.J.; Santosa, D.; Vom Dahl, S.; Häussinger, D.; Müller, M.N.; Canbay, A.; Rigoldi, M.; et al. Hepatocellular carcinoma in Gaucher disease: An international case series. J. Inherit. Metab. Dis. 2018, 41, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Ganne-Carrié, N.; Trinchet, J.C.; Beaugrand, M. Hepatic iron overload and risk of hepatocellular carcinoma in cirrhosis. Gastroenterol. Clin. Biol. 2010, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Elmberg, M.; Hultcrantz, R.; Ekbom, A.; Brandt, L.; Olsson, S.; Olsson, R.; Lindgren, S.; Lööf, L.; Stål, P.; Wallerstedt, S.; et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 2003, 125, 1733–1741. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Su, G.L.; Conjeevaram, H.S.; Emick, D.M.; Lok, A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002, 36, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; van Karnebeek, C.D.M.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K.; et al. Diagnosis and treatment of tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. 2017, 19, 1380–1395. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Puranik, S. Hypertyrosinemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bitzer, M.; Voesch, S.; Albert, J.; Bartenstein, P.; Bechstein, W.; Blödt, S.; Brunner, T.; Dombrowski, F.; Evert, M.; Follmann, M.; et al. S3-Leitlinie: Diagnostik und Therapie biliärer Karzinome. Z. Gastroenterol. 2022, 60, 219–238. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.M.; Lee, J.S.; Lee, H.Y.; Park, B.H.; Kim, Y.H.; Han, J.K.; Choi, B.I. Hepatocellular carcinoma: Diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015, 275, 97–109. [Google Scholar] [CrossRef]
- Schellhaas, B.; Bernatik, T.; Bohle, W.; Borowitzka, F.; Chang, J.; Dietrich, C.F.; Dirks, K.; Donoval, R.; Drube, K.; Friedrich-Rust, M.; et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma—A Prospective Multicenter DEGUM Study. Ultraschall Med. 2021, 42, e20. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Jung, E.M.; Ziesch, M.; Praktiknjo, M.; Link, A.; Dietrich, C.F.; Klinger, C.; Schultheiß, M.; Jesper, D.; Schellhaas, B. Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS®/ESCULAP) in hepatic nodules in cirrhotic patients-a prospective multicenter study. Eur. Radiol. 2021, 31, 7614–7625. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; De Filippis, G.; De Santis, A.; Geiger, D.; Del Monte, M.; Lombardo, C.V.; Rossi, M.; Corradini, S.G.; Mennini, G.; Catalano, C. Hepatocellular carcinoma in cirrhotic patients: Prospective comparison of US, CT and MR imaging. Eur. Radiol. 2013, 23, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef] [PubMed]
- Mitchell, D.G.; Bruix, J.; Sherman, M.; Sirlin, C.B. LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015, 61, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Lièvre, M.A.; Franco, D.; Gervais, P.; Kuhnast, B.; Agostini, H.; Marthey, L.; Désarnaud, S.; Helal, B.O. Diagnostic value of combining ¹¹C-choline and ¹⁸F-FDG PET/CT in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 852–859. [Google Scholar] [CrossRef]
- Roskams, T. Anatomic pathology of hepatocellular carcinoma: Impact on prognosis and response to therapy. Clin. Liver Dis. 2011, 15, 245–259, vii–x. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Solé, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008, 47, 97–104. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1701. [Google Scholar] [CrossRef]
- Hameed, B.; Mehta, N.; Sapisochin, G.; Roberts, J.P.; Yao, F.Y. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014, 20, 945–951. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Halazun, K.J.; Tabrizian, P.; Najjar, M.; Florman, S.; Schwartz, M.; Michelassi, F.; Samstein, B.; Brown, R.S., Jr.; Emond, J.C.; Busuttil, R.W.; et al. Is it Time to Abandon the Milan Criteria?: Results of a Bicoastal US Collaboration to Redefine Hepatocellular Carcinoma Liver Transplantation Selection Policies. Ann. Surg. 2018, 268, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.B.; Klee, M.; Palshof, T.; Hansen, H.H. Performance status assessment in cancer patients. An inter-observer variability study. Br. J. Cancer 1993, 67, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Sidali, S.; Trépo, E.; Sutter, O.; Nault, J.C. New concepts in the treatment of hepatocellular carcinoma. United Eur. Gastroenterol. J. 2022, 10, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Sharma, R.; Allara, E.; Yen, C.; Arizumi, T.; Kubota, K.; Bettinger, D.; Jang, J.W.; Smirne, C.; Kim, Y.W.; et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatol. 2017, 66, 338–346. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Hoshida, Y.; Pinato, D.J.; Marrero, J.; Nault, J.C.; Paradis, V.; Tayob, N.; Sherman, M.; Lim, Y.S.; Feng, Z.; et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021, 160, 2572–2584. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e983, quiz e914–985. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Guy, J.; Frenette, C.T.; Dodge, J.L.; Osorio, R.W.; Minteer, W.B.; Roberts, J.P.; Yao, F.Y. Excellent Outcomes of Liver Transplantation Following Down-Staging of Hepatocellular Carcinoma to within Milan Criteria: A Multicenter Study. Clin. Gastroenterol. Hepatol. 2018, 16, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Mehta, N.; Flemming, J.; Dodge, J.; Hameed, B.; Fix, O.; Hirose, R.; Fidelman, N.; Kerlan, R.K., Jr.; Roberts, J.P. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology 2015, 61, 1968–1977. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Lencioni, R.; Kudo, M.; Erinjeri, J.; Qin, S.; Ren, Z.; Chan, S.; Arai, Y.; Heo, J.; Mai, A.; Escobar, J.; et al. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J. Clin. Oncol. 2024, 42, LBA432. [Google Scholar] [CrossRef]
- Toni, E.N.D.; Kubisch, I.; Khaled, N.B.; Ricke, J.; Mayerle, J.; Ehmer, U.; Waldschmidt, D.; Rössler, D.C.; Oehrle, B.; Bitzer, M.; et al. Atezolizumab and bevacizumab with transarterial chemoembolization in hepatocellular carcinoma: The DEMAND randomized phase II clinical trial. J. Clin. Oncol. 2022, 40, TPS492. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Semela, D.; Dufour, J.F. Angiogenesis and hepatocellular carcinoma. J. Hepatol. 2004, 41, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Dufour, J.F.; Peck-Radosavljevic, M.; Trojan, J.; Vogel, A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021, 17, 1237–1251. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Villanueva, A.; Newell, P.; Chiang, D.Y.; Friedman, S.L.; Llovet, J.M. Genomics and signaling pathways in hepatocellular carcinoma. Semin. Liver Dis. 2007, 27, 55–76. [Google Scholar] [CrossRef]
- Cervello, M.; Bachvarov, D.; Lampiasi, N.; Cusimano, A.; Azzolina, A.; McCubrey, J.A.; Montalto, G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle 2012, 11, 2843–2855. [Google Scholar] [CrossRef]
- Stotz, M.; Gerger, A.; Haybaeck, J.; Kiesslich, T.; Bullock, M.D.; Pichler, M. Molecular Targeted Therapies in Hepatocellular Carcinoma: Past, Present and Future. Anticancer. Res. 2015, 35, 5737–5744. [Google Scholar] [PubMed]
- Patt, Y.; Rojas-Hernandez, C.; Fekrazad, H.M.; Bansal, P.; Lee, F.C. Phase II Trial of Sorafenib in Combination with Capecitabine in Patients with Hepatocellular Carcinoma: INST 08-20. Oncologist 2017, 22, 1158-e116. [Google Scholar] [CrossRef]
- Azim, H.A.; Omar, A.; Atef, H.; Zawahry, H.; Shaker, M.K.; Abdelmaksoud, A.K.; EzzElarab, M.; Abdel-Rahman, O.; Ismail, M.; Kassem, L.; et al. Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: A Phase II trial (ESLC01 study). J. Hepatocell. Carcinoma 2018, 5, 109–119. [Google Scholar] [CrossRef]
- Assenat, E.; Pageaux, G.P.; Thézenas, S.; Peron, J.M.; Bécouarn, Y.; Seitz, J.F.; Merle, P.; Blanc, J.F.; Bouché, O.; Ramdani, M.; et al. Sorafenib alone vs. sorafenib plus GEMOX as 1st-line treatment for advanced HCC: The phase II randomised PRODIGE 10 trial. Br. J. Cancer 2019, 120, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Srimuninnimit, V.; Sriuranpong, V.; Suwanvecho, S. Efficacy and safety of sorafenib in combination with gemcitabine in patients with advanced hepatocellular carcinoma: A multicenter, open-label, single-arm phase II study. Asia Pac. J. Clin. Oncol. 2014, 10, 255–260. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K.; et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef]
- Kondo, M.; Morimoto, M.; Kobayashi, S.; Ohkawa, S.; Hidaka, H.; Nakazawa, T.; Aikata, H.; Hatanaka, T.; Takizawa, D.; Matsunaga, K.; et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer 2019, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; He, M.; Bu, X.; Xu, Y.; Huang, Y.; Wen, D.; Li, Q.; Xu, L.; Zhang, Y.; Wei, W.; et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur. J. Cancer 2022, 174, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Hoshi, T.; Yamamoto, Y.; Ikemori-Kawada, M.; Minoshima, Y.; Funahashi, Y.; Matsui, J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018, 7, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Cheng, A.L.; Vogel, A.; Tovoli, F.; Ueshima, K.; Aikata, H.; et al. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: A subanalysis of the REFLECT study. J. Hepatol. 2023, 78, 133–141. [Google Scholar] [CrossRef]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Abd El Aziz, M.A.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar]
- Luo, J.; Gao, B.; Lin, Z.; Fan, H.; Ma, W.; Yu, D.; Yang, Q.; Tian, J.; Yang, X.; Li, B. Efficacy and safety of lenvatinib versus sorafenib in first-line treatment of advanced hepatocellular carcinoma: A meta-analysis. Front. Oncol. 2022, 12, 1010726. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ryoo, B.Y.; Merle, P.; Park, J.W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020, 5, e000714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Finn, R.S.; Kim, J.S.; Karwal, M.; Li, R.K.; Ismail, F.; Thomas, M.; Harris, R.; Baudelet, C.; Walters, I.; et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.C.; Chen, P.J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013, 119, 380–387. [Google Scholar] [CrossRef]
- Zhu, A.X.; Sahani, D.V.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Catalano, O.A.; Sindhwani, V.; Blaszkowsky, L.S.; Yoon, S.S.; Lahdenranta, J.; et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol. 2009, 27, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Qin, S.; Park, J.W.; Poon, R.T.; Raoul, J.L.; Philip, P.A.; Hsu, C.H.; Hu, T.H.; Heo, J.; Xu, J.; et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Lin, D.Y.; Park, J.W.; Kudo, M.; Qin, S.; Chung, H.C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, C.; Gupta, M.; Lee, S.; Krishnamurthi, S.; Estfan, B.; Wang, K.; Attwood, K.; Wilton, J.; Bies, R.; Bshara, W.; et al. A multicentre phase 1b/2 study of tivozanib in patients with advanced inoperable hepatocellular carcinoma. Br. J. Cancer 2020, 122, 963–970. [Google Scholar] [CrossRef]
- Alberts, S.R.; Fitch, T.R.; Kim, G.P.; Morlan, B.W.; Dakhil, S.R.; Gross, H.M.; Nair, S. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II North Central Cancer Treatment Group Clinical Trial. Am. J. Clin. Oncol. 2012, 35, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Thongprasert, S.; Lim, H.Y.; Sukeepaisarnjaroen, W.; Yang, T.-S.; Wu, C.-C.; Chao, Y.; Chan, S.L.; Kudo, M.; Ikeda, M.; et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 2016, 64, 774–784. [Google Scholar] [CrossRef]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II–III Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wan, X.; Quan, H.; Zheng, M.; Fu, L.; Li, Y.; Lou, L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018, 109, 1207–1219. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, A.; Zhang, W.; Jiang, Z.; Chen, B.; Zhao, J.; Li, Z.; Wang, L.; Bi, X.; Zhao, H.; et al. Anlotinib in the treatment of advanced hepatocellular carcinoma: An open-label phase II study (ALTER-0802 study). Hepatol. Int. 2021, 15, 621–629. [Google Scholar] [CrossRef]
- Okusaka, T.; Kudo, M.; Ikeda, K.; Ikeda, M.; Okita, K.; Sugawara, M.; Tamai, T.; Ren, M.; Saito, K.; Kumada, H. Impact of bodyweight-based starting doses on the safety and efficacy of lenvatinib in primarily Japanese patients with hepatocellular carcinoma. Hepatol. Res. 2022, 52, 784–793. [Google Scholar] [CrossRef]
- Okusaka, T.; Ikeda, K.; Kudo, M.; Finn, R.; Qin, S.; Han, K.H.; Cheng, A.L.; Piscaglia, F.; Kobayashi, M.; Sung, M.; et al. Safety and efficacy of lenvatinib by starting dose based on body weight in patients with unresectable hepatocellular carcinoma in REFLECT. J. Gastroenterol. 2021, 56, 570–580. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Amaro, C.P.; Lee-Ying, R.; Sim, H.W.; Samwi, H.; Chan, K.K.; Knox, J.J.; Ko, Y.J.; Swiha, M.; Batuyong, E.; et al. Effect of sorafenib starting dose and dose intensity on survival in patients with hepatocellular carcinoma: Results from a Canadian Multicenter Database. Cancer Med. 2020, 9, 4918–4928. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y.; Endo, M.; Takeda, H.; Tsuchiya, K.; Joko, K.; Ogawa, C.; Taniguchi, H.; Orito, E.; Uchida, Y.; et al. Comparison of standard-dose and half-dose sorafenib therapy on clinical outcome in patients with unresectable hepatocellular carcinoma in field practice: A propensity score matching analysis. Int. J. Oncol. 2014, 45, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Numata, K.; Kondo, M.; Kobayashi, S.; Ohkawa, S.; Hidaka, H.; Nakazawa, T.; Okuwaki, Y.; Okuse, C.; Matsunaga, K.; et al. Field practice study of half-dose sorafenib treatment on safety and efficacy for hepatocellular carcinoma: A propensity score analysis. Hepatol. Res. 2015, 45, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Yu, S.; Mamtani, R.; Mehta, R.; D’Addeo, K.; Wileyto, E.P.; Taddei, T.H.; Kaplan, D.E. Starting Dose of Sorafenib for the Treatment of Hepatocellular Carcinoma: A Retrospective, Multi-Institutional Study. J. Clin. Oncol. 2017, 35, 3575–3581. [Google Scholar] [CrossRef]
