Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
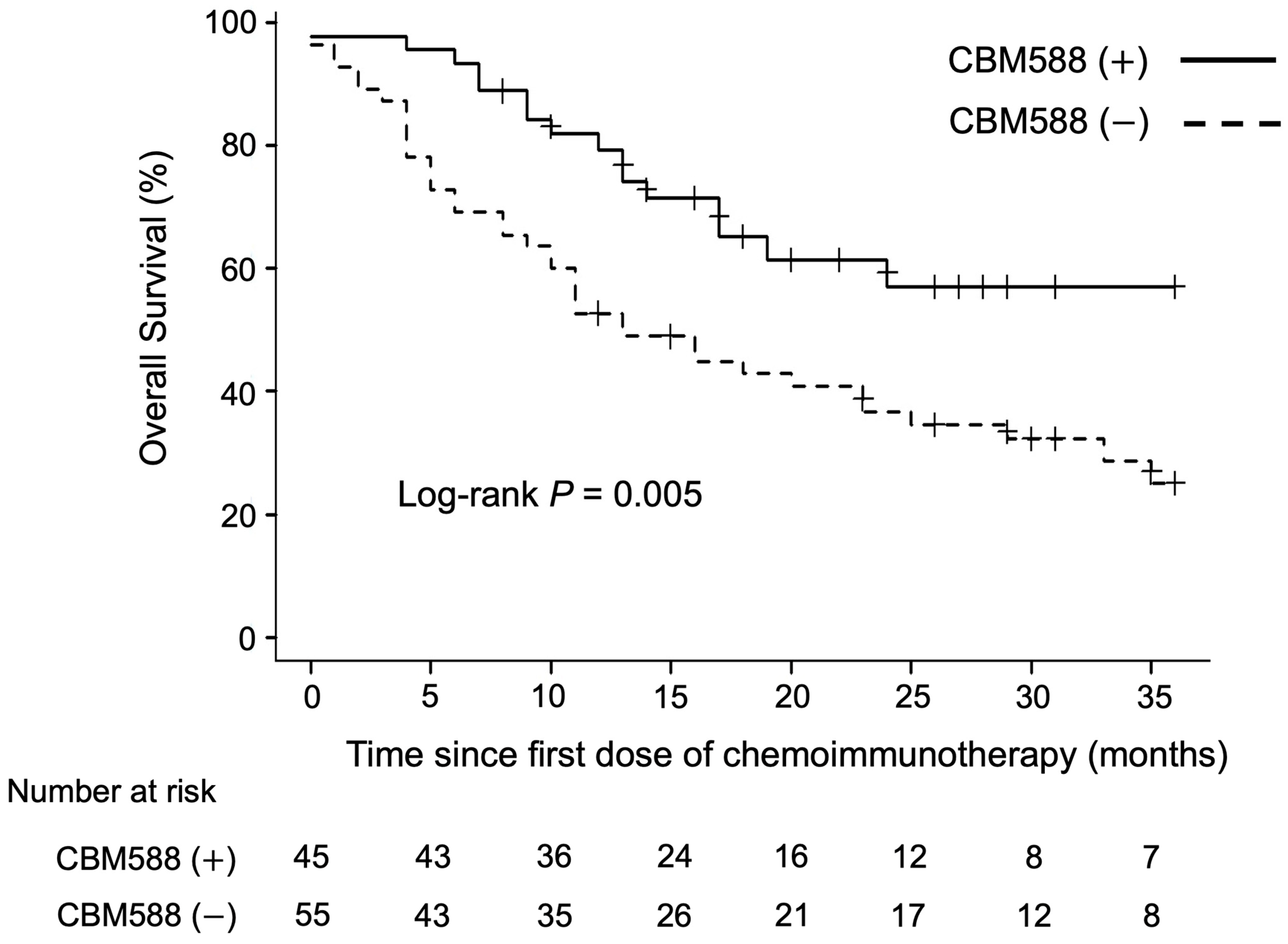
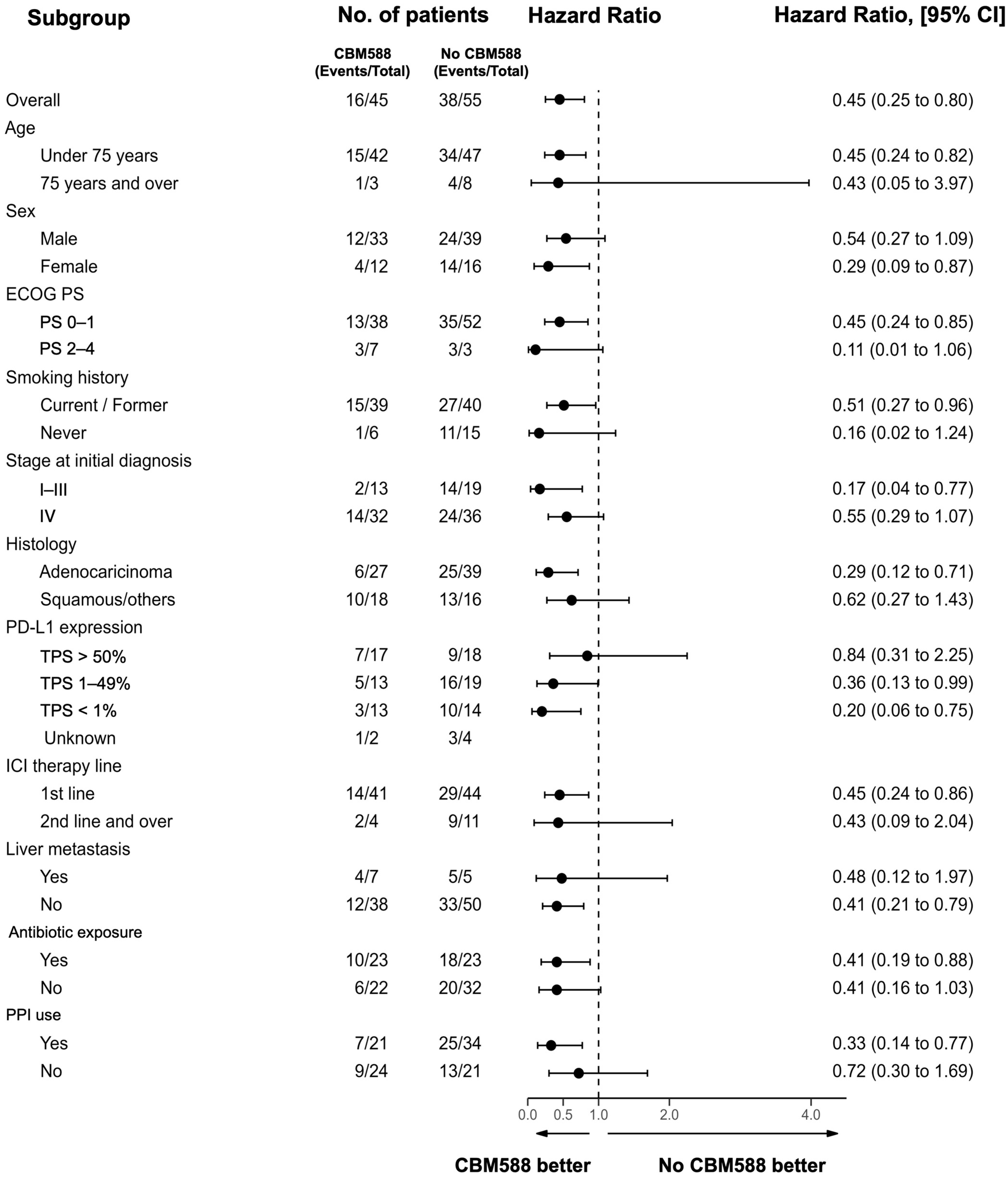
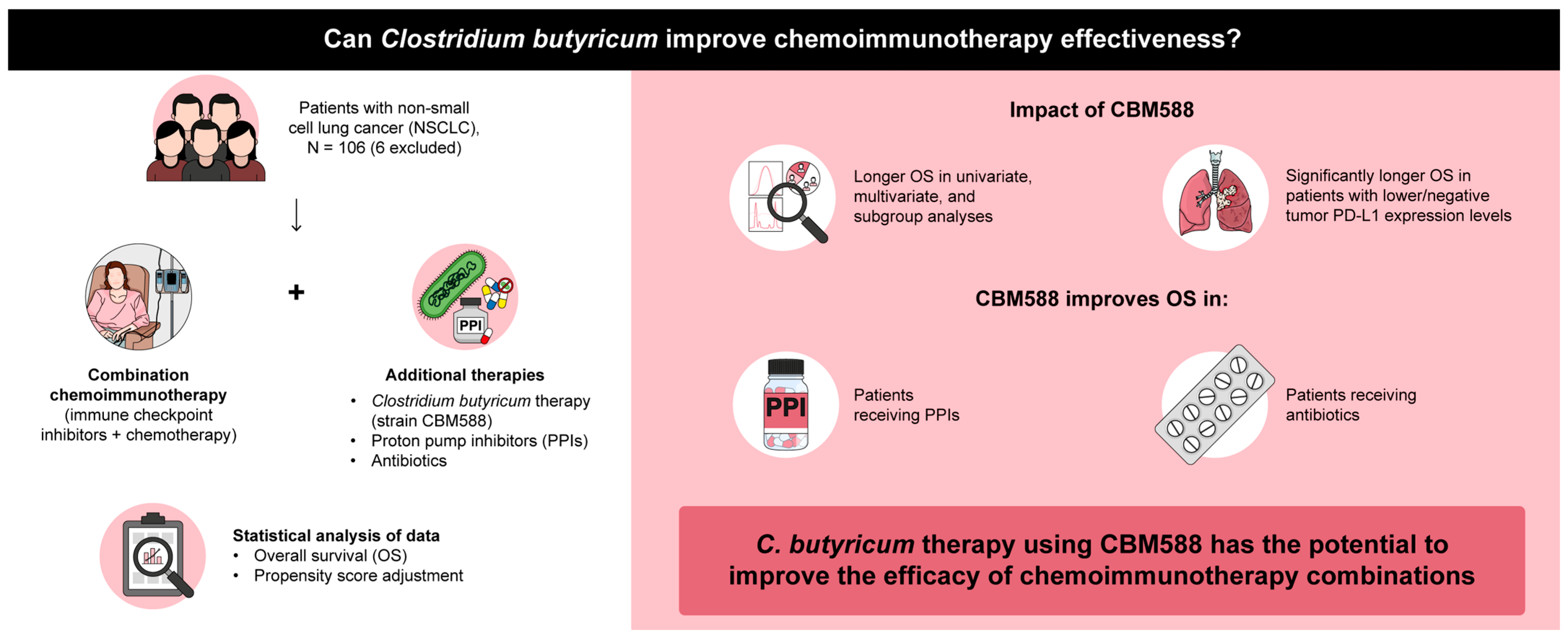
3.2. Impact of CBM588 on the Efficacy of Chemoimmunotherapy Combinations
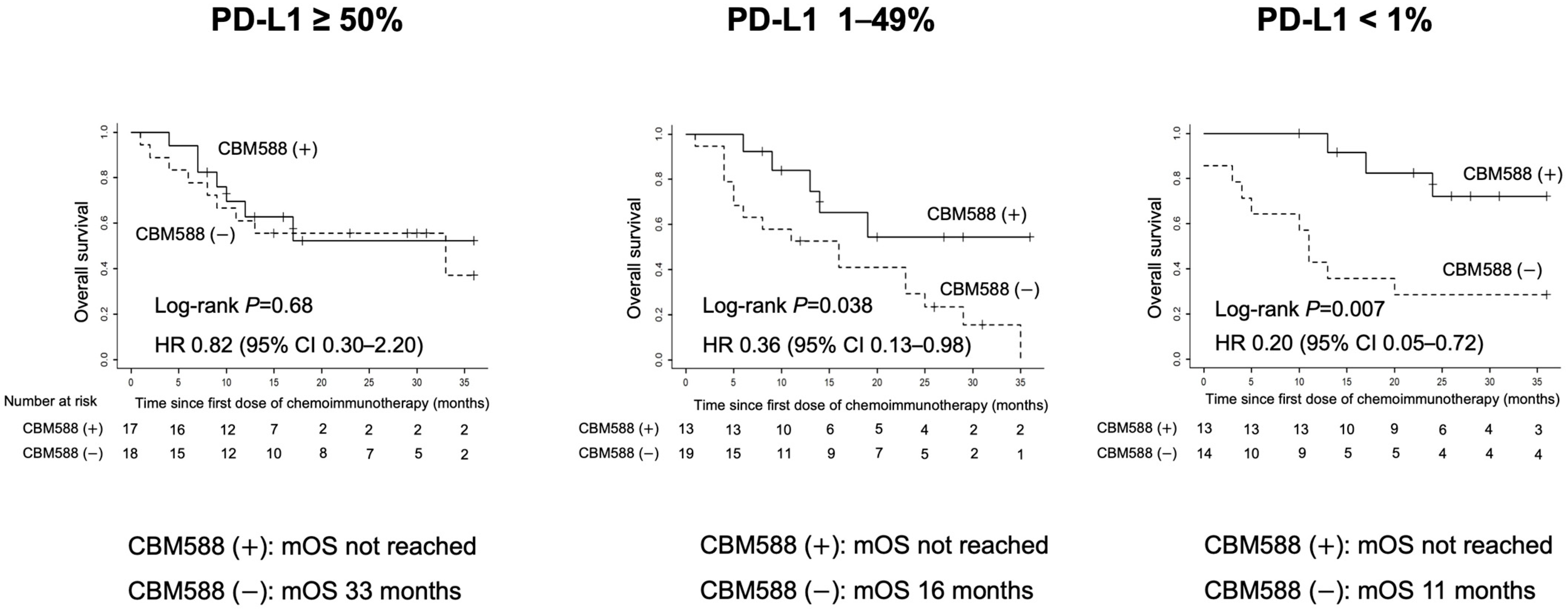
3.3. Impact of CBM588 on Tumor PD-L1 Expression-Based OS
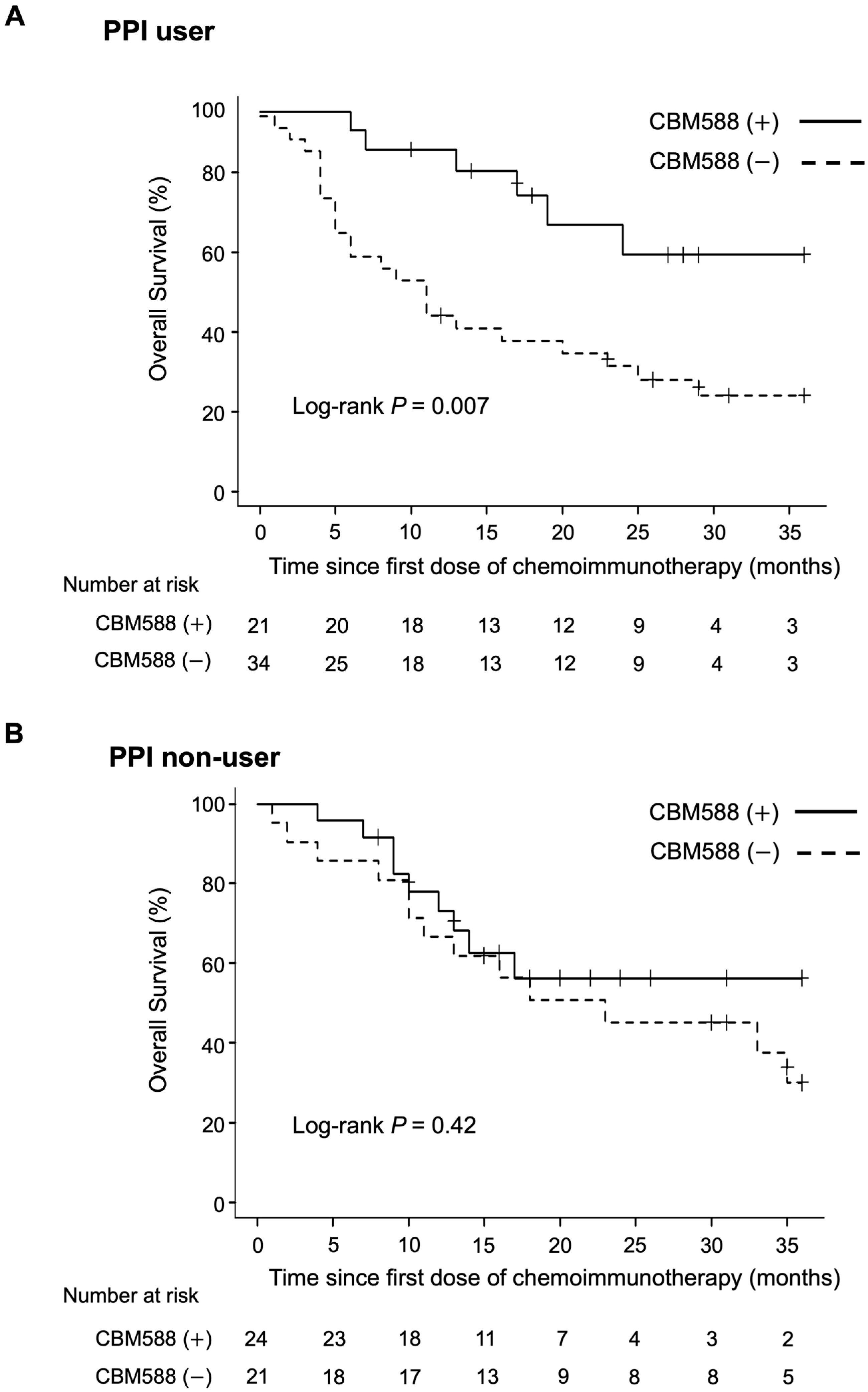
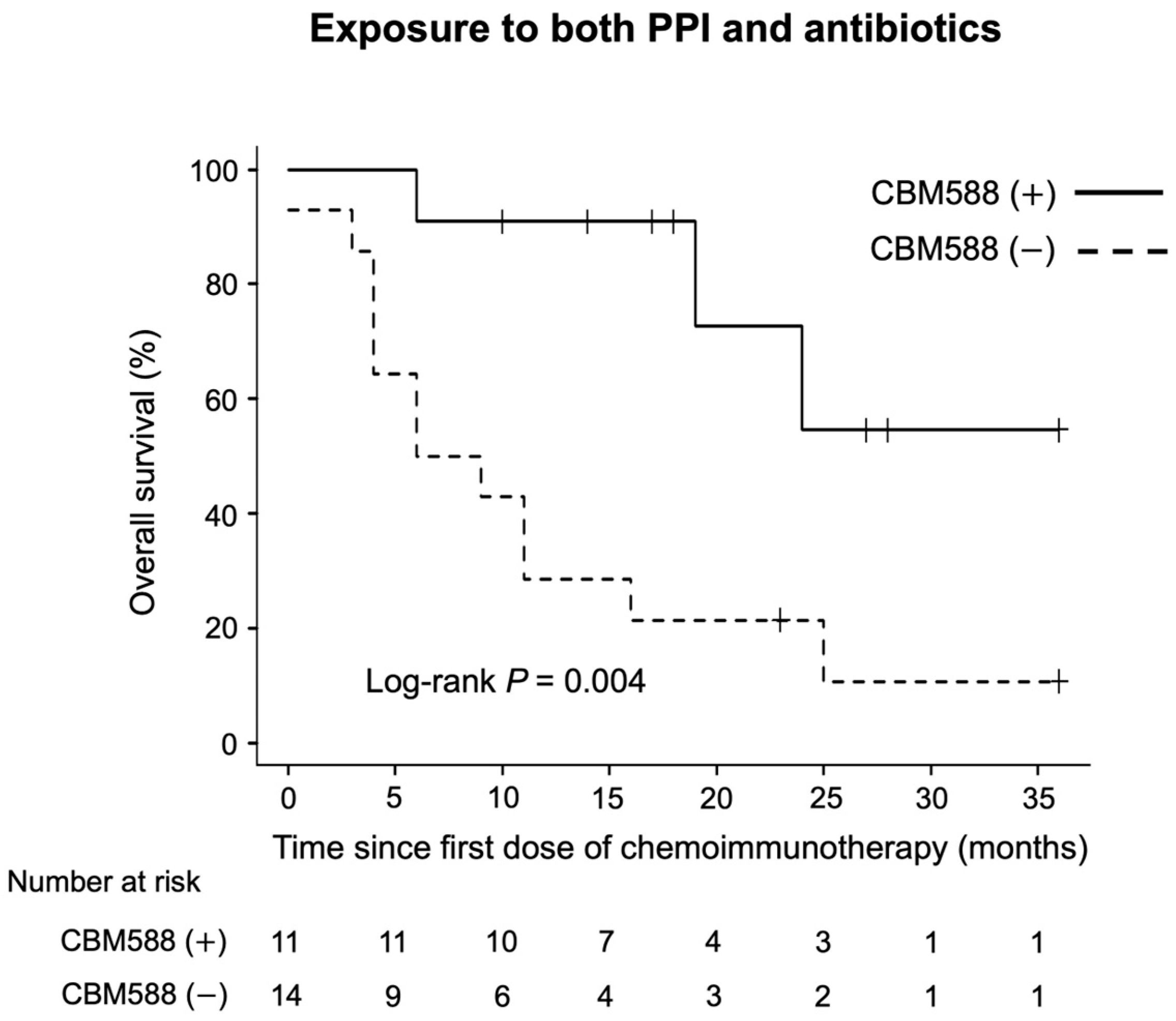
3.4. CBM588 Enhances the Efficacy of Chemoimmunotherapy Combinations in Patients with NSCLC Receiving PPIs
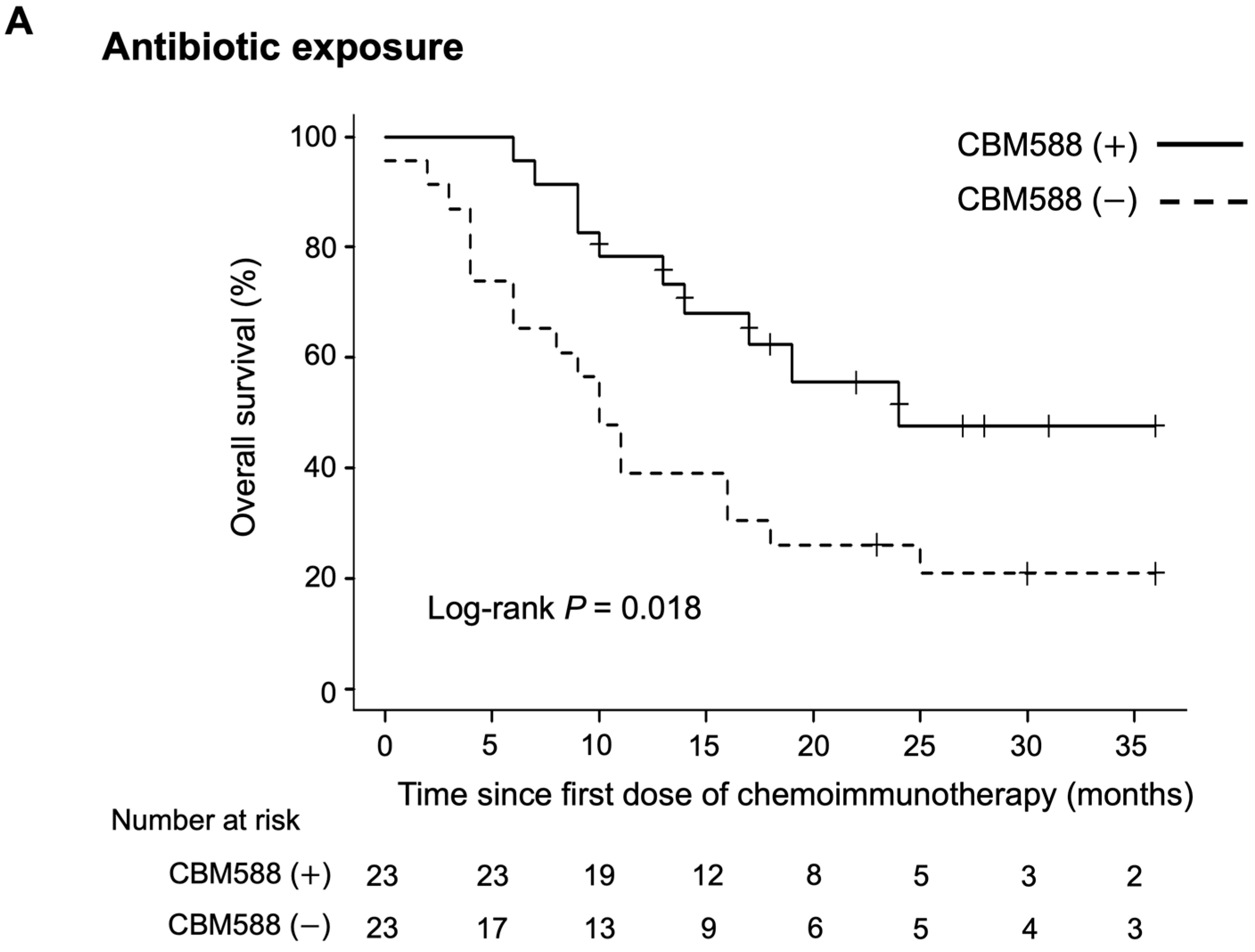
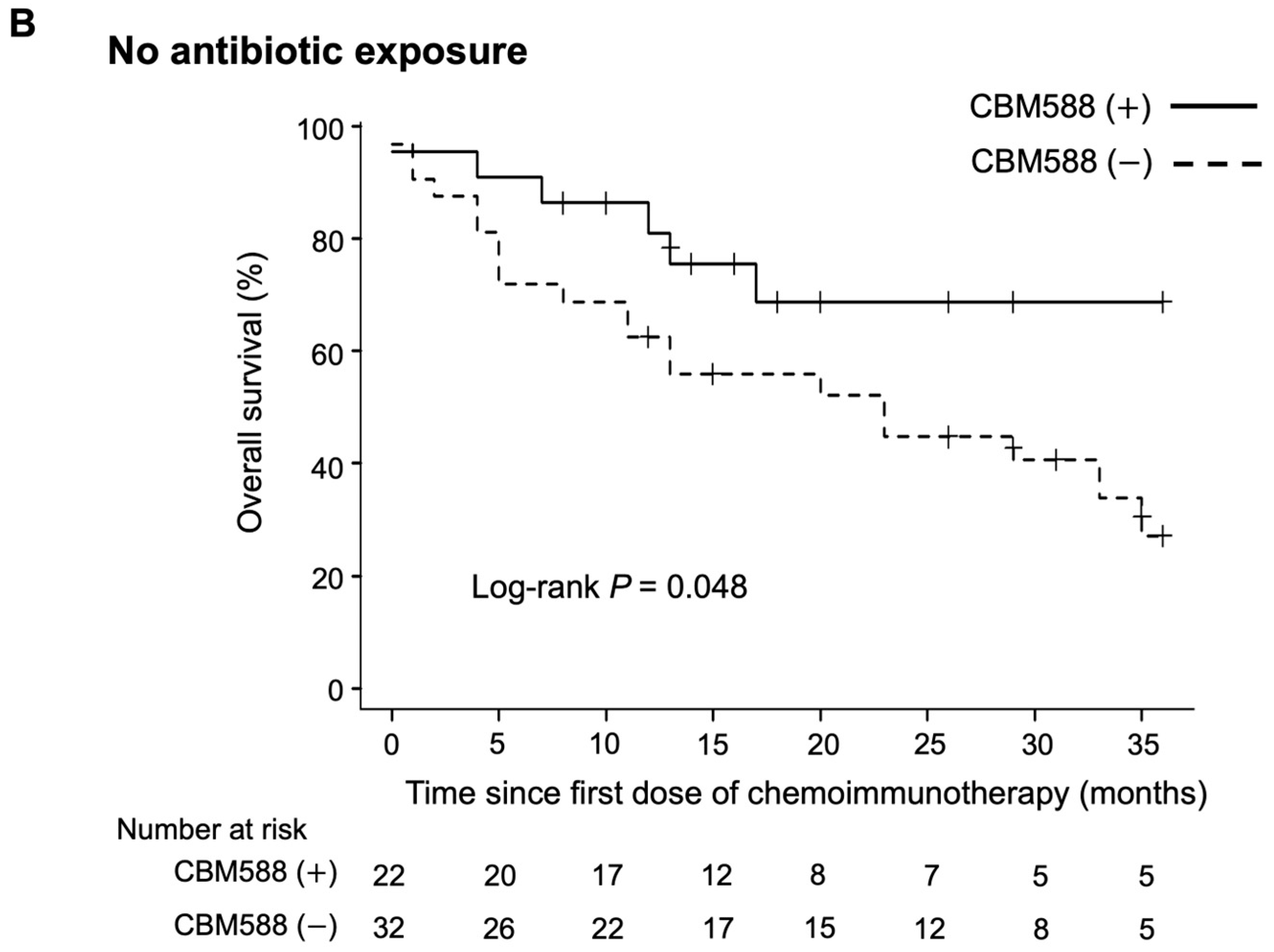
3.5. Impact of CBM588 on Survival Outcomes in Patients with NSCLC Receiving Chemoimmunotherapy Combinations Alongside Antibiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Remon, J.; Hellmann, M.D. First-line immunotherapy for non-small-cell lung cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Reck, M.; Smit, E.F.; Mok, T.; Hellmann, M.D. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann. Oncol. 2019, 30, 884–896. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Desilets, A.; Daillere, R.; Terrisse, S.; Kroemer, G.; Zitvogel, L. Microbiota-centered interventions: The next breakthrough in immuno-oncology? Cancer Discov. 2021, 11, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kroemer, G. Cross-reactivity between cancer and microbial antigens. Oncoimmunology 2021, 10, 1877416. