Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Classification, Epidemiology and Risk Factors
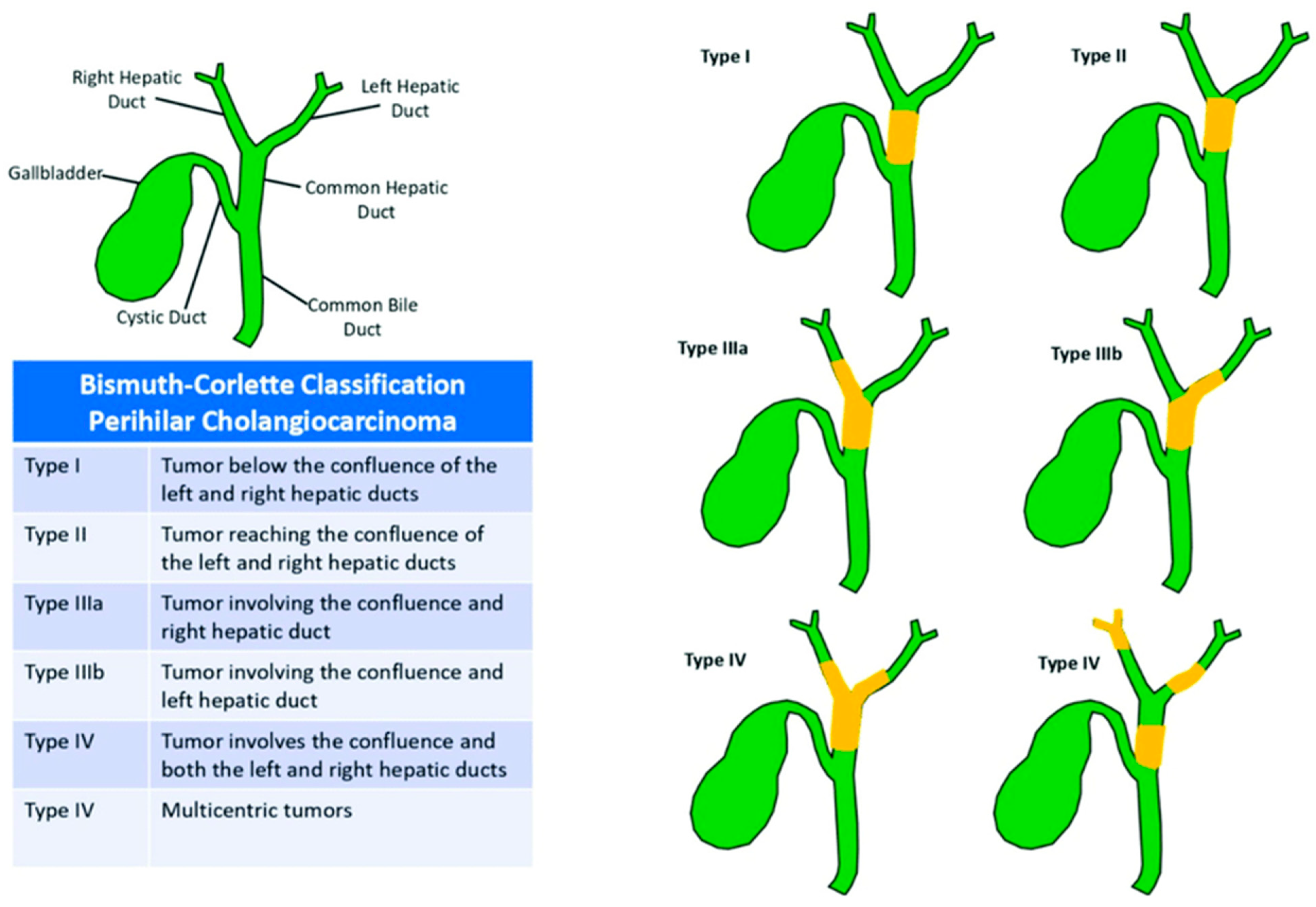
4. Clinical Presentation and Pathology
5. Diagnostic Testing and Staging
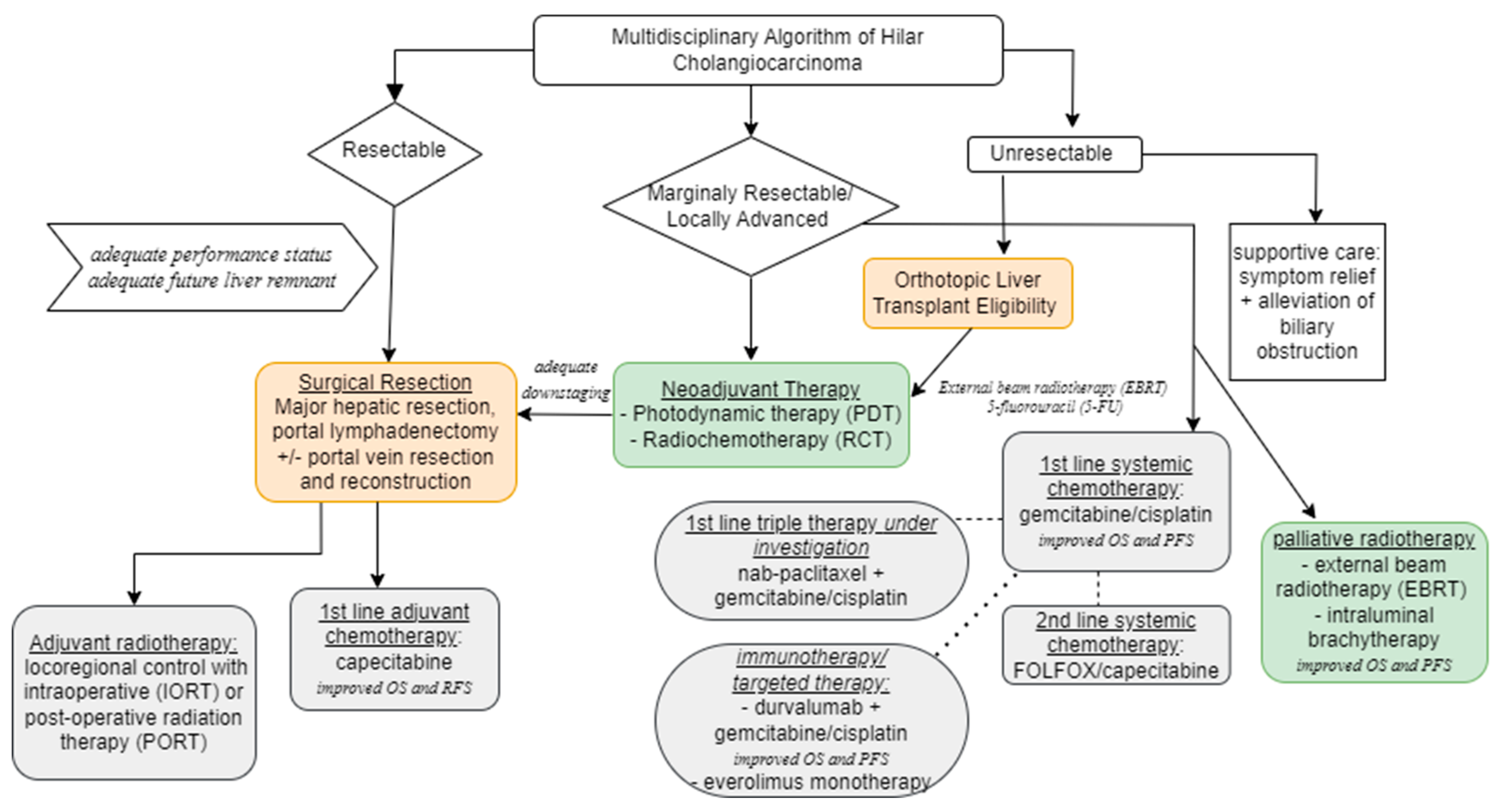
6. Principles of Surgical Resection
7. Orthotopic Liver Transplantation
8. Locoregional Therapy
8.1. Neoadjuvant Radiochemotherapy (RCT) in Resectable Cancer
8.2. Neoadjuvant Photodynamic Therapy
8.3. Radiation in the Adjuvant and Palliative Setting
9. Systemic Therapy
9.1. Adjuvant Chemotherapy
9.2. Systemic Chemotherapy for Locally Advanced or Metastatic HC
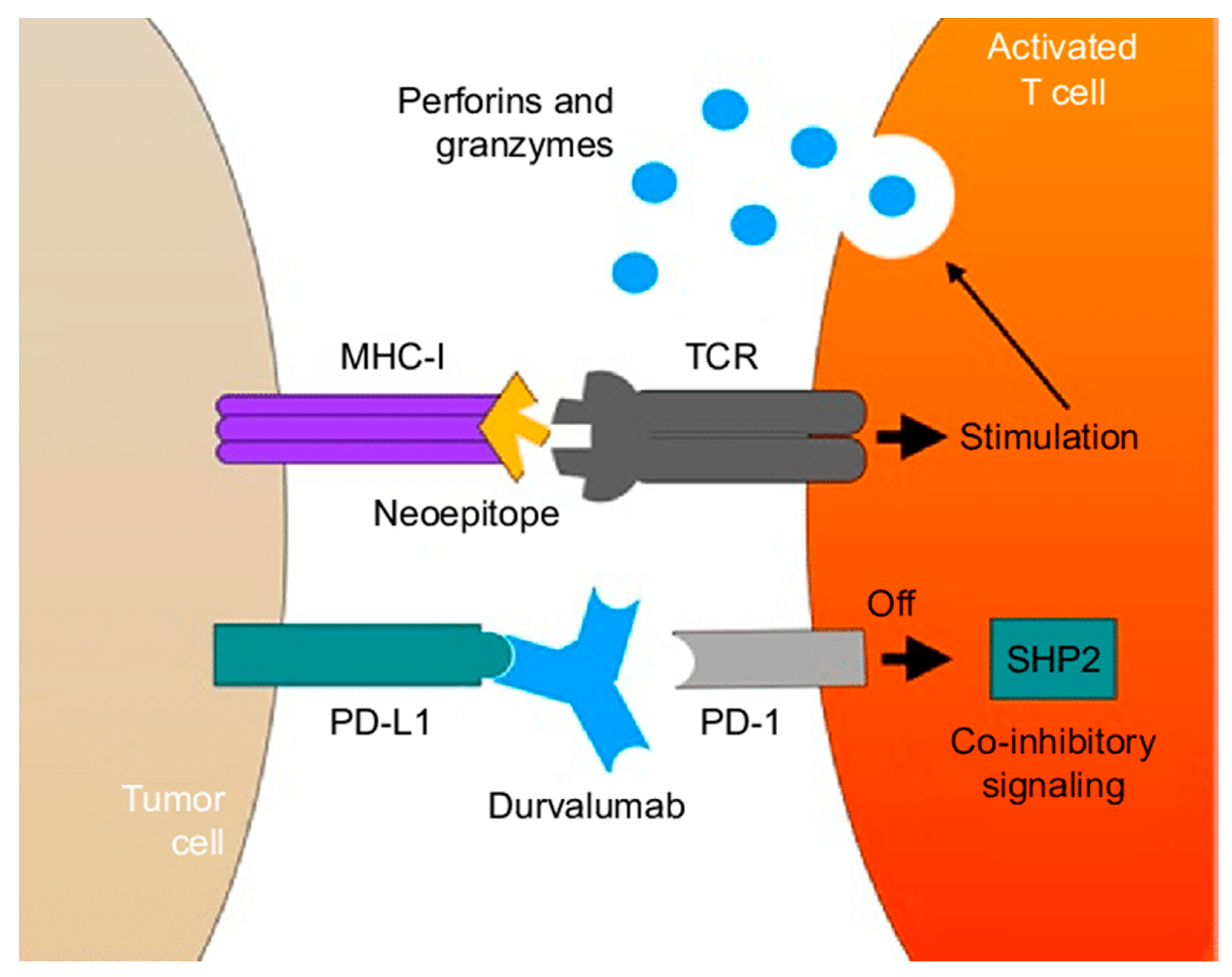
9.3. Immunotherapy
9.4. Targeted Therapy
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.J.; Cosgrove, D.; Herman, J.M.; Rastegar, N.; Kamel, I.; Pawlik, T.M. Multimodal treatment strategies for advanced hilar cholangiocarcinoma. Langenbeck’s Arch. Surg. 2014, 399, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.; Winston, C. Hilar cholangiocarcinoma: Diagnosis and staging. HPB 2005, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, Resectability, and Outcome in 225 Patients with Hilar Cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Beal, E.W.; Chakedis, J.; Chen, Q.; Lv, Y.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J. Surg. 2018, 42, 2919–2929. [Google Scholar] [CrossRef]
- Mizuno, T.; Ebata, T.; Nagino, M. Advanced hilar cholangiocarcinoma: An aggressive surgical approach for the treatment of advanced hilar cholangiocarcinoma: Perioperative management, extended procedures, and multidisciplinary approaches. Surg. Oncol. 2019, 33, 201–206. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Hemming, A.W.M.; Reed, A.I.; Fujita, S.; Foley, D.P.; Howard, R.J. Surgical Management of Hilar Cholangiocarcinoma. Ann. Surg. 2005, 241, 693–702. [Google Scholar] [CrossRef]
- Cannon, R.M.; Brock, G.; Buell, J.F. Surgical resection for hilar cholangiocarcinoma: Experience improves resectability. HPB 2012, 14, 142–149. [Google Scholar] [CrossRef][Green Version]
- National Comprehensive Cancer Network (NCCN). NCCN Clin Pract Guidel Oncol. Available online: https://www.nccn.org/professionals/physician_gls (accessed on 8 November 2023).