- Tomonari, T.; Tani, J.; Ogawa, C.; Deguchi, A.; Senoh, T.; Moriya, A.; Shibata, H.; Fukuno, H.; Tanaka, H.; Tanaka, T.; et al. Multicenter retrospective study of initial treatment outcome and feasibility of initiating dose reduction of cabozantinib in unresectable hepatocellular carcinoma. Hepatol. Res. 2023, 53, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Ogasawara, S.; Okubo, T.; Itokawa, N.; Yoshino, R.; Fujimoto, K.; Kogure, T.; Yumita, S.; Ishino, T.; Ogawa, K.; et al. Cabozantinib for Advanced Hepatocellular Carcinoma in the Latest Real-World Practice: A Multicenter Retrospective Analysis. Drugs Real. World Outcomes 2023, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhu, K.; Kang, H.; Lu, M.; Qu, Z.; Lu, L.; Song, T.; Zhou, W.; Wang, H.; Yang, W.; et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Nivolumab Approved for Liver Cancer. Cancer Discov. 2017, 7, Of3. [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 75, 600–609. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.H.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Updated efficacy and safety of KEYNOTE-224: A phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur. J. Cancer 2022, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Merle, P.; Kudo, M.; Edeline, J.; Bouattour, M.; Cheng, A.-L.; Chan, S.L.; Yau, T.; Garrido, M.; Knox, J.; Daniele, B.; et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: Longer Term Follow-Up from the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2023, 12, 309–320. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.-Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383. [Google Scholar] [CrossRef]
- Ren, Z.; Ducreux, M.; Abou-Alfa, G.K.; Merle, P.; Fang, W.; Edeline, J.; Li, Z.; Wu, L.; Assenat, E.; Hu, S.; et al. Tislelizumab in Patients with Previously Treated Advanced Hepatocellular Carcinoma (RATIONALE-208): A Multicenter, Non-Randomized, Open-Label, Phase 2 Trial. Liver Cancer 2023, 12, 72–84. [Google Scholar] [CrossRef]
- Qin, S.; Kudo, M.; Meyer, T.; Finn, R.S.; Vogel, A.; Bai, Y.; Guo, Y.; Meng, Z.; Zhang, T.; Satoh, T.; et al. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33, S1402–S1403. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; Toni, E.N.D.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Cillo, A.R.; Karapetyan, L.; Kirkwood, J.M.; Workman, C.J.; Vignali, D.A.A. Molecular Pathways and Mechanisms of LAG3 in Cancer Therapy. Clin. Cancer Res. 2022, 28, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Schöffski, P.; Tan, D.S.W.; Martín, M.; Ochoa-de-Olza, M.; Sarantopoulos, J.; Carvajal, R.D.; Kyi, C.; Esaki, T.; Prawira, A.; Akerley, W.; et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J. Immunother. Cancer 2022, 10, e003776. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Hsu, C.-H.; Li, D.; Burgoyne, A.; Cotter, C.; Badhrinarayanan, S.; Wang, Y.; Yin, A.; Edubilli, T.R.; et al. Results from the MORPHEUS-liver study: Phase Ib/II randomized evaluation of tiragolumab (tira) in combination with atezolizumab (atezo) and bevacizumab (bev) in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (uHCC). J. Clin. Oncol. 2023, 41, 4010. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Tian, J.-C.; Liu, H.; Yan, L.-J.; Ding, Z.-N.; Han, C.-L.; Tian, B.-W.; Tan, S.-Y.; Dong, Z.-R.; Wang, D.-X.; Xue, J.-S.; et al. Adverse events of immune checkpoint inhibitors in hepatocellular carcinoma: A systemic review and meta-analysis. Clin. Exp. Med. 2023, 23, 2115–2129. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Morimoto, M.; Kobayashi, S.; Ueno, M.; Uojima, H.; Hidaka, H.; Kusano, C.; Chuma, M.; Numata, K.; Tsuruya, K.; et al. Association between Immune-Related Adverse Events and Survival in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab. Oncologist 2023, 28, e526–e533. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yasui, Y.; Tsuchiya, K.; Matsumoto, H.; Yamazaki, Y.; Uchihara, N.; Tanaka, Y.; Miyamoto, H.; Yamada-Shimizu, M.; Keitoku, T.; et al. Impact of immune-related adverse events in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab. J. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Kudo, M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Knox, J.J.; Chen, H.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998. [Google Scholar] [CrossRef]
- Zhu, A.X.; Blaszkowsky, L.S.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Horgan, K.; Sheehan, S.; Hale, K.E.; Enzinger, P.C.; Bhargava, P.; et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J.; et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Foerster, F.; Kloeckner, R.; Reig, M.; Chan, S.L.; Chung, J.W.; Merle, P.; Park, J.-W.; Piscaglia, F.; Vogel, A.; Gaillard, V.; et al. ABC-HCC: A phase IIIb, randomized, multicenter, open-label trial of atezolizumab plus bevacizumab versus transarterial chemoembolization (TACE) in intermediate-stage hepatocellular carcinoma. J. Clin. Oncol. 2022, 40, TPS498. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur. J. Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Persano, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Survival outcomes from atezolizumab plus bevacizumab versus Lenvatinib in Child Pugh B unresectable hepatocellular carcinoma patients. J. Cancer Res. Clin. Oncol. 2023, 149, 7565–7577. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, V.; Pallozzi, M.; Cerrito, L.; Santopaolo, F.; Stella, L.; Gasbarrini, A.; Ponziani, F.R. Management of Portal Hypertension in Patients with Hepatocellular Carcinoma on Systemic Treatment: Current Evidence and Future Perspectives. Cancers 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first- line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S808–S869. [Google Scholar] [CrossRef]
- Kudo, M.; Ikeda, M.; Motomura, K.; Okusaka, T.; Kato, N.; Dutcus, C.E.; Hisai, T.; Suzuki, M.; Ikezawa, H.; Iwata, T.; et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117. J. Clin. Oncol. 2020, 38, 513. [Google Scholar] [CrossRef]
- Vogel, A.; Siegler, G.M.; Siebler, J.; Lindig, U.; Schultheiß, M.; Müller, T.; Simon, H.; Jöckel, C.; Mueller, D.W.; Al-Batran, S.-E.; et al. IMMUNIB trial (AIO-HEP-0218/ass): A single-arm, phase II study evaluating safety and efficacy of immunotherapy nivolumab in combination with lenvatinib in advanced-stage hepatocellular carcinoma (HCC). J. Clin. Oncol. 2022, 40, 4107. [Google Scholar] [CrossRef]
- Zhou, J.; Fan, J.; Gu, F.-M.; Li, T.; Bai, D.-S.; Sun, H.-C.; Wang, Z.; Qiu, S.-J.; Ye, Q.-H.; Shi, Y.-H.; et al. A phase II/III study of camrelizumab plus apatinib as perioperative treatment of resectable hepatocellular carcinoma at intermediate-high risk of recurrence: Primary results of major pathologic response from phase II stage. J. Clin. Oncol. 2023, 41, 4126. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Cubillo Gracian, A.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab Plus Cabozantinib with or without Ipilimumab for Advanced Hepatocellular Carcinoma: Results from Cohort 6 of the CheckMate 040 Trial. J. Clin. Oncol. 2023, 41, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Liu, B.; Li, Q.; Lu, Y.; Chen, Y.; et al. ORIENT-32: Updated characterization of response to sintilimab plus bevacizumab biosimilar (IBI305) vs sorafenib for unresectable hepatocellular carcinoma. J. Clin. Oncol. 2023, 41, 570. [Google Scholar] [CrossRef]
- Li, Q.; Cao, M.; Yuan, G.; Cheng, X.; Zang, M.; Chen, M.; Hu, X.; Huang, J.; Li, R.; Guo, Y.; et al. Lenvatinib Plus Camrelizumab vs. Lenvatinib Monotherapy as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Cohort Study. Front. Oncol. 2022, 12, 809709. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.Y.; Jin, Z.C.; Chen, J.J.; Zhu, H.D.; Zhu, X.L. Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. J. Clin. Transl. Hepatol. 2023, 11, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Saeki, I.; Ishikawa, T.; Inaba, Y.; Morimoto, N.; Aikata, H.; Tanabe, N.; Wada, Y.; Kondo, Y.; et al. A Phase 2, Prospective, Multicenter, Single-Arm Trial of Transarterial Chemoembolization Therapy in Combination Strategy with Lenvatinib in Patients with Unresectable Intermediate-Stage Hepatocellular Carcinoma: TACTICS-L Trial. Liver Cancer 2023, 13, 99–112. [Google Scholar] [CrossRef]
- Zhu, H.-D.; Li, H.-L.; Huang, M.-S.; Yang, W.-Z.; Yin, G.-W.; Zhong, B.-Y.; Sun, J.-H.; Jin, Z.-C.; Chen, J.-J.; Ge, N.-J.; et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct. Target. Ther. 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Chen, Y.; Huang, X.; Hu, W.; Li, X.; Sun, J.; Shen, Y.; Zhang, M.; Wu, J.; Gao, S.; et al. Efficacy and safety of envafolimab plus lenvatinib combined with TACE in unresectable hepatocellular carcinoma: An open-label, single-arm, phase 2 study—The CISLD-12 study. J. Clin. Oncol. 2023, 41, 558. [Google Scholar] [CrossRef]
- Cao, F.; Yang, Y.; Si, T.; Luo, J.; Zeng, H.; Zhang, Z.; Feng, D.; Chen, Y.; Zheng, J. The Efficacy of TACE Combined with Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front. Oncol. 2021, 11, 783480. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, G.; Mu, L.; Wang, H.; Zhou, C.; Yan, H.; Huang, M. TACE Combined with Lenvatinib and Camrelizumab for Unresectable Multiple Nodular and Large Hepatocellular Carcinoma (>5 cm). Technol. Cancer Res. Treat. 2023, 22, 15330338231200320. [Google Scholar] [CrossRef] [PubMed]
- Schwacha-Eipper, B.; Minciuna, I.; Banz, V.; Dufour, J.F. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology 2020, 72, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.S.; Cheung, N.V. Immunotherapy of hepatocellular carcinoma using chimeric antigen receptors and bispecific antibodies. Cancer Lett. 2017, 399, 44–52. [Google Scholar] [CrossRef]
- Chan, A.W.; Tong, J.H.; Chan, S.L.; Lai, P.B.; To, K.F. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology 2014, 64, 935–950. [Google Scholar] [CrossRef]
- Guo, Z.; Li, L.Q.; Jiang, J.H.; Ou, C.; Zeng, L.X.; Xiang, B.D. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 2098–2106. [Google Scholar] [CrossRef]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Qin, L.X.; Wauthier, E.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef]
- Hoh, A.; Dewerth, A.; Vogt, F.; Wenz, J.; Baeuerle, P.A.; Warmann, S.W.; Fuchs, J.; Armeanu-Ebinger, S. The activity of γδ T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int. 2013, 33, 127–136. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, B.; Gao, H.; Jiang, H.; Kong, J.; Yan, J.; Pan, X.; Li, K.; Zhang, P.; Yao, M.; et al. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer Immunol. Immunother. 2014, 63, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Kebenko, M.; Goebeler, M.E.; Wolf, M.; Hasenburg, A.; Seggewiss-Bernhardt, R.; Ritter, B.; Rautenberg, B.; Atanackovic, D.; Kratzer, A.; Rottman, J.B.; et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology 2018, 7, e1450710. [Google Scholar] [CrossRef]
- Lima, L.D.P.; Machado, C.J.; Rodrigues, J.; Vasconcellos, L.S.; Junior, E.P.; Vidigal, P.V.T.; Resende, V. Immunohistochemical Coexpression of Epithelial Cell Adhesion Molecule and Alpha-Fetoprotein in Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5970852. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Bin, C.; Na, H.; Peng, S.; Yi, D.; Xiang-hua, Y.; Fang-yin, Z.; Da-yong, Z.; Rong-cheng, L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol. Biol. Rep. 2012, 39, 351–357. [Google Scholar] [CrossRef]
- Shirakawa, H.; Suzuki, H.; Shimomura, M.; Kojima, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T.; Konishi, M.; Kobayashi, N.; Kinoshita, T.; et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009, 100, 1403–1407. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Li, N.; Wei, L.; Liu, X.; Bai, H.; Ye, Y.; Li, D.; Li, N.; Baxa, U.; Wang, Q.; Lv, L.; et al. A Frizzled-Like Cysteine-Rich Domain in Glypican-3 Mediates Wnt Binding and Regulates Hepatocellular Carcinoma Tumor Growth in Mice. Hepatology 2019, 70, 1231–1245. [Google Scholar] [CrossRef]
- Yu, L.; Huang, N.; Sun, H.; Yang, X.; Fu, Y.; Lang, Q.; Wang, J.; Ge, L. Development of a Tetravalent T-Cell Engaging Bispecific Antibody against Glypican-3 for Hepatocellular Carcinoma. J. Immunother. 2021, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, Q.; Luo, C.; Wei, J.; Xu, Y.; Zhao, L.; Wang, Y. A novel bispecific antibody as an immunotherapeutic agent in hepatocellular carcinoma. Mol. Immunol. 2023, 162, 125–132. [Google Scholar] [CrossRef]