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal akkermansia muciniphila predicts clinical response to pd-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Fluckiger, A.; Daillere, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.G.; et al. Cross-reactivity between tumor mhc class i-restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Nagata, N.; Nishijima, S.; Miyoshi-Akiyama, T.; Kojima, Y.; Kimura, M.; Aoki, R.; Ohsugi, M.; Ueki, K.; Miki, K.; Iwata, E.; et al. Population-level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology 2022, 163, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Savage, N. The complex relationship between drugs and the microbiome. Nature 2020, 577, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Kichenadasse, G.; McKinnon, R.A.; Abuhelwa, A.Y.; Logan, J.M.; Badaoui, S.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of impower150. Br. J. Cancer 2022, 126, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 2020, 26, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaifaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Cardona, A.; Nagarkar, D.R.; Scala, A.D.; Gandara, D.R.; Rittmeyer, A.; Albert, M.L.; Powles, T.; Kok, M.; Herrera, F.G.; et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the oak and poplar trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef]
- Tomita, Y.; Goto, Y.; Sakata, S.; Imamura, K.; Minemura, A.; Oka, K.; Hayashi, A.; Jodai, T.; Akaike, K.; Anai, M.; et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology 2022, 11, 2081010. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-pd-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol. Res. 2020, 8, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Ricciuti, B.; Facchinetti, F.; Alessi, J.V.M.; Venkatraman, D.; Dall, F.G.; Cravero, P.; Vaz, V.R.; Ottaviani, D.; Majem, M.; et al. Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann. Oncol. 2021, 32, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.; Sutradhar, R.; Niu, Y.; Liu, N.; Liu, Y.; Kaliwal, Y.; Powis, M.L.; Liu, G.; Peppercorn, J.M.; Bedard, P.L.; et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: A population-based study. J. Clin. Oncol. 2023, 41, 3122–3134. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Badaoui, S.; Kichenadasse, G.; Karapetis, C.S.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Efficacy of atezolizumab in patients with advanced nsclc receiving concomitant antibiotic or proton pump inhibitor treatment: Pooled analysis of five randomized control trials. J. Thorac. Oncol. 2022, 17, 758–767. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-pd-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-pd-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of pd-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.L.; van de Haar, J.; Veninga, V.; Chalabi, M.; Ijsselsteijn, M.E.; van der Ploeg, M.; van den Bulk, J.; Ruano, D.; van den Berg, J.G.; Haanen, J.B.; et al. Gammadelta t cells are effectors of immunotherapy in cancers with hla class i defects. Nature 2023, 613, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dahling, S.; Kastenmuller, W.; et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated cd8(+) t cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic cd8(+) t cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-pd-l1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M.; Yamada, T.; Miki, T.; Sakai, T. Clostridium butyricum miyairi 588 shows antitumor effects by enhancing the release of trail from neutrophils through mmp-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef] [PubMed]
| Total n = 100 | CBM588 n = 45 | No CBM588 n = 55 | p-Value (CBM588 vs. No CBM588) | |
|---|---|---|---|---|
| Median age (IQR) | 67.0 (63.0–71.0) | 67.0 (64.5–70.5) | 66.0 (61.0–72.0) | 0.57 |
| Sex, n (%) | ||||
| Male | 72 (72) | 33 (73) | 39 (71) | 0.83 |
| Female | 28 (28) | 12 (27) | 16 (29) | |
| ECOG performance status, n (%) | ||||
| 0–1 | 90 (90) | 38 (84) | 52 (95) | 0.11 |
| 2–4 | 10 (10) | 7 (16) | 3 (5) | |
| Smoking history, n (%) | ||||