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA A Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus state-ment from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Goenka, M.K.; Goenka, U. Palliation: Hilar cholangiocarcinoma. World J. Hepatol. 2014, 6, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Ruff, S.M.; Cloyd, J.M.; Pawlik, T.M. Annals of Surgical Oncology Practice Guidelines Series: Management of Primary Liver and Biliary Tract Cancers. Ann. Surg. Oncol. 2023, 30, 7935–7949. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Pizzo, C.; Botticelli, A.; Hahne, J.C.; Passalacqua, R.; Tomasello, G.; Petrelli, F. Biliary tract cancer: Current challenges and future prospects. Cancer Manag. Res. 2018, 11, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Kamel, I.; Cosgrove, D.P.; Herman, J.M.; Pawlik, T.M. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management. Hepatobiliary Surg. Nutr. 2014, 3, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.-N. Hilar Cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef]
- Aljiffry, M.; Walsh, M.J.; Molinari, M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J. Gastroenterol. 2009, 15, 4240–4262. [Google Scholar] [CrossRef]
- Inchingolo, R.; Acquafredda, F.; Ferraro, V.; Laera, L.; Surico, G.; Surgo, A.; Fiorentino, A.; Marini, S.; De’Angelis, N.; Memeo, R.; et al. Non-surgical treatment of hilar cholangiocarcinoma. World J. Gastrointest. Oncol. 2021, 13, 1696–1708. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uesaka, K.; Kakuda, Y.; Sugino, T.; Kubota, K.; Furukawa, T.; Fukumura, Y.; Isayama, H.; Terada, T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J. Clin. Med. 2020, 9, 3991. [Google Scholar] [CrossRef]
- Castellano-Megías, V.M. Pathological aspects of so called “hilar cholangiocarcinoma”. World J. Gastrointest. Oncol. 2013, 5, 159. [Google Scholar] [CrossRef]
- Bickenbach, K.; Galka, E.; Roggin, K.K. Molecular Mechanisms of Cholangiocarcinogenesis: Are Biliary Intraepithelial Neoplasia and Intraductal Papillary Neoplasms of the Bile Duct Precursors to Cholangiocarcinoma? Surg. Oncol. Clin. N. Am. 2009, 18, 215–224. [Google Scholar] [CrossRef]