- Du, K.; Li, Y.; Liu, J.; Chen, W.; Wei, Z.; Luo, Y.; Liu, H.; Qi, Y.; Wang, F.; Sui, J. A bispecific antibody targeting GPC3 and CD47 induced enhanced antitumor efficacy against dual antigen-expressing HCC. Mol. Ther. 2021, 29, 1572–1584. [Google Scholar] [CrossRef]
- Fu, M.; He, Q.; Guo, Z.; Zhou, X.; Li, H.; Zhao, L.; Tang, H.; Zhou, X.; Zhu, H.; Shen, G.; et al. Therapeutic Bispecific T-Cell Engager Antibody Targeting the Transferrin Receptor. Front. Immunol. 2019, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, N.; Li, Z.; Shen, L.; Ji, K.; Zheng, Z.; Liu, D.; Lou, H.; Bai, L.; Liu, T.; et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): A multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023, 24, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Yoshitake, Y.; Senju, S.; Monji, M.; Komori, H.; Motomura, Y.; Hosaka, S.; Beppu, T.; Ishiko, T.; Kamohara, H.; et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun. 2003, 306, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Urban, D.J.; Nani, R.R.; Zhang, Y.F.; Li, N.; Fu, H.; Shah, H.; Gorka, A.P.; Guha, R.; Chen, L.; et al. Glypican-3-Specific Antibody Drug Conjugates Targeting Hepatocellular Carcinoma. Hepatology 2019, 70, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.R.; Hsu, H.C. Cloning and expression of CD24 gene in human hepatocellular carcinoma: A potential early tumor marker gene correlates with p53 mutation and tumor differentiation. Cancer Res. 1995, 55, 4717–4721. [Google Scholar]
- Ma, Z.; He, H.; Sun, F.; Xu, Y.; Huang, X.; Ma, Y.; Zhao, H.; Wang, Y.; Wang, M.; Zhang, J. Selective targeted delivery of doxorubicin via conjugating to anti-CD24 antibody results in enhanced antitumor potency for hepatocellular carcinoma both in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2017, 143, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Pan, G.; Ni, J.; Xie, F.; Jiang, B.; Wei, L.; Gao, J.; Zhou, W. Enhanced doxorubicin delivery to hepatocellular carcinoma cells via CD147 antibody-conjugated immunoliposomes. Nanomedicine 2018, 14, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, G.; Gao, Q.; Sun, J.; Zhang, Q.; Du, L.; Qiu, Z.; Wang, C.; Zheng, F.; Sun, B.; et al. A human anti-c-Met Fab fragment conjugated with doxorubicin as targeted chemotherapy for hepatocellular carcinoma. PLoS ONE 2013, 8, e63093. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.E.; Li, G.M.; Tang, Y.Q.; Xi, S.Y.; Loong, J.H.C.; Li, M.M.; Li, H.L.; Cheng, W.; Zhu, W.J.; Mo, J.Q.; et al. Targeting tumor lineage plasticity in hepatocellular carcinoma using an anti-CLDN6 antibody-drug conjugate. Sci. Transl. Med. 2021, 13, abb6282. [Google Scholar] [CrossRef]
- Smith, L.M.; Nesterova, A.; Ryan, M.C.; Duniho, S.; Jonas, M.; Anderson, M.; Zabinski, R.F.; Sutherland, M.K.; Gerber, H.P.; Van Orden, K.L.; et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br. J. Cancer 2008, 99, 100–109. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Nejadmoghaddam, M.R.; Minai-Tehrani, A.; Ghahremanzadeh, R.; Mahmoudi, M.; Dinarvand, R.; Zarnani, A.H. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J. Med. Biotechnol. 2019, 11, 3–23. [Google Scholar] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Invest. New Drugs 2018, 36, 121–135. [Google Scholar] [CrossRef]
- Murali, M.; Kumar, A.R.; Nair, B.; Pavithran, K.; Devan, A.R.; Pradeep, G.K.; Nath, L.R. Antibody-drug conjugate as targeted therapeutics against hepatocellular carcinoma: Preclinical studies and clinical relevance. Clin. Transl. Oncol. 2022, 24, 407–431. [Google Scholar] [CrossRef]
- Bollard, J.; Miguela, V.; Ruiz de Galarreta, M.; Venkatesh, A.; Bian, C.B.; Roberto, M.P.; Tovar, V.; Sia, D.; Molina-Sánchez, P.; Nguyen, C.B.; et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 2017, 66, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, G.; Fumarola, C.; La Monica, S.; Bonelli, M.A.; Cretella, D.; Alfieri, R.; Cavazzoni, A.; Galetti, M.; Bertolini, P.; Missale, G.; et al. Simultaneous Combination of the CDK4/6 Inhibitor Palbociclib with Regorafenib Induces Enhanced Anti-tumor Effects in Hepatocarcinoma Cell Lines. Front. Oncol. 2020, 10, 563249. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, G.; Fumarola, C.; La Monica, S.; Bonelli, M.; Cavazzoni, A.; Galetti, M.; Terenziani, R.; Eltayeb, K.; Volta, F.; Zoppi, S.; et al. CDK4/6 inhibitors improve the anti-tumor efficacy of lenvatinib in hepatocarcinoma cells. Front. Oncol. 2022, 12, 942341. [Google Scholar] [CrossRef]
- Littman, S.J.; Brus, C.; Burkart, A. A phase II study of palbociclib (PD-0332991) in adult patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 277. [Google Scholar] [CrossRef]
- Villa, E.; Piscaglia, F.; Geva, R.; Dalecos, G.; Papatheodoridis, G.; Ciomei, M.; Davite, C.; Crivori, P.; Palejwala, V.; Jacob, J.; et al. Phase IIa safety and efficacy of milciclib, a pan-cyclin dependent kinase inhibitor, in unresectable, sorafenib-refractory or -intolerant hepatocellular carcinoma patients. J. Clin. Oncol. 2020, 38, e16711. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Schaer, D.A.; Beckmann, R.P.; Dempsey, J.A.; Huber, L.; Forest, A.; Amaladas, N.; Li, Y.; Wang, Y.C.; Rasmussen, E.R.; Chin, D.; et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. 2018, 22, 2978–2994. [Google Scholar] [CrossRef]
- Ahn, S.M.; Jang, S.J.; Shim, J.H.; Kim, D.; Hong, S.M.; Sung, C.O.; Baek, D.; Haq, F.; Ansari, A.A.; Lee, S.Y.; et al. Genomic portrait of resectable hepatocellular carcinomas: Implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 2014, 60, 1972–1982. [Google Scholar] [CrossRef]
- Bang, J.; Jun, M.; Lee, S.; Moon, H.; Ro, S.W. Targeting EGFR/PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 2130. [Google Scholar] [CrossRef]
- Harada, K.; Shiota, G.; Kawasaki, H. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver 1999, 19, 318–325. [Google Scholar] [CrossRef]
- Thomas, M.B.; Garrett-Mayer, E.; Anis, M.; Anderton, K.; Bentz, T.; Edwards, A.; Brisendine, A.; Weiss, G.; Siegel, A.B.; Bendell, J.; et al. A Randomized Phase II Open-Label Multi-Institution Study of the Combination of Bevacizumab and Erlotinib Compared to Sorafenib in the First-Line Treatment of Patients with Advanced Hepatocellular Carcinoma. Oncology 2018, 94, 329–339. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Holen, K.D.; Northfelt, D.W.; Pitot, H.C.; Picus, J.; Flynn, P.J.; Erlichman, C. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 2012, 118, 2424–2430. [Google Scholar] [CrossRef]
- Yau, T.; Wong, H.; Chan, P.; Yao, T.J.; Pang, R.; Cheung, T.T.; Fan, S.T.; Poon, R.T. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Investig. New Drugs 2012, 30, 2384–2390. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Morris, J.S.; Iwasaki, M.; Al-Shamsi, H.O.; Raghav, K.P.; Girard, L.; Cheung, S.; Nguyen, V.; Elsayes, K.M.; Xiao, L.; et al. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. OncoTargets Ther. 2016, 9, 773–780. [Google Scholar] [CrossRef]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef]
- Zhu, A.X.; Stuart, K.; Blaszkowsky, L.S.; Muzikansky, A.; Reitberg, D.P.; Clark, J.W.; Enzinger, P.C.; Bhargava, P.; Meyerhardt, J.A.; Horgan, K.; et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 2007, 110, 581–589. [Google Scholar] [CrossRef]
- Asnacios, A.; Fartoux, L.; Romano, O.; Tesmoingt, C.; Louafi, S.S.; Mansoubakht, T.; Artru, P.; Poynard, T.; Rosmorduc, O.; Hebbar, M.; et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: Results of a multicenter phase 2 study. Cancer 2008, 112, 2733–2739. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Bernard, S.; Goldberg, R.M.; Morse, M.A.; Garcia, R.; Woods, L.; Moore, D.T.; O’Neil, B.H. Phase II Study of Capecitabine, Oxaliplatin, and Cetuximab for Advanced Hepatocellular Carcinoma. Gastrointest. Cancer Res. 2011, 4, 78–83. [Google Scholar]
- Hu, B.; Zou, T.; Qin, W.; Shen, X.; Su, Y.; Li, J.; Chen, Y.; Zhang, Z.; Sun, H.; Zheng, Y.; et al. Inhibition of EGFR Overcomes Acquired Lenvatinib Resistance Driven by STAT3-ABCB1 Signaling in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 3845–3857. [Google Scholar] [CrossRef]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef]
- Ke, A.W.; Shi, G.M.; Zhou, J.; Wu, F.Z.; Ding, Z.B.; Hu, M.Y.; Xu, Y.; Song, Z.J.; Wang, Z.J.; Wu, J.C.; et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology 2009, 49, 491–503. [Google Scholar] [CrossRef]
- Bayard, Q.; Caruso, S.; Couchy, G.; Rebouissou, S.; Bioulac Sage, P.; Balabaud, C.; Paradis, V.; Sturm, N.; de Muret, A.; Guettier, C.; et al. Recurrent chromosomal rearrangements of ROS1, FRK and IL6 activating JAK/STAT pathway in inflammatory hepatocellular adenomas. Gut 2020, 69, 1667–1676. [Google Scholar] [CrossRef]
- Jia, S.W.; Fu, S.; Wang, F.; Shao, Q.; Huang, H.B.; Shao, J.Y. ALK gene copy number gain and its clinical significance in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 183–192. [Google Scholar] [CrossRef]
- Santoro, A.; Rimassa, L.; Borbath, I.; Daniele, B.; Salvagni, S.; Van Laethem, J.L.; Van Vlierberghe, H.; Trojan, J.; Kolligs, F.T.; Weiss, A.; et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: A randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013, 14, 55–63. [Google Scholar] [CrossRef]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Rota Caremoli, E.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Kudo, M.; Morimoto, M.; Moriguchi, M.; Izumi, N.; Takayama, T.; Yoshiji, H.; Hino, K.; Oikawa, T.; Chiba, T.; Motomura, K.; et al. A randomized, double-blind, placebo-controlled, phase 3 study of tivantinib in Japanese patients with MET-high hepatocellular carcinoma. Cancer Sci. 2020, 111, 3759–3769. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Sukeepaisarnjaroen, W.; Han, G.; Choo, S.P.; Sriuranpong, V.; Pan, H.; Yau, T.; Guo, Y.; Chen, M.; et al. A phase II study of the efficacy and safety of the MET inhibitor capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919889001. [Google Scholar] [CrossRef]
- Harding, J.J.; Zhu, A.X.; Bauer, T.M.; Choueiri, T.K.; Drilon, A.; Voss, M.H.; Fuchs, C.S.; Abou-Alfa, G.K.; Wijayawardana, S.R.; Wang, X.A.; et al. A Phase Ib/II Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Cancer. Clin. Cancer Res. 2019, 25, 5202–5211. [Google Scholar] [CrossRef]
- Ryoo, B.Y.; Cheng, A.L.; Ren, Z.; Kim, T.Y.; Pan, H.; Rau, K.M.; Choi, H.J.; Park, J.W.; Kim, J.H.; Yen, C.J.; et al. Randomised Phase 1b/2 trial of tepotinib vs sorafenib in Asian patients with advanced hepatocellular carcinoma with MET overexpression. Br. J. Cancer 2021, 125, 200–208. [Google Scholar] [CrossRef]
- Hsu, C.; Chang, Y.-F.; Yen, C.-J.; Lu, L.-C.; Zhu, X.; Xu, Y.; Zhou, Q.; Dong, X.; Tong, Y. Safety and efficacy of combination of GT90001, an anti-activin receptor-like kinase-1 (ALK-1) antibody, and nivolumab in patients with metastatic hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 326. [Google Scholar] [CrossRef]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef]
- Choo, S.P.; Chowbay, B.; Ng, Q.S.; Thng, C.H.; Lim, C.; Hartono, S.; Koh, T.S.; Huynh, H.; Poon, D.; Ang, M.K.; et al. A Phase 1 dose-finding and pharmacodynamic study of rapamycin in combination with bevacizumab in patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2013, 49, 999–1008. [Google Scholar] [CrossRef]
- Rizell, M.; Andersson, M.; Cahlin, C.; Hafström, L.; Olausson, M.; Lindnér, P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int. J. Clin. Oncol. 2008, 13, 66–70. [Google Scholar] [CrossRef]
- Decaens, T.; Luciani, A.; Itti, E.; Hulin, A.; Roudot-Thoraval, F.; Laurent, A.; Zafrani, E.S.; Mallat, A.; Duvoux, C. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig. Liver Dis. 2012, 44, 610–616. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.K.; Lim, H.Y.; Poon, R.T.; Blanc, J.F.; Vogel, A.; Chen, C.L.; et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. Jama 2014, 312, 57–67. [Google Scholar] [CrossRef]
- Koeberle, D.; Dufour, J.F.; Demeter, G.; Li, Q.; Ribi, K.; Samaras, P.; Saletti, P.; Roth, A.D.; Horber, D.; Buehlmann, M.; et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. [Google Scholar] [CrossRef]
- Grabinski, N.; Ewald, F.; Hofmann, B.T.; Staufer, K.; Schumacher, U.; Nashan, B.; Jücker, M. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol. Cancer 2012, 11, 85. [Google Scholar] [CrossRef]
- Ye, L.; Mayerle, J.; Ziesch, A.; Reiter, F.P.; Gerbes, A.L.; De Toni, E.N. The PI3K inhibitor copanlisib synergizes with sorafenib to induce cell death in hepatocellular carcinoma. Cell Death Discov. 2019, 5, 86. [Google Scholar] [CrossRef]
- Patra, T.; Meyer, K.; Ray, R.B.; Ray, R. A combination of AZD5363 and FH5363 induces lethal autophagy in transformed hepatocytes. Cell Death Dis. 2020, 11, 540. [Google Scholar] [CrossRef]
- Ashling, W. Nab-sirolimus under Investigation in TSC1/TSC2-Mutated GI Solid Tumors. Suppl. Featur. Publ. 2022, 1. Available online: https://www.onclive.com/view/nab-sirolimus-under-investigation-in-tsc1-tsc2-mutated-gi-solid-tumors (accessed on 2 May 2024).