| Current/Former | 79 (79) | 39 (87) | 40 (73) | 0.14 |
| Never | 21 (21) | 6 (13) | 15 (27) | |
| Stage at initial diagnosis, n (%) | ||||
| I–III | 32 (32) | 13 (29) | 19 (35) | 0.67 |
| IV | 68 (68) | 32 (71) | 36 (65) | |
| Histology, n (%) | ||||
| Adenocarcinoma | 66 (66) | 27 (60) | 39 (71) | 0.29 |
| Squamous/others | 34 (34) | 18 (12/6, 40%) | 16 (12/4, 29%) | |
| EGFR/ALK mutation status, n (%) | ||||
| Wild-type | 73 (73) | 34 (76) | 39 (71) | 0.015 |
| Mutant | 11 (11) | 1 (2) | 10 (18) | |
| Unknown | 16 (16) | 10 (22) | 6 (11) | |
| PD-L1 status, n (%) | ||||
| TPS ≥ 50% | 35 (35) | 17 (38) | 18 (33) | 0.85 |
| TPS 1–49% | 32 (32) | 13 (29) | 19 (35) | |
| TPS < 1% | 27 (27) | 13 (29) | 14 (25) | |
| Unknown/Undeterminable | 6 (6) | 2 (4) | 4 (7) | |
| Therapy line, n (%) | ||||
| 1st line | 85 (85) | 41 (91) | 44 (80) | 0.16 |
| ≥2nd line | 15 (15) | 4 (9) | 11 (20) | |
| Immune checkpoint inhibitor, n (%) | ||||
| Pembrolizumab | 75 (75) | 33 (73) | 42 (76.4) | 0.08 |
| Atezolizumab | 15 (15) | 5 (11) | 10 (18.2) | |
| Nivolumab + Ipilimumab | 10 (10) | 7 (16) | 3 (5.4) | |
| Liver metastasis, n (%) | ||||
| Yes | 12 (12) | 7 (16) | 5 (9) | 0.37 |
| No | 88 (88) | 38 (84) | 50 (91) | |
| Antibiotic use, n (%) | ||||
| Yes | 46 (46) | 23 (51) | 23 (42) | 0.42 |
| No | 53 (54) | 22 (49) | 32 (58) | |
| PPI use, n (%) | ||||
| Yes | 55 (55) | 21 (47) | 34 (62) | 0.16 |