- Bosman, F.T. WHO Classification of Tumours of the Digestive System, 4th ed.; World Health Organization, International Agency for Research on Cancer, Eds.; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Hucl, T. Precursors to Cholangiocarcinoma. Gastroenterol. Res. Pract. 2019, 2019, 1389289. [Google Scholar] [CrossRef]
- Shin, D.W.; Moon, S.-H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Mocan, T.; Horhat, A.; Mois, E.; Graur, F.; Tefas, C.; Craciun, R.; Nenu, I.; Spârchez, M.; Sparchez, Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World J. Gastrointest. Oncol. 2021, 13, 2050–2063. [Google Scholar] [CrossRef]
- Schulick, R. Criteria of unresectability and the decision-making process. HPB 2008, 10, 122–125. [Google Scholar] [CrossRef][Green Version]
- Parikh, A.A.; Abdalla, E.K.; Vauthey, J.-N. Operative considerations in resection of hilar cholangiocarcinoma. HPB 2005, 7, 254–258. [Google Scholar] [CrossRef]
- Lidsky, M.E.; Jarnagin, W.R. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann. Gastroenterol. Surg. 2018, 2, 304–312. [Google Scholar] [CrossRef]
- Ebata, T.; Mizuno, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Nagino, M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br. J. Surg. 2018, 105, 829–838. [Google Scholar] [CrossRef]
- Ersan, V.; Usta, S.; Aydin, C.; Carr, B.I.; Karatoprak, S.; Yilmaz, S. Critical overview of resection for Bismuth-Corlette type IV perihilar cholangiocarcinoma. Acta Chir. Belg. 2023, 123, 489–496. [Google Scholar] [CrossRef]
- Shindoh, J.; Tzeng, C.-W.D.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Wei, S.H.; Huang, S.Y.; Mahvash, A.; Gupta, S.; Wallace, M.J.; et al. Optimal Future Liver Remnant in Patients Treated with Extensive Preoperative Chemotherapy for Colorectal Liver Metastases. Ann. Surg. Oncol. 2013, 20, 2493–2500. [Google Scholar] [CrossRef]
- Thirunavukarasu, P.; Aloia, T.A. Preoperative Assessment and Optimization of the Future Liver Remnant. Surg. Clin. N. Am. 2016, 96, 197–205. [Google Scholar] [CrossRef]
- Zorzi, D.; Laurent, A.; Pawlik, T.M.; Lauwers, G.Y.; Vauthey, J.; Abdalla, E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br. J. Surg. 2007, 94, 274–286. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Diniz, A.L.; Zhu, A.X. Consensus Conference on Hilar Cholangiocarcinoma. HPB 2015, 17, 666–668. [Google Scholar] [CrossRef][Green Version]