- Schmidt, C.M.; McKillop, I.H.; Cahill, P.A.; Sitzmann, J.V. Increased MAPK expression and activity in primary human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 1997, 236, 54–58. [Google Scholar] [CrossRef]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Goff, L.W.; Kauh, J.S.; Strosberg, J.R.; Bekaii-Saab, T.S.; Lee, R.M.; Kazi, A.; Moore, D.T.; Learoyd, M.; Lush, R.M.; et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2011, 29, 2350–2356. [Google Scholar] [CrossRef]
- Tai, W.M.; Yong, W.P.; Lim, C.; Low, L.S.; Tham, C.K.; Koh, T.S.; Ng, Q.S.; Wang, W.W.; Wang, L.Z.; Hartano, S.; et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann. Oncol. 2016, 27, 2210–2215. [Google Scholar] [CrossRef]
- Huynh, H.; Ngo, V.C.; Koong, H.N.; Poon, D.; Choo, S.P.; Toh, H.C.; Thng, C.H.; Chow, P.; Ong, H.S.; Chung, A.; et al. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC). J. Hepatol. 2010, 52, 79–87. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.-C.; Sung, Y.-C.; Ramjiawan, R.R.; Lin, T.-T.; Chang, C.-C.; Jeng, K.-S.; Chang, C.-F.; Liu, C.-H.; Gao, D.-Y.; et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci. Rep. 2017, 7, 44123. [Google Scholar] [CrossRef]
- Kim, R.; Tan, E.; Wang, E.; Mahipal, A.; Chen, D.T.; Cao, B.; Masawi, F.; Machado, C.; Yu, J.; Kim, D.W. A Phase I Trial of Trametinib in Combination with Sorafenib in Patients with Advanced Hepatocellular Cancer. Oncologist 2020, 25, e1893–e1899. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.Y.; Yoon, J.H.; Hsu, C.; Rau, K.M.; Poon, R.T.; Yeo, W.; Park, J.W.; et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 5976–5985. [Google Scholar] [CrossRef]
- Lim, H.Y.; Merle, P.; Weiss, K.H.; Yau, T.; Ross, P.; Mazzaferro, V.; Blanc, J.F.; Ma, Y.T.; Yen, C.J.; Kocsis, J.; et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 4650–4661. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Song, X.; Utpatel, K.; Zhang, Y.; Wang, P.; Lu, X.; Zhang, J.; Xu, M.; Su, T.; et al. Combined Treatment with MEK and mTOR Inhibitors is Effective in In Vitro and In Vivo Models of Hepatocellular Carcinoma. Cancers 2019, 11, 930. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Z.; Zhang, Y.; Evert, M.; Calvisi, D.F.; Chen, X. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Investig. 2022, 132, 1–6. [Google Scholar] [CrossRef]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer 2010, 126, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, M.; Le, D.T.; Pishvaian, M.J.; Weinberg, B.A.; Papadopoulos, K.P.; Shen, L.; Gong, J.; Li, J.; Strickler, J.H.; Zhou, A.; et al. Phase 1 study of WNT pathway Porcupine inhibitor CGX1321 and phase 1b study of CGX1321 + pembrolizumab (pembro) in patients (pts) with advanced gastrointestinal (GI) tumors. J. Clin. Oncol. 2023, 41, 3514. [Google Scholar] [CrossRef]
- Giakoustidis, A.; Giakoustidis, D.; Mudan, S.; Sklavos, A.; Williams, R. Molecular signalling in hepatocellular carcinoma: Role of and crosstalk among WNT/ß-catenin, Sonic Hedgehog, Notch and Dickkopf-1. Can. J. Gastroenterol. Hepatol. 2015, 29, 209–217. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, H.M.; Broering, R.; Shi, X.D.; Yu, X.H.; Xu, L.B.; Wu, W.R.; Liu, C. Dickkopf-1 contributes to hepatocellular carcinoma tumorigenesis by activating the Wnt/β-catenin signaling pathway. Signal Transduct. Target. Ther. 2019, 4, 54. [Google Scholar] [CrossRef]
- Suda, T.; Yamashita, T.; Sunagozaka, H.; Okada, H.; Nio, K.; Sakai, Y.; Yamashita, T.; Mizukoshi, E.; Honda, M.; Kaneko, S. Dickkopf-1 Promotes Angiogenesis and is a Biomarker for Hepatic Stem Cell-like Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 2801. [Google Scholar] [CrossRef]
- Tao, Y.M.; Liu, Z.; Liu, H.L. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig. Liver Dis. 2013, 45, 251–257. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, X.R.; Tan, Y.; You, H.; Xu, Y.; Chu, W.; Ge, T.; Zhou, J.; Qiu, S.J.; Shi, Y.H.; et al. High level of serum protein DKK1 predicts poor prognosis for patients with hepatocellular carcinoma after hepatectomy. Hepat. Oncol. 2015, 2, 231–244. [Google Scholar] [CrossRef]
- Wall, J.A.; Klempner, S.J.; Arend, R.C. The anti-DKK1 antibody DKN-01 as an immunomodulatory combination partner for the treatment of cancer. Expert. Opin. Investig. Drugs 2020, 29, 639–644. [Google Scholar] [CrossRef]
- Seo, S.H.; Cho, K.J.; Park, H.J.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Cheon, J.H.; Yook, J.I.; et al. Inhibition of Dickkopf-1 enhances the anti-tumor efficacy of sorafenib via inhibition of the PI3K/Akt and Wnt/β-catenin pathways in hepatocellular carcinoma. Cell Commun. Signal 2023, 21, 339. [Google Scholar] [CrossRef]
- Awad, A.E.; Ebrahim, M.A.; Eissa, L.A.; El-Shishtawy, M.M. Dickkopf-1 and Amphiregulin as Novel Biomarkers and Potential Therapeutic Targets in Hepatocellular Carcinoma. Int. J. Hematol. Oncol. Stem Cell Res. 2019, 13, 153–163. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Kummar, S.; Lassen, U.N. TRK Inhibition: A New Tumor-Agnostic Treatment Strategy. Target. Oncol. 2018, 13, 545–556. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016, 1, e000023. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Hendrickson, P.G.; Olson, M.; Luetkens, T.; Weston, S.; Han, T.; Atanackovic, D.; Fine, G.C. The promise of adoptive cellular immunotherapies in hepatocellular carcinoma. Oncoimmunology 2020, 9, 1673129. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y.; et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Jiang, S.S.; Tang, Y.; Zhang, Y.J.; Weng, D.S.; Zhou, Z.G.; Pan, K.; Pan, Q.Z.; Wang, Q.J.; Liu, Q.; He, J.; et al. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget 2015, 6, 41339–41349. [Google Scholar] [CrossRef]
- Wada, Y.; Nakashima, O.; Kutami, R.; Yamamoto, O.; Kojiro, M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998, 27, 407–414. [Google Scholar] [CrossRef]
- Brunner, S.M.; Rubner, C.; Kesselring, R.; Martin, M.; Griesshammer, E.; Ruemmele, P.; Stempfl, T.; Teufel, A.; Schlitt, H.J.; Fichtner-Feigl, S. Tumor-infiltrating, interleukin-33–producing effector-memory CD8+ T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 2015, 61, 1957–1967. [Google Scholar] [CrossRef]
- Gao, X.; Mi, Y.; Guo, N.; Xu, H.; Xu, L.; Gou, X.; Jin, W. Cytokine-Induced Killer Cells As Pharmacological Tools for Cancer Immunotherapy. Front. Immunol. 2017, 8, 774. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, B.; Tang, Z.R.; Lei, Z.Y.; Wang, H.F.; Feng, Y.Y.; Fan, Z.P.; Xu, D.P.; Wang, F.S. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 1146–1151. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391.e1386. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: An extended 5-year follow-up. Cancer Immunol. Immunother. 2019, 68, 23–32. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Kim, Y.; Shuang, Z.Y.; Zhang, Y.J.; Lao, X.M.; Li, Y.Q.; Chen, M.S.; Pawlik, T.M.; Xia, J.C.; et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology 2016, 5, e1083671. [Google Scholar] [CrossRef]
- Hao, M.Z.; Lin, H.L.; Chen, Q.; Ye, Y.B.; Chen, Q.Z.; Chen, M.S. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: A comparative study. Chin. J. Cancer 2010, 29, 172–177. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, H.; Liu, L.; Cao, S.; Ren, B.; Zhang, N.; An, X.; Yu, J.; Li, H.; Ren, X. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocelluar carcinoma. J. Clin. Immunol. 2014, 34, 194–203. [Google Scholar] [CrossRef]
- Huang, Z.M.; Li, W.; Li, S.; Gao, F.; Zhou, Q.M.; Wu, F.M.; He, N.; Pan, C.C.; Xia, J.C.; Wu, P.H.; et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J. Immunother. 2013, 36, 287–293. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Gao, D.; Zhang, B.; Zheng, M.; Lun, M.; Wei, M.; Duan, R.; Guo, M.; Hua, J.; et al. A prognosis and impact factor analysis of DC-CIK cell therapy for patients with hepatocellular carcinoma undergoing postoperative TACE. Cancer Biol. Ther. 2018, 19, 475–483. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Li, H.Y.; Wang, Z.H.; Wu, J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188441. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Liu, Z.; Wei, D.; Wu, X.; Bian, H.; Chen, Z. Adoptive cell transfer therapy for hepatocellular carcinoma. Front. Med. 2019, 13, 3–11. [Google Scholar] [CrossRef]
- Zhou, F.; Shang, W.; Yu, X.; Tian, J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med. Res. Rev. 2018, 38, 741–767. [Google Scholar] [CrossRef]
- Gao, H.; Li, K.; Tu, H.; Pan, X.; Jiang, H.; Shi, B.; Kong, J.; Wang, H.; Yang, S.; Gu, J.; et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6418–6428. [Google Scholar] [CrossRef]
- Wu, X.; Luo, H.; Shi, B.; Di, S.; Sun, R.; Su, J.; Liu, Y.; Li, H.; Jiang, H.; Li, Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 1483–1494. [Google Scholar] [CrossRef]