| No | 45 (45) | 24 (53) | 21 (38) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| CBM588 | ||||
| No | Reference | Reference | ||
| Yes | 0.62 (0.39–0.97) | 0.036 | 0.55 (0.34–0.87) | 0.011 |
| Age (years) | 1.00 (0.97–1.03) | 0.83 | ||
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.59 (0.36–0.95) | 0.030 | 0.54 (0.33–0.88) | 0.013 |
| ECOG performance status | ||||
| 0–1 | Reference | |||
| 2–4 | 1.83 (0.94–3.55) | 0.08 | ||
| Smoking history | ||||
| Current/Former | 0.75 (0.44–1.26) | 0.28 | ||
| Never | Reference | |||
| Stage at initial diagnosis | ||||
| I–III | Reference | |||
| IV | 1.31 (0.80–2.16) | 0.28 | ||
| Histology | ||||
| Adenocarcinoma | 0.70 (0.44–1.12) | 0.14 | ||
| Squamous/others | Reference | |||
| EGFR/ALK mutation status | ||||
| Wild-type | Reference | |||
| Mutant | 1.96 (0.99–3.88) | 0.05 | ||
| Unknown | 0.87 (0.45–1.66) | 0.67 | ||
| PD-L1 status | ||||
| TPS < 1% | Reference | |||
| TPS 1–49% | 1.13 (0.65–1.98) | 0.66 | ||
| TPS ≥ 50% | 0.70 (0.39–1.27) | 0.25 | ||
| Unknown/Undeterminable | 1.20 (0.45–3.18) | 0.71 | ||
| Therapy line | ||||
| 1st line | Reference | |||
| ≥2nd line | 1.34 (0.74–2.44) | 0.34 | ||
| Liver metastasis | ||||
| Yes | 2.54 (1.32–4.89) | 0.005 | 3.33 (1.69–6.58) | 0.001 |
| No | Reference | Reference | ||
| Antibiotic use | ||||
| Yes | 1.25 (0.80–1.95) | 0.33 | ||
| No | Reference | |||