- Geers, J.; Jaekers, J.; Topal, H.; Aerts, R.; Vandoren, C.; Boer, G.V.; Topal, B. Predictors of survival after surgery with curative intent for perihilar cholangiocarcinoma. World J. Surg. Oncol. 2020, 18, 286. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.; Klümpen, H.; Malka, D.; Primrose, J.; Rimassa, L.; Stenzinger, A.; Valle, J.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver Transplantation with Neoadjuvant Chemoradiation is More Effective than Resection for Hilar Cholangiocarcinoma. Ann. Surg. 2005, 242, 451–461. [Google Scholar] [CrossRef]
- Bruer, E.; Mueller, M. Liver transplantation as a new standard of care in patients with perihilar cholangiocarcinoma? Results from an international benchmark study. Ann. Surg. 2022, 276, 846–853. [Google Scholar] [CrossRef]
- Machairas, N.; Kostakis, I.D.; Tsilimigras, D.I.; Prodromidou, A.; Moris, D. Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant. Rev. 2020, 34, 100516. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef]
- Murad, S.D.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarci-noma at 12 US centers. Gastroenterology 2012, 143, 88–98. [Google Scholar] [CrossRef]
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–609. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Shima, Y.; Okabayashi, T.; Negoro, Y.; Shimada, Y.; Iwata, J.; Matsumoto, M.; Hata, Y.; Noda, Y.; Sui, K.; et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J. Surg. 2018, 42, 2910–2918. [Google Scholar] [CrossRef]
- Frosio, F.; Mocchegiani, F.; Conte, G.; Bona, E.D.; Vecchi, A.; Nicolini, D.; Vivarelli, M. Neoadjuvant therapy in the treatment of hilar cholangiocarcinoma: Review of the literature. World J. Gastrointest. Surg. 2019, 11, 279–286. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Xiao, H.; Chen, S.; Zhu, W.; Lu, H.; Cao, L.; Xue, P.; Li, H.; Zhang, D. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma. Surg. Endosc. 2021, 35, 5655–5664. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Wiedmann, M.; Caca, K.; Berr, F.; Schiefke, I.; Tannapfel, A.; Wittekind, C.; Mössner, J.; Hauss, J.; Witzigmann, H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma. Cancer 2003, 97, 2783–2790. [Google Scholar] [CrossRef]