- Fang, W.; Fu, Q.; Zhao, Q.; Zheng, Y.; Liu, L.; Li, Z.; Dai, X.; Wang, H.; Zhu, X.; Zhao, P.; et al. Phase I trial of fourth-generation chimeric antigen receptor T-cells targeting glypican-3 for advanced hepatocellular carcinoma. J. Clin. Oncol. 2021, 39, 4088. [Google Scholar] [CrossRef]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Lu, J.; Cheng, N.; Horan, L.H.; Liu, B.; et al. Abstract 2299: ET1402L1-CART, a T cell therapy targeting the intracellular tumor antigen AFP, demonstrates potent antitumor activity in hepatocellular carcinoma models. Cancer Res. 2016, 76, 2299. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H.; et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, T.; Guo, J.; Wang, J.; Jia, L.; Shi, X.; Yang, T.; Jiao, R.; Wei, X.; Feng, Z.; et al. Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 546586. [Google Scholar] [CrossRef]
- Sun, B.; Yang, D.; Dai, H.; Liu, X.; Jia, R.; Cui, X.; Li, W.; Cai, C.; Xu, J.; Zhao, X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol. Res. 2019, 7, 1813–1823. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wei, D.; Liu, Z.K.; Yong, Y.L.; Wei, W.; Zhang, Z.Y.; Lv, J.J.; Zhang, Z.; Chen, Z.N.; Bian, H. Doxycycline Inducible Chimeric Antigen Receptor T Cells Targeting CD147 for Hepatocellular Carcinoma Therapy. Front. Cell Dev. Biol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. Oncoimmunology 2020, 9, 1846926. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Kalafateli, M.; Triantos, C. Chimeric Antigen Receptor T Cell Therapy for Hepatocellular Carcinoma: Where Do We Stand? Int. J. Mol. Sci. 2024, 25, 2631. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Mizukoshi, E.; Kobayashi, E.; Tamai, T.; Hamana, H.; Ozawa, T.; Kishi, H.; Kitahara, M.; Yamashita, T.; Arai, K.; et al. Association between High-Avidity T-Cell Receptors, Induced by α-Fetoprotein-Derived Peptides, and Anti-Tumor Effects in Patients with Hepatocellular Carcinoma. Gastroenterology 2017, 152, 1395–1406.e1310. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Nobuoka, D.; Shirakawa, H.; Kuronuma, T.; Motomura, Y.; Mizuno, S.; Ishii, H.; Nakachi, K.; Konishi, M.; et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: Immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012, 18, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016, 5, e1129483. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Nakagawa, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Iida, N.; Fushimi, K.; Kaneko, S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015, 369, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Nakagawa, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Fushimi, K.; Kobayashi, E.; Kishi, H.; Muraguchi, A.; et al. Immunological features of T cells induced by human telomerase reverse transcriptase-derived peptides in patients with hepatocellular carcinoma. Cancer Lett. 2015, 364, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Forner, A.; Korangy, F.; N’Kontchou, G.; Barget, N.; Ayuso, C.; Ormandy, L.A.; Manns, M.P.; Beaugrand, M.; Bruix, J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010, 10, 209. [Google Scholar] [CrossRef]
- Löffler, M.W.; Gori, S.; Izzo, F.; Mayer-Mokler, A.; Ascierto, P.A.; Königsrainer, A.; Ma, Y.T.; Sangro, B.; Francque, S.; Vonghia, L.; et al. Phase I/II Multicenter Trial of a Novel Therapeutic Cancer Vaccine, HepaVac-101, for Hepatocellular Carcinoma. Clin. Cancer Res. 2022, 28, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, Y.; Tahara, K.; Goto, S.; Sasaki, A.; Kai, S.; Seike, M.; Chen, C.L.; Kawano, K.; Kitano, S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol. Immunother. 2003, 52, 155–161. [Google Scholar] [CrossRef]
- Palmer, D.H.; Midgley, R.S.; Mirza, N.; Torr, E.E.; Ahmed, F.; Steele, J.C.; Steven, N.M.; Kerr, D.J.; Young, L.S.; Adams, D.H. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009, 49, 124–132. [Google Scholar] [CrossRef]
- Lee, W.C.; Wang, H.C.; Hung, C.F.; Huang, P.F.; Lia, C.R.; Chen, M.F. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: A clinical trial. J. Immunother. 2005, 28, 496–504. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.; Lee, M.; Heo, M.K.; Song, J.S.; Kim, K.H.; Lee, H.; Yi, N.J.; Lee, K.W.; Suh, K.S.; et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br. J. Cancer 2015, 113, 1666–1676. [Google Scholar] [CrossRef]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.C.; Lee, W.B.; Lee, H.S.; Bae, Y.S.; et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef]
- El Ansary, M.; Mogawer, S.; Elhamid, S.A.; Alwakil, S.; Aboelkasem, F.; Sabaawy, H.E.; Abdelhalim, O. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J. Cancer Res. Clin. Oncol. 2013, 139, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H.; Ribas, A.; Dissette, V.B.; Lee, Y.; Yang, J.Q.; De la Rocha, P.; Duran, S.D.; Hernandez, J.; Seja, E.; Potter, D.M.; et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006, 12, 2817–2825. [Google Scholar] [CrossRef]
- Rizell, M.; Sternby Eilard, M.; Andersson, M.; Andersson, B.; Karlsson-Parra, A.; Suenaert, P. Phase 1 Trial with the Cell-Based Immune Primer Ilixadencel, Alone, and Combined with Sorafenib, in Advanced Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Calmeiro, J.; Carrascal, M.A.; Tavares, A.R.; Ferreira, D.A.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics 2020, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef]
- Repáraz, D.; Aparicio, B.; Llopiz, D.; Hervás-Stubbs, S.; Sarobe, P. Therapeutic Vaccines against Hepatocellular Carcinoma in the Immune Checkpoint Inhibitor Era: Time for Neoantigens? Int. J. Mol. Sci. 2022, 23, 2022. [Google Scholar] [CrossRef]
- Zhang, W.; Song, T.Q.; Zhang, T.; Wu, Q.; Kong, D.L.; Li, Q.; Sun, H.C. Adjuvant interferon for early or late recurrence of hepatocellular carcinoma and mortality from hepatocellular carcinoma following curative treatment: A meta-analysis with comparison of different types of hepatitis. Mol. Clin. Oncol. 2014, 2, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Romito, R.; Schiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G.; et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yin, Z.; Cao, L.; Xu, X.; Yan, T.; Liu, C.; Li, D. Adjuvant pegylated interferon therapy improves the survival outcomes in patients with hepatitis-related hepatocellular carcinoma after curative treatment: A meta-analysis. Medicine 2018, 97, e11295. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ten Dijke, P. Immunoregulation by members of the TGFβ superfamily. Nat. Rev. Immunol. 2016, 16, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Gane, E.; Assenat, E.; Siebler, J.; Galle, P.R.; Merle, P.; Hourmand, I.O.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; et al. A Phase 2 Study of Galunisertib (TGF-β1 Receptor Type I Inhibitor) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00056. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Saleh, M.; Aix, S.P.; Ochoa-de-Olza, M.; Patel, S.P.; Antonia, S.; Zhao, Y.; Gueorguieva, I.; Man, M.; Estrem, S.T.; et al. A phase Ib/II study of galunisertib in combination with nivolumab in solid tumors and non-small cell lung cancer. BMC Cancer 2023, 23, 708. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Cao, L.; Rahma, O.E.; Makarova-Rusher, O.V.; Kleiner, D.E.; Fioravanti, S.; Walker, M.; Carey, S.; Yu, Y.; et al. A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib. United Eur. Gastroenterol. J. 2015, 3, 453–461. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ma, C.; Ulahannan, S.V.; Rahma, O.E.; Makarova-Rusher, O.; Cao, L.; Yu, Y.; Kleiner, D.E.; Trepel, J.; Lee, M.J.; et al. Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma. Clin. Cancer Res. 2017, 23, 4633–4641. [Google Scholar] [CrossRef]
- Diab, A.; Hamid, O.; Thompson, J.A.; Ros, W.; Eskens, F.; Doi, T.; Hu-Lieskovan, S.; Klempner, S.J.; Ganguly, B.; Fleener, C.; et al. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clin. Cancer Res. 2022, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; O’Donnell, R.; Semrad, T.J.; Mack, P.; Blanchard, S.; Bahary, N.; Jiang, Y.; Yen, Y.; Wright, J.; Chen, H.; et al. A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2018, 81, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Capanu, M.; O’Reilly, E.M.; Ma, J.; Chou, J.F.; Gansukh, B.; Shia, J.; Kalin, M.; Katz, S.; Abad, L.; et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J. Hepatol. 2014, 60, 319–324. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, Y.; Cai, X.B.; Liu, B. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wu, L.L.; Lau, W.Y.; Huan, H.B.; Wen, X.D.; Ma, K.S.; Li, X.W.; Bie, P. Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J. Gastroenterol. 2016, 22, 5384–5392. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, J.; Zheng, S.M.; Wang, Y.; Xiang, X.; Cheng, Q.; Zhu, J. The efficacy of sorafenib in preventing hepatocellular carcinoma recurrence after resection: A systematic review and meta-analysis. Rev. Esp. Enferm. Dig. 2020, 112, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Hu, L.; Liu, J.; Sun, M.; Sun, Y.; Xue, F. Prognostic Nomograms Combined Adjuvant Lenvatinib for Hepatitis B Virus-related Hepatocellular Carcinoma with Microvascular Invasion after Radical Resection. Front. Oncol. 2022, 12, 919824. [Google Scholar] [CrossRef]