| PPI use | ||||
| Yes | 1.46 (0.93–2.29) | 0.10 | ||
| No | Reference | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| CBM588 | ||||
| No | Reference | Reference | ||
| Yes | 0.44 (0.25–0.79) | 0.006 | 0.41 (0.22–0.76) | 0.004 |
| Age (years) | 1.00 (0.97–1.04) | 0.94 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.61 (0.35–1.08) | 0.09 | ||
| ECOG performance status | ||||
| 0–1 | Reference | |||
| 2–4 | 1.64 (0.70–3.85) | 0.26 | ||
| Smoking history | ||||
| Current/Former | 0.92 (0.49–1.76) | 0.81 | ||
| Never | Reference | |||
| Stage at initial diagnosis | ||||
| I–III | Reference | |||
| IV | 1.13 (0.63–2.02) | 0.69 | ||
| Histology | ||||
| Adenocarcinoma | 0.53 (0.31–0.92) | 0.024 | 0.32 (0.15–0.69) | 0.003 |
| Squamous/others | Reference | Reference | ||
| EGFR/ALK mutation status | ||||
| Wild-type | Reference | Reference | ||
| Mutant | 2.42 (1.16–5.07) | 0.019 | 2.13 (0.93–4.86) | 0.07 |
| Unknown | 1.24 (0.61–2.51) | 0.55 | 0.52 (0.22–1.26) | 0.15 |
| PD-L1 status | ||||
| TPS < 1% | Reference | |||
| TPS 1–49% | 1.59 (0.79–3.18) | 0.19 | ||
| TPS ≥ 50% | 1.10 (0.53–2.29) | 0.80 | ||
| Unknown/Undeterminable | 1.87 (0.61–5.76) | 0.27 | ||
| Therapy line | ||||
| 1st line | Reference | |||
| ≥2nd line | 1.51 (0.78–2.93) | 0.23 | ||
| Liver metastasis | ||||
| Yes | 2.77 (1.32–5.82) | 0.007 | 2.02 (0.90–4.53) | 0.09 |
| No | Reference | |||