- Todoroki, T. Chemotherapy for bile duct carcinoma in the light of adjuvant chemotherapy to surgery. Hepatogastroenterology 2000, 47, 644–649. [Google Scholar]
- Mahadevan, A.; Dagoglu, N.; Mancias, J.; Raven, K.; Khwaja, K.; Tseng, J.F.; Ng, K.; Enzinger, P.; Miksad, R.; Bullock, A.; et al. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J. Cancer 2015, 6, 1099–1104. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Valle, J.W.; Wasan, H.; Johnson, P.; Jones, E.; Dixon, L.; Swindell, R.; Baka, S.; Maraveyas, A.; Corrie, P.; Falk, S.; et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: A multicentre randomised phase II study—The UK ABC-01 Study. Br. J. Cancer 2009, 101, 621–627. [Google Scholar] [CrossRef]
- Shroff, R.T.; A Guthrie, K.; Scott, A.J.; Borad, M.J.; Goff, L.W.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; Aghajanian, C.; et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J. Clin. Oncol. 2023, 41, LBA490. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Shroff, R.T. New Treatment Options for Advanced Biliary Tract Cancer. Curr. Treat. Options Oncol. 2020, 21, 63. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, H.P.; Wasan, H.S. A randomised phase III, multi-centre, open-label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract can-cers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. [abstract ABC-06]. J. Clin. Oncol. 2019, 37, 4003. [Google Scholar]
- Junior, P.L.S.U.; Araujo, R.L. Immunotherapy in biliary tract cancers: Current evidence and future perspectives. World J. Gastrointest. Oncol. 2022, 14, 1446–1455. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Évid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Gray, S.; Lamarca, A.; Edeline, J.; Klümpen, H.-J.; Hubner, R.A.; McNamara, M.G.; Valle, J.W. Targeted Therapies for Perihilar Cholangiocarcinoma. Cancers 2022, 14, 1789. [Google Scholar] [CrossRef]