- Cai, L.; Li, H.; Guo, J.; Zhao, W.; Li, Y.; Duan, Y.; Hou, X.; Cheng, L.; Du, H.; Shao, X.; et al. 176P Effect of adjuvant lenvatinib (LEN) on tumour recurrence in patients with hepatocellular carcinoma (HCC) and high residual alpha-fetoprotein (AFP) following resection or ablation: A single-center, retrospective study. Ann. Oncol. 2020, 31, S1308. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Nakahira, S.; Nishida, N.; Ida, H.; Minami, Y.; Nakai, T.; Wada, H.; Kubo, S.; Ohkawa, K.; et al. Final results of adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. J. Clin. Oncol. 2022, 40. [Google Scholar]
- Chen, W.; Hu, S.; Liu, Z.; Sun, Y.; Wu, J.; Shen, S.; Peng, Z. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol. Int. 2023, 17, 406–416. [Google Scholar] [CrossRef]
- Kudo, M.; Chen, M.; Chow, P.K.H.; Kaseb, A.O.; Lee, H.C.; Yopp, A.C.; Becker, L.; Painter, S.H.; Kovic, B.; Lian, Q.; et al. Efficacy, safety and patient reported outcomes (PROs) from the phase III IMbrave050 trial of adjuvant atezolizumab (atezo) + bevacizumab (bev) vs active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. J. Clin. Oncol. 2023, 41, 4002. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; Cheng, A.-L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef]
- Wei, W.; Jian, P.E.; Li, S.H.; Guo, Z.X.; Zhang, Y.F.; Ling, Y.H.; Lin, X.J.; Xu, L.; Shi, M.; Zheng, L.; et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: A randomized clinical trial of efficacy and safety. Cancer Commun. 2018, 38, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, Z.; Chen, Y.; Hu, J.; Yang, G.; Yu, L.; Yang, X.; Huang, A.; Zhang, X.; Zhou, S.; et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma after Resection: A Randomized Controlled Study. Clin. Cancer Res. 2018, 24, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, T.; Zhang, J.; Zhang, X.; Chen, W.; Shen, Y.; Bai, X.; Liang, T. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB 2020, 22, 795–808. [Google Scholar] [CrossRef]
- Hsiao, J.H.; Tsai, C.C.; Liang, T.J.; Chiang, C.L.; Liang, H.L.; Chen, I.S.; Chen, Y.C.; Chang, P.M.; Chou, N.H.; Wang, B.W. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int. J. Surg. 2017, 45, 35–41. [Google Scholar] [CrossRef]
- Hirokawa, F.; Komeda, K.; Taniguchi, K.; Asakuma, M.; Shimizu, T.; Inoue, Y.; Kagota, S.; Tomioka, A.; Yamamoto, K.; Uchiyama, K. Is Postoperative Adjuvant Transcatheter Arterial Infusion Therapy Effective for Patients with Hepatocellular Carcinoma who Underwent Hepatectomy? A Prospective Randomized Controlled Trial. Ann. Surg. Oncol. 2020, 27, 4143–4152. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mei, J.; Wang, Q.; Guo, Z.; Lu, L.; Ling, Y.; Xu, L.; Chen, M.; Zheng, L.; Lin, W.; et al. Postoperative Adjuvant Transarterial Infusion Chemotherapy with FOLFOX Could Improve Outcomes of Hepatocellular Carcinoma Patients with Microvascular Invasion: A Preliminary Report of a Phase III, Randomized Controlled Clinical Trial. Ann. Surg. Oncol. 2020, 27, 5183–5190. [Google Scholar] [CrossRef]
- Huang, S.X.; Wu, Y.L.; Tang, C.W.; Feng, W.M.; Xu, Y.Q.; Bao, Y.; Zheng, Y.Y. Prophylactic hepatic artery infusion chemotherapy improved survival after curative resection in patients with hepatocellular carcinoma. Hepatogastroenterology 2015, 62, 122–125. [Google Scholar]
- Ke, Q.; Wang, L.; Wu, W.; Huang, X.; Li, L.; Liu, J.; Guo, W. Meta-Analysis of Postoperative Adjuvant Hepatic Artery Infusion Chemotherapy versus Surgical Resection Alone for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 720079. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Wen, T.F.; Lu, C.D.; Zeng, Y.Y.; Xiang, B.D.; Xu, X.; Huang, Z.Y.; Li, X.C.; Zhang, T.; et al. 945P Adjuvant lenvatinib in combination with TACE for hepatocellular carcinoma patients with high risk of postoperative relapse (LANCE): Updated results from a multi-center prospective cohort study. Ann. Oncol. 2021, 32, S824–S825. [Google Scholar] [CrossRef]
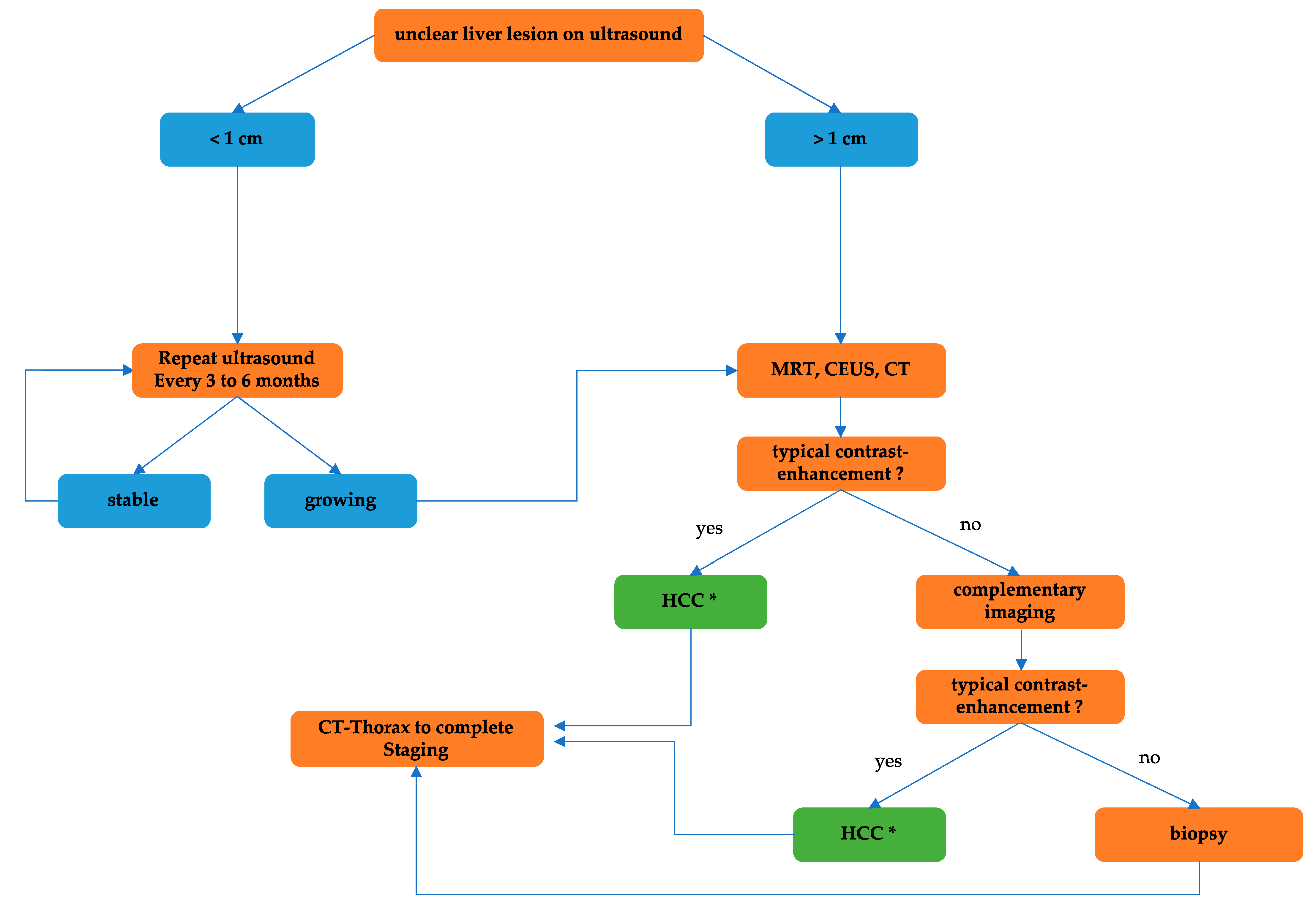
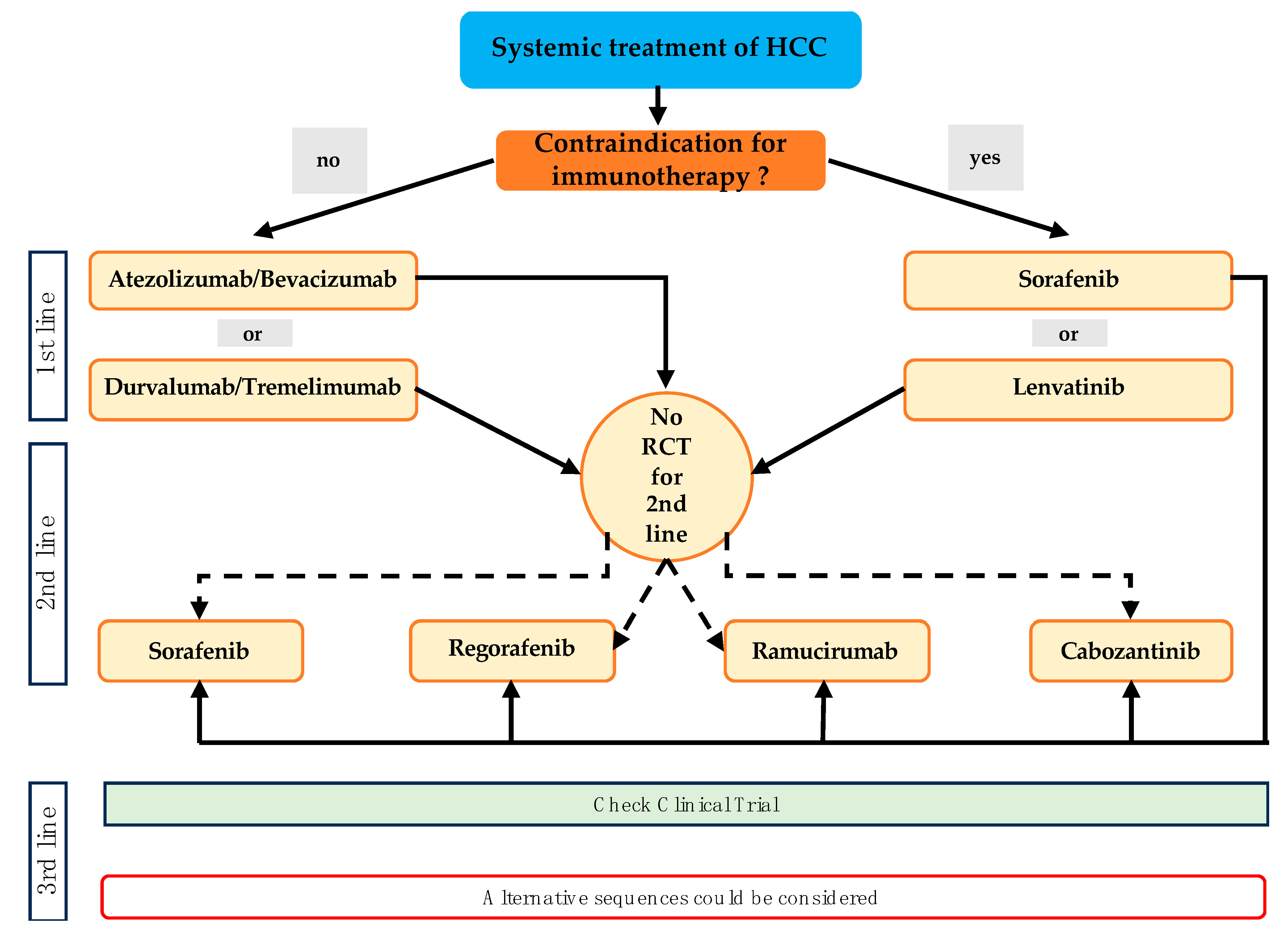
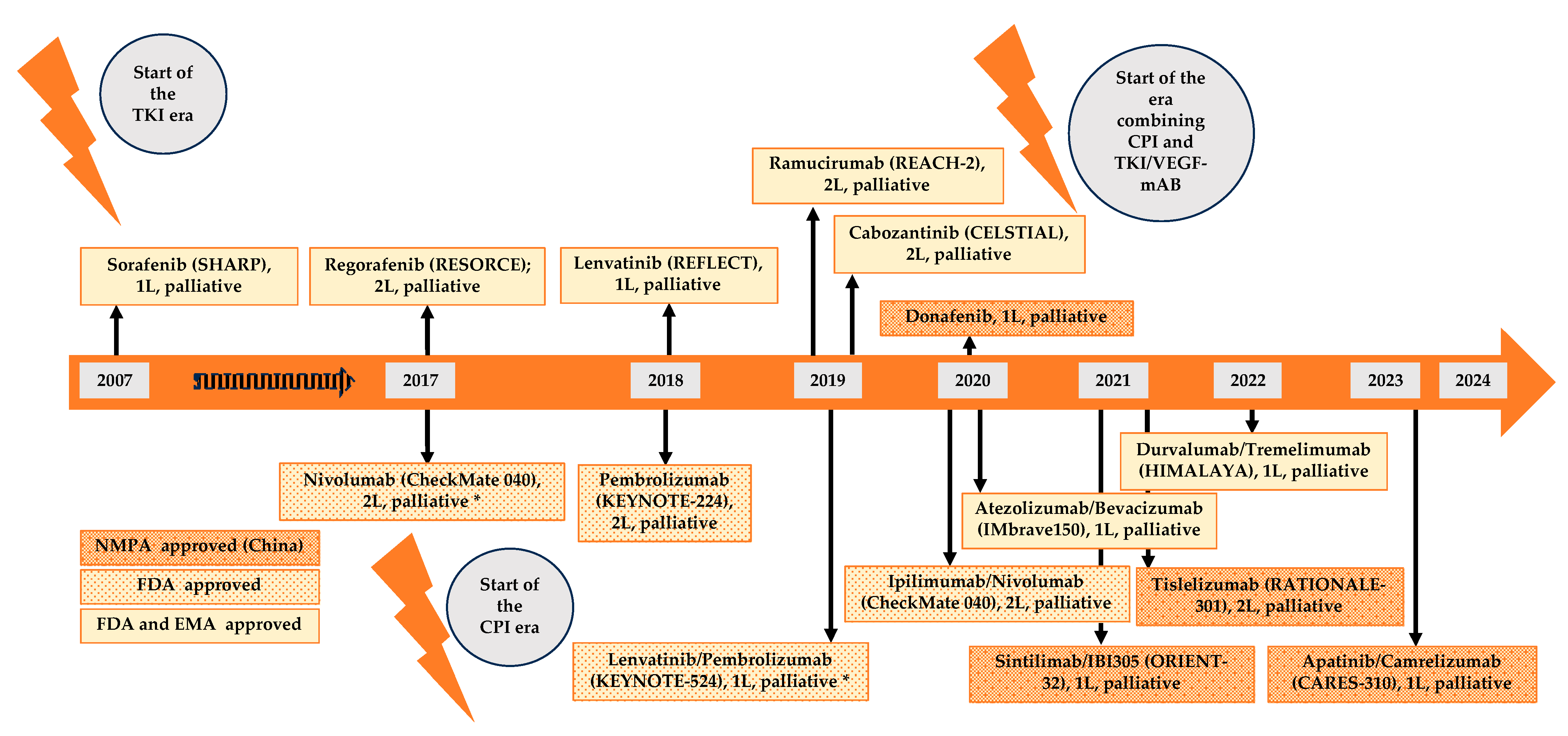
| Risk-Factor | Study-Design | Risk Estimate (95% CI) | Reference |
|---|---|---|---|
| acute intermittent porphyria | case-control | 8.0 (24.3–59.3) *; 5.15 (2.90–9.12) | [15,16,17,18] |
| aflatoxin | case-control | 6.37 (3.74–10.86) | [19,20,21] |
| alcohol abuse | case-control | 7.3 (6.8–7.8) | [22,23] |
| α1-antitrypsin deficiency | case-control | 5 (N/A); 3.91 (2.67–5.74) | [18,24,25,26,27] |
| biliary cirrhosis | case-control | 12.28 (8.86–17.02) | [18] |
| chronic hepatitis B viral infection | case-control | 21.6 (17.9–26.0); 7.65 (4.6–24.95) | [23,28,29,30,31,32,33] |
| chronic hepatitis C viral infection | case-control | 59.9 (55.1–65.1); 109.88 (71.12–207.10) | [23,28,31,32,33,34,35] |
| cirrhosis of the liver | case-control | 808.37 (257.52–2537.52) | [33,36,37] |
| diabetes mellitus | case-control | 3.00 (2.75–4.41) | [33,38,39,40] |
| Gaucher disease | N/A | N/A | [41,42] |
| glycogen storage disease | N/A | N/A | [43] |
| hemochromatosis | case-control | 10.89 (4.38–27.09) | [18,44] |
| non-alcoholic fatty liver disease | case-control | 7.55 (4.89–11.26) | [33,45,46,47] |
| tyrosinemia type I | N/A | N/A | [48,49] |
| Wilson disease | case-control | 4.1 (1.80–9.33) | [18] |
| Study | Year | Phase | mOS | mPFS | NCT |
|---|---|---|---|---|---|
| SHARP | 2008 | III | 10.7 | - | NCT00105443 |
| BRSIK-FL | 2013 | III | 9.9 | - | NCT00858871 |
| SUN1170 | 2013 | III | 10.2 | 3.0 | NCT00699374 |
| LiGHT | 2015 | III | 9.8 | - | NCT01009593 |
| REFLECT | 2018 | III | 12.3 | 3.7 | NCT01761266 |
| IMbrave150 | 2020 | III | 13.4 | 4.3 | NCT03434379 |
| CheckMate 459 | 2022 | III | 14.7 | - | NCT02576509 |
| COSMIC-312 | 2022 | III | 15.5 | 4.2 | NCT03755791 |
| HIMALAYA | 2022 | III | 13.8 | 4.1 | NCT03298451 |
| RATIONALE-301 | 2023 | III | 14.1 | 3.4 | NCT03412773 |
| CARES-310 | 2023 | III | 15.2 | 3.7 | NCT03764293 |
| Study | Phase | Status | NCT |
|---|---|---|---|
| Predictors of sorafenib response in patients with advanced HCC | IV | Not yet recruiting | NCT05967429 |
| Real-world study of efficacy and safety of ICIs and TKIs therapy for HCC | IV | Active, recruiting | NCT05420922 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | II | 1L, palliative + PVTT | HAIC plus Tor | HAIC + Sor | PFS | NCT04135690 |
| - | III | 1L, palliative | HAIC + Sor | TACE + Sor | OS | NCT02856126 |
| HAICPD1-HCC | II | neoadjuvant | HAIC + Sin | HAIC | PFS | NCT03869034 |
| - | II | Palliative | HAIC + Dur | - | OS | NCT04945720 |
| - | II | 1L, palliative | HAIC + Sin + B | - | ORR | NCT05029973 |
| - | II | neoadjuvant | HAIC + Dur + Tre + B | - | ORR | NCT05864755 |
| - | II | 1L, palliative | HAIC + Len + Tis | - | ORR | NCT05954897 |
| - | II | 1L, palliative | HAIC + Len + PD-1 | Len + PD-1- | PFS | NCT05166239 |
| - | II | perioperative | Sin + Len | HAIC | DFS | NCT05519410 |
| - | II | Palliative | HAIC + Sin + B | - | PFS | NCT05617430 |
| - | II | neoadjuvant | HAIC + Cam + Apa | - | R0-rate; SCR | NCT05099848 |
| - | II | Palliative | HAIC+ Cam + Len or Rego | - | PFS | NCT05135364 |
| D-TRIPLET | II | Palliative | HAIC + B + Sin | - | ORR | NCT05214339 |
| - | III | Palliative | HAIC + Len + PD-1 | HAIC (Len + PD-1 sequential) | OS | NCT06041477 |
| - | III | Palliative | HAIC + Cam + Apa | Cam + Apa | OS | NCT05198609 |
| TRIPLET | II | Palliative | HAIC + Cam + Apa | - | ORR | NCT04191889 |
| - | II | 1L, palliative | HAIC + A + B | - | PFS | NCT05886465 |
| TRIPLET-III | III | 1L, palliative | HAIC + Cam + Apa | Cam + Apa | PFS | NCT05313282 |
| - | II | Palliative | HAIC + Len + Cam | - | ORR | NCT05003700 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| STELLAR | IV | palliative | Len or Sor | - | Safety | NCT04763408 |
| - | IV | 1L palliative | Len | - | Safety | NCT04297254 |
| - | II | recurrence after LTx | Len | - | ORR | NCT05103904 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| REGONEXT | II | 2L, palliative (after A/B) | Rego | - | PFS | NCT05134532 |
| CaPture | II | 2L, palliative, refractory to PD-1 | Cabo | - | ToT | NCT04767906 |
| AURORA | II | 2L, palliative (after Len or Len + IO) | Cabo | - | ToT | NCT04511455 |
| - | I/II | 2L, palliative, CP B | Cabo | - | MTD, RP2D | NCT04497038 |
| Study | Phase | Treatment | mOS Experimental | mOS Control | NCT |
|---|---|---|---|---|---|
| BRISK-FL | III | brivanib vs. sorafenib | 9.5 months | 9.9 months | NCT00858871 |
| LiGHT | III | linifanib vs. sorafenib | 9.1 months | 9.8 months | NCT01009593 |
| SUN1170 | III | sunitinib vs. sorafenib | 7.9 months | 10.2 months | NCT00699374 |