| Antibiotic use | ||||
| Yes | 1.40 (0.82–2.40) | 0.22 | ||
| No | Reference | |||
| PPI use | ||||
| Yes | 1.30 (0.76–2.24) | 0.34 | ||
| No | Reference | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, Y.; Sakata, S.; Imamura, K.; Iyama, S.; Jodai, T.; Saruwatari, K.; Hamada, S.; Akaike, K.; Anai, M.; Fukusima, K.; et al. Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations. Cancers 2024, 16, 47. https://doi.org/10.3390/cancers16010047
Tomita Y, Sakata S, Imamura K, Iyama S, Jodai T, Saruwatari K, Hamada S, Akaike K, Anai M, Fukusima K, et al. Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations. Cancers. 2024; 16(1):47. https://doi.org/10.3390/cancers16010047
Chicago/Turabian StyleTomita, Yusuke, Shinya Sakata, Kosuke Imamura, Shinji Iyama, Takayuki Jodai, Koichi Saruwatari, Shohei Hamada, Kimitaka Akaike, Moriyasu Anai, Kazuaki Fukusima, and et al. 2024. "Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations" Cancers 16, no. 1: 47. https://doi.org/10.3390/cancers16010047
APA StyleTomita, Y., Sakata, S., Imamura, K., Iyama, S., Jodai, T., Saruwatari, K., Hamada, S., Akaike, K., Anai, M., Fukusima, K., Takaki, A., Tsukamoto, H., Goto, Y., Motozono, C., Sugata, K., Satou, Y., Ueno, T., Ikeda, T., & Sakagami, T. (2024). Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations. Cancers, 16(1), 47. https://doi.org/10.3390/cancers16010047