- Pei, S.-N.; Liao, C.-K.; Chen, Y.-S.; Tseng, C.-H.; Hung, C.-M.; Chiu, C.-C.; Hsieh, M.-C.; Tsai, Y.-F.; Liao, H.-Y.; Liu, W.-C.; et al. A Novel Combination of Bevacizumab with Chemotherapy Improves Therapeutic Effects for Advanced Biliary Tract Cancer: A Retrospective, Observational Study. Cancers 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.M.; Haldenby, S.; Antzcak, P.; Fowler, A.; Bullock, K.; Kenny, J.; Gilbert, T.; Andrews, T.; Diaz-Nieto, R.; Fenwick, S.; et al. Genomic profiling of idiopathic peri-hilar cholangiocarcinoma reveals new targets and mutational pathways. Sci. Rep. 2023, 13, 6681. [Google Scholar] [CrossRef]
- Lau, D.K.; Tay, R.Y.; Yeung, Y.H.; Chionh, F.; Mooi, J.; Murone, C.; Skrinos, E.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: The RADiChol Study. Br. J. Cancer 2018, 118, 966–971. [Google Scholar] [CrossRef]
- Queiroz, M.M.; Lima, N.F.; de Castria, T.B. Immunotherapy and Targeted Therapy for Advanced Biliary Tract Cancer: Adding New Flavors to the Pizza. Cancers 2023, 15, 1970. [Google Scholar] [CrossRef]
| Blood Tests | Imaging | Endoscopy | Tissue Sampling |
|---|---|---|---|
|
|
|
|
Local invasion
|
Inadequate future liver remnant
|
Distant metastatic disease
|
| Medically unfit patients unable to tolerate a major hepatic resection |
| Recommended Treatments | ESMO European Society for Medical Oncology | ENS-CCA European Network for the Study of Cholangiocarcinoma | NCCN National Comprehensive Cancer Network | AHPBA Americas Hepato-Pancreato-Biliary Association | ASCO American Society of Clinical Oncology |
| Neoadjuvant Treatment | - durvalumab + gemcitabine-cisplatin recommended to downstage locally advanced cancers | - recommended in setting of transplantation | - no preferred treatments recommended | - recommending in setting of transplantation | Guidelines address adjuvant treatments for resected malignancies |
| Adjuvant/Locoregional Therapies | - capecitabine - adjuvant radiation considered in limited cases after adjuvant capecitabine in select patients (R1 resection) | - transarterial chemoembolization (TACE) - intraductal radiofrequency ablation (RFA) | - capecitabine - fluoropyridine-based chemoradiotherapy | - chemoradiation recommended for node positive and margin positive disease - role of adjuvant chemotherapy not clearly defined | - capecitabine moderate recommendation for chemoradiation - chemoradiotherapy (R1 resection) |