| - | II | dovotinib vs. sorafenib | 8.0 months | 8.4 months | - |
| - | I/II | tivozanib | 9.0 months | - | NCT01835223 |
| - | II | cediranib | 5.8 months | - | NCT00238394 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| CheckMate 9DW | III | 1L, palliative | Ipi plus Nivo | Sor/Len | OS | NCT04039607 |
| PRIME-HCC | I/II | neoadjuvant | Ipi plus Nivo | - | safety + delay to surgery | NCT03682276 |
| - | II | 2L, palliative (after A/B) | Ipi plus Nivo | - | ORR | NCT05199285 |
| NEOTOMA | II | neoadjuvant/adjuvant | Dur plus Tre | - | safety | NCT05440864 |
| SIERRA | III | 1L, palliative | Dur plus Tre | - | safety, ORR | NCT05883644 |
| - | II | neoadjuvant before liver transplantation | Dur plus Tre | - | cellular rejection rates | NCT05027425 |
| - | I | neoadjuvant (resection) | Nivo | Nivo + Rela | complete pre-op treatment and proceed to surgery | NCT04658147 |
| - | III | 1L, palliative | Cam + FOLFOX4 | Cam | OS | NCT03605706 |
| ACROPOLI | II | palliative | Spartalizumab | Tis | ORR | NCT04802876 |
| - | II | palliative | INCB086550 | - | ORR | NCT04629339 |
| - | I | palliative | MT-8421 plus Nivolumab | MT-8421 | Safety, ORR | NCT06034860 |
| - | IV | palliative | Cam | - | safety | NCT04947956 |
| - | III | 1L, palliative | Cam plus FOLFOX4 | FOLFOX4 | OS | NCT03605706 |
| - | I/II | 1L, palliative | CHS-006 (anti-TIGIT) + Tor | - | safety | NCT05757492 |
| - | II | 1L, palliative | Nivo + Cabi | Nivo | ORR | NCT04050462 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | II | palliative | Sor + Nivo | - | ORR, MTD | NCT03439891 |
| - | I/II | palliative | Sor + Pembro | - | ORR | NCT03211416 |
| GOING | II | 2L, palliative | Rego + Nivo | - | safety | NCT04170556 |
| REGOMUNE | I/II | 2L, palliative | Rego + Ave | - | safety | NCT03475953 |
| - | II/III | 1L, palliative | Fino + SCT510 | Sor | OS, PFS | NCT04560894 |
| PRIMER-1 | II | perioperative | Pembro | Lenv (Arm C: Len + Pembro) | MPR | NCT05185739 |
| - | II/III | 1L, palliative | Rulonilimab + Len | Len | ORR | NCT05408221 |
| - | I/II | 1L, palliative | Len + Cam | - | ORR | NCT04443309 |
| - | II | 1L, palliative | Len + Tori | - | ORR | NCT04368078 |
| - | III | 1L, palliative | Len + Tori | Len | OS | NCT04523493 |
| - | I/II | 1L, palliative | Cadonilimab + Len | - | ORR | NCT04444167 |
| - | II | 2L, palliative | Candolilimab + B | - | DCR | NCT05760599 |
| - | II | 1L, palliative | Sinti + Len | - | ORR | NCT04042805 |
| - | II | 1L, palliative | Dur + Len | - | ORR | NCT05312216 |
| Dulect2020-1 | - | 1L, palliative or prä LTx | Dur + Len | - | PFS, RFS | NCT04443322 |
| - | II | perioperative | Tis + Len | - | safety | NCT04834986 |
| - | II | palliative | A + Cabo or Len | Cabo or Len | OS, PFS | NCT05168163 |
| PLENTY202001 | - | prä LTx | Len + Pembro | - | RFS | NCT04425226 |
| TALENT | II | neoadjuvant | Tis + Len | Tis | DFS | NCT04615143 |
| - | II | 1L, palliative | Tis + Rego | Rego | Safety, ORR, PFS | NCT04183088 |
| - | II | neoadjuvant | Dur + Rego | - | ORR | NCT05194293 |
| - | 2L, palliative | PD-1 + Rego | - | PFS | NCT05048017 | |
| - | II | 1L, palliative | Tori + B | - | Safety, ORR | NCT04605796 |
| IMbrave152/ SKYSCRAPER-14 | III | 1L, palliative | Tira + A + B | A + B | PFS, OS | NCT05904886 |
| - | II | 2L, palliative | Rego + Pembro | - | ORR | NCT04696055 |
| TRIPLET | II/III | 1L, palliative | A + B | A + B + Ipi | ORR, OS | NCT05665348 |
| AB7 Trial | II | 1L, palliative Child–Pugh B | A + B | - | safety | NCT04829383 |
| - | II | neoadjuvant | A + B | - | Safety, pCR | NCT04721132 |
| MONTBLANC | II | 1L, palliative | Dur + Tre and B in case of progress | Dur + Tre + B | ORR | NCT05844046 |
| CAPT | II | neoadjuvant | Cam + Apa | Cam | RFR | NCT04930315 |
| - | II | 1L, palliative | Cam plus Apa plus mFOLFOX7 | - | ORR | NCT05412589 |
| - | I/II | 1L, palliative | Adebrelimab plus Cam plus Apa | - | Safety, ORR | NCT05924997 |
| - | II | neoadjuvant | Cam plus Apa plus Oxaliplatin | - | MPR | NCT04850040 |
| - | II | Perioperative | Cam plus Apa | - | ORR | NCT04701060 |
| - | II | 1L, palliative | Dona + Sinti | - | PFS | NCT05162352 |
| - | III | 1L, palliative | Sinti + IBI310 | Sor | OS, ORR | NCT04720716 |
| - | I/II | 1L, palliative | Dona + Tori | - | Safety, ORR | NCT04503902 |
| - | II | neoadjuvant | Tis | Tis + Len | MPR | NCT05807776 |
| - | II | palliative | Pembro + Quavonlimab + Len | - | Safety, ORR | NCT04740307 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| CHECKMATE 74W | III | locoregional | Nivo + Ipi + TACE | Nivo + TACE (Arm C: TACE alone) | Safety | NCT04340193 |
| LEAP-012 | III | locoregional | Len + Pembro + TACE | TACE | PFS, OS | NCT04246177 |
| TACE-3 | II/III | locoregional | TACE | TACE + Nivo | OS, TTTP | NCT04268888 |
| CHANCE2202 | IV | locoregional | TACE + PD-1/PD-L1 + TKI or B | TACE | PFS, OS | NCT05332496 |
| CHANCE2201 | IV | locoregional | TACE + PD-1/PD-L1 + TKI or B | PD-1/PD-L1 + TKI or B | OS | NCT05332821 |
| - | I | locoregional | PD-1 + Len + TACE | - | Resection rate | NCT04974281 |
| EMERALD-3 | III | locoregional | Tre + Dur+ Len +TACE | Tre + Dur +TACE (Arm C: TACE alone) | PFS | NCT05301842 |
| - | I/II | 1L, palliative | Len + Tis | Len + Tis + TACE | ORR | NCT05842317 |
| - | III | 1L, palliative | TACE + Len synchron | TACE + Len sequential | OS | NCT05220020 |
| - | III | palliative | TACE + Cam + Apa | TACE | PFS | NCT05320692 |
| - | III | palliative | TACE + Sinti + B | TACE + Len | OS | NCT05985798 |
| - | II | palliative | TACE + Sinti | - | ORR | NCT04297280 |
| TASK-02 | II | palliative | TACE + Sinti + B | - | ORR | NCT04954794 |
| - | III | palliative | TACE + Len + Sinti | TACE + Len | OS | NCT05608200 |
| MORNING | II | neoadjuvant | TACE + Cadonilimab | - | MPR | NCT05578430 |
| - | II | palliative | TACE + Sinti + Fru | - | PFS | NCT05971199 |
| - | II | palliative | TACE + Tis + Sor | - | OS | NCT04992143 |
| - | II | palliative | TACE + Ipi + Nivo + Cabo | - | PFS | NCT04472767 |
| - | III | perioperative | TACE + Cam + Apa | TACE | RFS | NCT05613478 |
| DEMAND | II | palliative | TACE + A +B synchron | TACE + A + B sequential | OS | NCT04224636 |
| T-Double | II | palliative | TACE + Sinti + B | - | ORR | NCT04796025 |
| - | II | - | Dur + Tre +B | TACE + Dur+ Tre + B | PFS | NCT03937830 |
| - | II | Prä LTx | Don + TACE | - | DSR | NCT05576909 |
| - | I | palliative | TACE + Don + Tori | - | safety | NCT04605185 |
| - | II | palliative | TACE + Cam + Apa | - | ORR | NCT05550025 |
| - | II | intermediate stage | TACE + Dur + Trem | - | ORR | NCT03638141 |
| - | II | intermediate stage | TACE + A + B | - | safety | NCT05776875 |
| ROSE | IV | palliative | TACE + Rego | Rego | OS | NCT05811481 |
| LEN-TAC | III | palliative | TACE + Len + Cam | Len | OS | NCT05738616 |
| - | II | palliative | TACE + Cam | - | TTP | NCT04652492 |
| Study | Phase | Treatment | Target 1 | Target 2 | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | II | AK104 | PD-1 | CTLA-4 | ORR | NCT04728321 |
| - | I/II | AK104 | PD-1 | CTLA-4 | ORR | NCT04444167 |
| DUET-4 | I | Bavunalimab | LAG-3 | CTLA-4 | safety | NCT03849469 |
| Study | Phase | Treatment | Target | Drug | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | I | TORL-4-500 | N/A | N/A | safety | NCT06005740 |
| - | I | MGC018-02 | B7-H3 | Duocarmycin | safety | NCT05293496 |
| Study | Phase | Setting | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|
| MATCH | II | palliative | Crizotinib for patients with MET amplification, ALK translocation or ROS1 translocation/inversion | ORR | NCT02465060 |
| Study | Phase | Setting | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|
| - | II | palliative | Lenvatinib, Trametinib, Everolimus | safety | NCT04803318 |
| MATCH | II | palliative | Taselisib (PI3K) | ORR | NCT02465060 |
| MATCH | II | palliative | Copanlisib (PI3K) | ORR | NCT02465060 |
| MATCH | II | palliative | Sapanisertib (mTOR) | ORR | NCT02465060 |
| MATCH | II | palliative | Capivasertib (AKT) | ORR | NCT02465060 |
| MATCH | II | palliative | Ipatasertib (AKT) | ORR | NCT02465060 |
| Study | Phase | Setting | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|
| - | I/II | palliative | DKN-01 + Sorafenib | Safety, time to progression | NCT03645980 |
| Study | Phase | Setting | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|
| MATCH | II | palliative | Larotrectinib for patients with NTRK fusion | ORR | NCT02465060 |
| Study | Phase | Treatment | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - 1 | II | palliative | CIK | No treatment | RFS | NCT02856815 |
| - 2 | II | - | TACE + CIK | TACE + follow-up | PFS | NCT01821482 |
| - 1 | II/III | BCLC A/B | MWA + CIK | MWA | survival | NCT02851784 |
| - 2 | II | palliative | TACE + CIK | TACE | OS | NCT02487017 |
| - 2 | III | BCLC B | TACE + CIK | TACE | - | NCT02568748 |
| - 2 | III | BCLC C/B | CIK | Best supportive care | - | NCT02568748 |
| - 2 | I/II | palliative | CIK + PD-1 Inhibitor | - | PFS | NCT02886897 |
| Active, recruiting | I | palliative | CIK | - | safety | NCT04282044 |
| Study | Phase | Setting | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|
| - 2 | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT04121273 |
| - 1 | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT02905188 |
| - 1 | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT03884751 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT04951141 |
| - 2 | I/II | palliative | GPC3-targeted CAR-T Cell | safety | NCT03084380 |
| - 2 | I/II | palliative | GPC3-targeted CAR-T Cell applied by transarterial infusion | safety | NCT02715362 |
| - 2 | I/II | palliative | GPC3-targeted CAR-T Cell applied by intratumor injection | safety | NCT03130712 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT03198546 |
| - | - | palliative | GPC3-targeted CAR-T Cell | safety | NCT03302403 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT04506983 |
| - | - | palliative | GPC3-targeted CAR-T Cell | safety | NCT05620706 |
| - | - | palliative | GPC3-targeted CAR-T Cell | safety | NCT05926726 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT05344664 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT05003895 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT05783570 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT05070156 |
| - | I/II | palliative | GPC3-targeted CAR-T Cell | safety | NCT05120271 |
| - | I | palliative | GPC3-targeted CAR-T Cell | safety | NCT05155189 |
| - | I/II | palliative | GPC3-targeted CAR-T Cell | safety | NCT05652920 |
| ATHENA | I/II | palliative | GPC3-targeted CAR-T Cell | safety | NCT06084884 |
| - 2 | I | palliative | c-Met/PD-L1 CAR-T Cell | OS | NCT03672305 |
| - 2 | I | palliative | NKG2D CAR-T cells | safety | NCT04550663 |
| - | I | palliative | NKG2D CAR T-cells | safety | NCT05131763 |
| - 2 | I/II | palliative | multi-target CAR-T cells | safety | NCT03638206 |
| - 2 | I/II | palliative | multi-target CAR-T cells | safety | NCT03941626 |
| - 2 | I | palliative | CD147 CAR-T cells applied by hepatic artery infusion | safety | NCT03993743 |
| - 2 | I/II | palliative | MUC1 CAR-T cells | safety | NCT02587689 |
| - 2 | I/II | palliative | EpCAM CAR-T cells | safety | NCT03013712 |
| - 2 | - | palliative | EpCAM CAR-T cells | DCR | NCT02729493 |
| - | I | palliative | EpCAM CAR-T cells | safety | NCT05028933 |
| - | I/II | palliative | B7/H3 CAR-T cells | safety, ORR | NCT05323201 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | I | palliative | Nivo + Ipi + Peptide Vaccine against DNAJB1-PRKACA Fusion Kinase | - | Safety, immune response | NCT04248569 |
| Fusion VAC22 | I | palliative | A + Peptide Vaccine against DNAJB1-PRKACA Fusion Kinase | - | Safety, immune response | NCT05937295 |
| - | I | palliative | Pembro + Peptide Vaccine against P53 | - | Safety | NCT02432963 |
| - | I/II | palliative | Peptide Vaccine against HER-2/neu | - | Safety | NCT04246671 |
| - | I/II | palliative | GNOS-PV02 Personalized Neoantigen Vaccine + Pembro + Plasmid-encoded IL-12 | - | Safety, immune response | NCT04251117 |
| - | - | palliative | mRNA Vaccine (ABOR2014/IPM511) | - | Safety | NCT05981066 |
| TERTIO | II | palliative | A + B + Anti-telomerase Vaccine | A + B | ORR | NCT05528952 |
| PNeoVCA | I | palliative | Pembro + Neoantigen Peptide Vaccine | - | Safety | NCT05269381 |
| - | II | adjuvant | Nivo + Neoantigen Dendritic Cell Vaccine | - | RFS, immune response | NCT04912765 |
| - 2 | I | BCLC B | MWA + Neoantigen-based Dendritic Cell Vaccine | MWA | safety | NCT03674073 |
| - 2 | I | adjuvant | Neoantigen-primed DC Vaccine | - | DFS | NCT04147078 |
| - 2 | II | - | Autologous Dendritic Cell Vaccine + Surgery or TACE or Len/Sor | Surgery or TACE or Len/Sor | PFS | NCT04317248 |
| - 2 | I | palliative | Oncolytic Virus M1 (M1-c6v1) + Apatinib + SHR-1210 (Anti-PD-1) | - | Safety | NCT04665362 |
| - | II | palliative | VG161 (Oncolytic Virus) | VG161 + Nivo | Safety, ORR, PFS | NCT05223816 |
| - | I | palliative | Synov1.1 (Oncolytic Virus) | - | Safety, response | NCT04612504 |
| - | II | palliative | RP3 (Oncolytic Virus) + A + B | - | ORR | NCT05733598 |
| - | I | palliative | Oncorine (Oncolytic Virus) + Len + Tis | - | safety | NCT05675462 |
| Study | Phase | Setting | Target | Treatment | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | II | palliative | IL-8 | BMS-986253 | ORR | NCT04050462 |
| - | II | palliative | IL-27 | Atezolizumab + Bevacizumab + SRF388 | Safety, PFS | NCT05359861 |
| - | I | palliative | IL-27 | Pembrolizumab/SRF388 | Safety, ORR | NCT04374877 |
| KEYNOTE-D13 | II | palliative | IL-15 | Pembrolizumab/SOT101 | ORR | NCT05256381 |
| - | I | palliative | OX40 | HFB301001 | Safety | NCT05229601 |
| GDFATHER | II | palliative | GDF-15 | Visugromab | Safety, response | NCT04725474 |
| Study | Phase | Setting | Treatment Arm A | Treatment Arm B | Primary Endpoint | NCT |
|---|---|---|---|---|---|---|
| - | II | adjuvant post LTx | Sor | placebo | 1-year RFS | NCT06041490 |
| - | II | LLTHVV | Len | - | 3-year RFS | NCT04319484 |
| - | - | LDLT | Len | - | recurrence rate | NCT05572528 |
| CheckMate 9DX | III | adjuvant | Nivo | placebo | RFS | NCT03383458 |
| KEYNOTE-937 | III | adjuvant | Pembro | placebo | RFS + OS | NCT03867084 |
| - | I | HCC after liver transplantation | Cam | - | ORR | NCT04564313 |
| JUPITER 04 | II/III | adjuvant (resection) | Tor | placebo | RFS | NCT03859128 |
| EMERALD-2 | III | adjuvant | Dur + B | Dur + placebo (Arm C: placebo) | RFS | NCT03847428 |
| PREVENT-2 | III | adjuvant | Tis + Len | Tis | RFS | NCT05910970 |
| CISLD-8 | I | adjuvant | Dona + PD-1 | - | RFS | NCT04418401 |
| NEOTOMA | II | adjuvant | Dur plus Tre | - | safety | NCT05440864 |
| DaDaLi | III | adjuvant | Sinti + B | - | RFS | NCT04682210 |
| - | II | adjuvant | Tis + Sitravantinib | - | RFS | NCT05407519 |
| - | II | adjuvant | Dona + Tis | - | RFS | NCT05545124 |
| - | II | adjuvant | Cam + Apa | Cam | DFS | NCT05367687 |
| - | III | adjuvant | Cam + Apa | placebo | DFS | NCT04639180 |
| - | II | adjuvant | Tor | placebo | DFS | NCT05240404 |
| - | I/II | adjuvant | TACE | Len + TACE | Safety, RFS | NCT04911959 |
| - | II | adjuvant | TACE + Don | - | RFS | NCT05161143 |
| ICMJE A | II | adjuvant | TACE + Tis | - | RFS | NCT04981665 |
| ALTER-H006 | II | adjuvant | TQB2450(PD L-1) + Anlotinib | - | DFS | NCT05111366 |
| - | II | adjuvant | HAIC + Anlotinib + TQB2450 (4 cycles) | HAIC + Anlotinib + TQB2450 (8 cycles) | DFS | NCT05311319 |
| Study | Phase | Setting | Drug | Target | FDA | EMA | NMPA | NCT |
|---|---|---|---|---|---|---|---|---|
| SHARP | III | palliative | Sorafenib | Multi-TKI | X | X | X | NCT00105443 |
| REFLECT | III | palliative | Lenvatinib | Multi-TKI | X | X | X | NCT01761266 |
| CELESTIAL | III | palliative | Cabozantinib | Multi-TKI | X | X | X | NCT01908426 |
| RESORCE | III | palliative | Regorafenib | Multi-TKI | X | X | N/A | NCT01774344 |
| IMbrave150 | III | palliative | Atezolizumab/Bevacizumab | PD-L1/VEGF | X | X | X | NCT03434379 |
| HIMALAYA | III | palliative | Durvalumab/Tremelimumab | PD-L1/CTLA-4 | X | X | X | NCT03298451 |
| KEYNOTE-224 KEYNOTE-394 | II III | palliative | Pembrolizumab | PD-1 | X | - | X | NCT02702414 NCT03062358 |
| CheckMate 040 | I/II | palliative | Ipilimumab/Nivolumab | CTLA-4/PD-1 | X | - | N/A | NCT01658878 |
| STARTRK-1/2 | I/II | palliative | Entrectinib | NTRK | X | X | N/A | NCT02097810 NCT02568267 |
| NAVIGATE SCOUT | I II | palliative | Larotrectinib | NTRK | X | X | N/A | NCT02576431 NCT02637687 |
| - | II/III | palliative | Donafenib | Multi-TKI | - | - | X | NCT02645981 |
| RESCUE CARES-310 | II III | palliative | Camrelizumab/Apatinib | PD-L1/VEGF | - | - | X | NCT03463876 NCT03764293 |
| ORIENT-32 | II/III | palliative | Sintilimab/IBI305 | PD-1/VEGF | - | - | X | NCT03794440 |
| RATIONALE-301 | III | palliative | Tislelizumab | PD-1 | - | - | X | NCT03419897 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heumann, P.; Albert, A.; Gülow, K.; Tümen, D.; Müller, M.; Kandulski, A. Insights in Molecular Therapies for Hepatocellular Carcinoma. Cancers 2024, 16, 1831. https://doi.org/10.3390/cancers16101831
Heumann P, Albert A, Gülow K, Tümen D, Müller M, Kandulski A. Insights in Molecular Therapies for Hepatocellular Carcinoma. Cancers. 2024; 16(10):1831. https://doi.org/10.3390/cancers16101831
Chicago/Turabian StyleHeumann, Philipp, Andreas Albert, Karsten Gülow, Deniz Tümen, Martina Müller, and Arne Kandulski. 2024. "Insights in Molecular Therapies for Hepatocellular Carcinoma" Cancers 16, no. 10: 1831. https://doi.org/10.3390/cancers16101831
APA StyleHeumann, P., Albert, A., Gülow, K., Tümen, D., Müller, M., & Kandulski, A. (2024). Insights in Molecular Therapies for Hepatocellular Carcinoma. Cancers, 16(10), 1831. https://doi.org/10.3390/cancers16101831