| Primary Systemic Treatment of Unresectable/Metastatic Disease + Immunotherapy | - durvalumab + gemcitabine-cisplatin - photodynamic therapy(PDT) and intraductal radiofrequency ablation are considered investigational | - Gemcitabine-cisplatin - Capecitabine - Gemcitabine-oxaliplatin - mFOLFOX - Fluorouracil-cisplatin No recommendations for immunotherapy, citing need for additional research | - durvalumab + gemcitabine-cisplatin - pembrolizumab + gemcitabine + cisplatin - PDT | - gemcitabine + cisplatin - recommend definitive chemoradiation | Guidelines address adjuvant treatments for resected malignancies |
| Targeted Therapy | - molecular analysis recommended during first line-therapy for advanced disease - ivosidenib (IDH1 mutations), FGFR inhibitors, pemrolizumab (MSI-H/dMMR), dabrafenib-trametinib (BRAF mutations) recommended, awaiting European Medicines Agency approval | - recommend additional clinical trials; no or only very modest surviual benefits | - useful in certain circumstances - pemrolizumab (panel states that data is limit) - dabrafenib and trametinib - comprehensive molecular profiling recommended in unresectable/metastatic disease | Not commented in guidelines | Guidelines address adjuvant treatments for resected malignancies |
| Transplantation | neoadjuvant chemoradiation followed by orthotopic liver transplantation | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmanaban, V.; Ruff, S.M.; Pawlik, T.M. Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements. Cancers 2024, 16, 30. https://doi.org/10.3390/cancers16010030
Padmanaban V, Ruff SM, Pawlik TM. Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements. Cancers. 2024; 16(1):30. https://doi.org/10.3390/cancers16010030
Chicago/Turabian StylePadmanaban, Vennila, Samantha M. Ruff, and Timothy M. Pawlik. 2024. "Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements" Cancers 16, no. 1: 30. https://doi.org/10.3390/cancers16010030
APA StylePadmanaban, V., Ruff, S. M., & Pawlik, T. M. (2024). Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements. Cancers, 16(1), 30. https://doi.org/10.3390/cancers16010030







