Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. GSCs Derived from GBM Biopsy Specimens
2.2. RNA Sequencing
2.3. Bioinformatic Analysis
2.4. Human XL Proteome Profiler Array (Cytokines Array)
2.5. SiRNA Transfection, RNA Extraction, Reverse Transcription and Real-Time PCR
2.6. Western-Blot Analysis
2.7. GSCs Irradiations
2.8. Neurosphere-Forming Analysis
3. Results
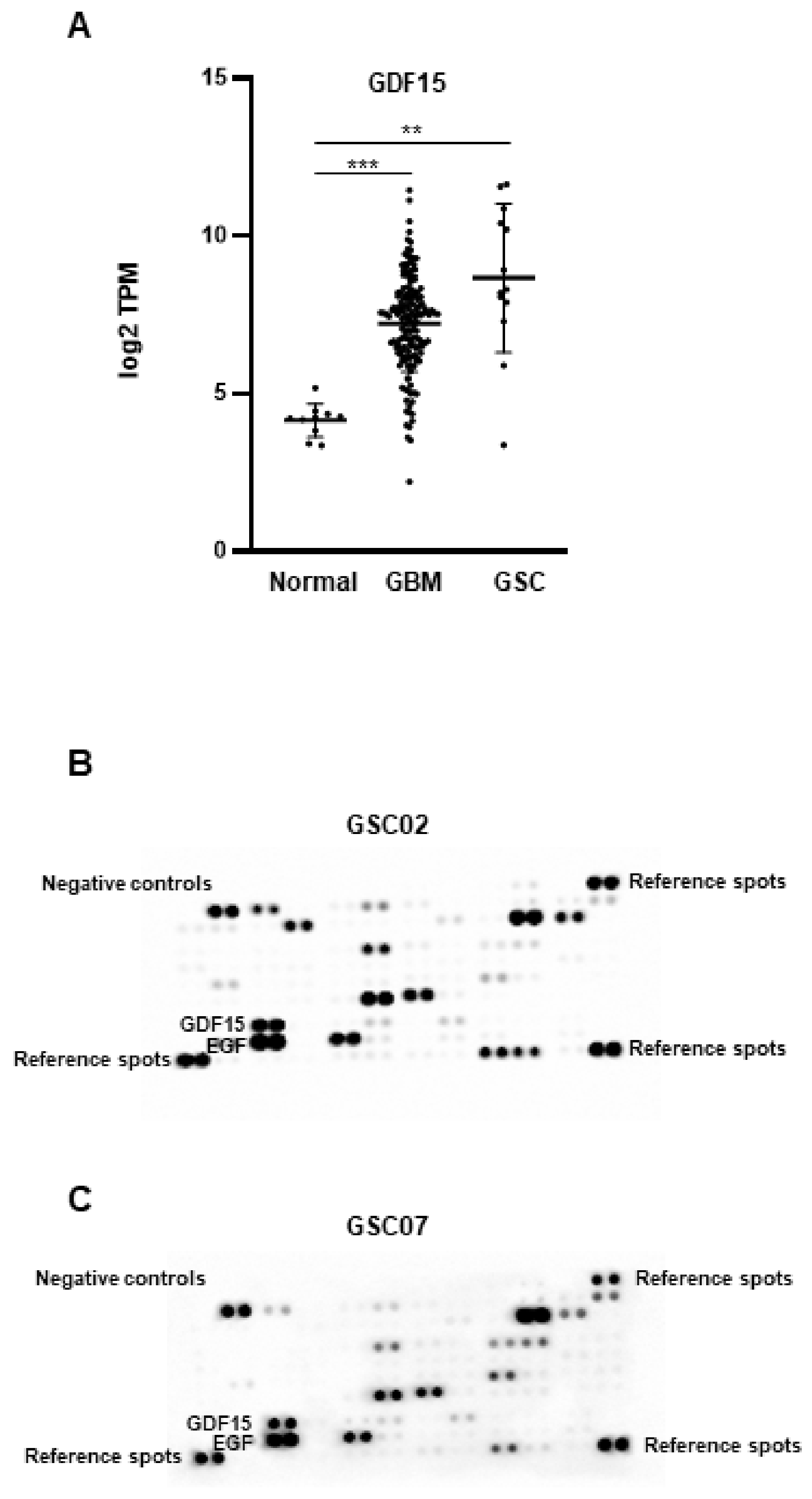
3.1. GDF15 Expression in GBM and GSCs
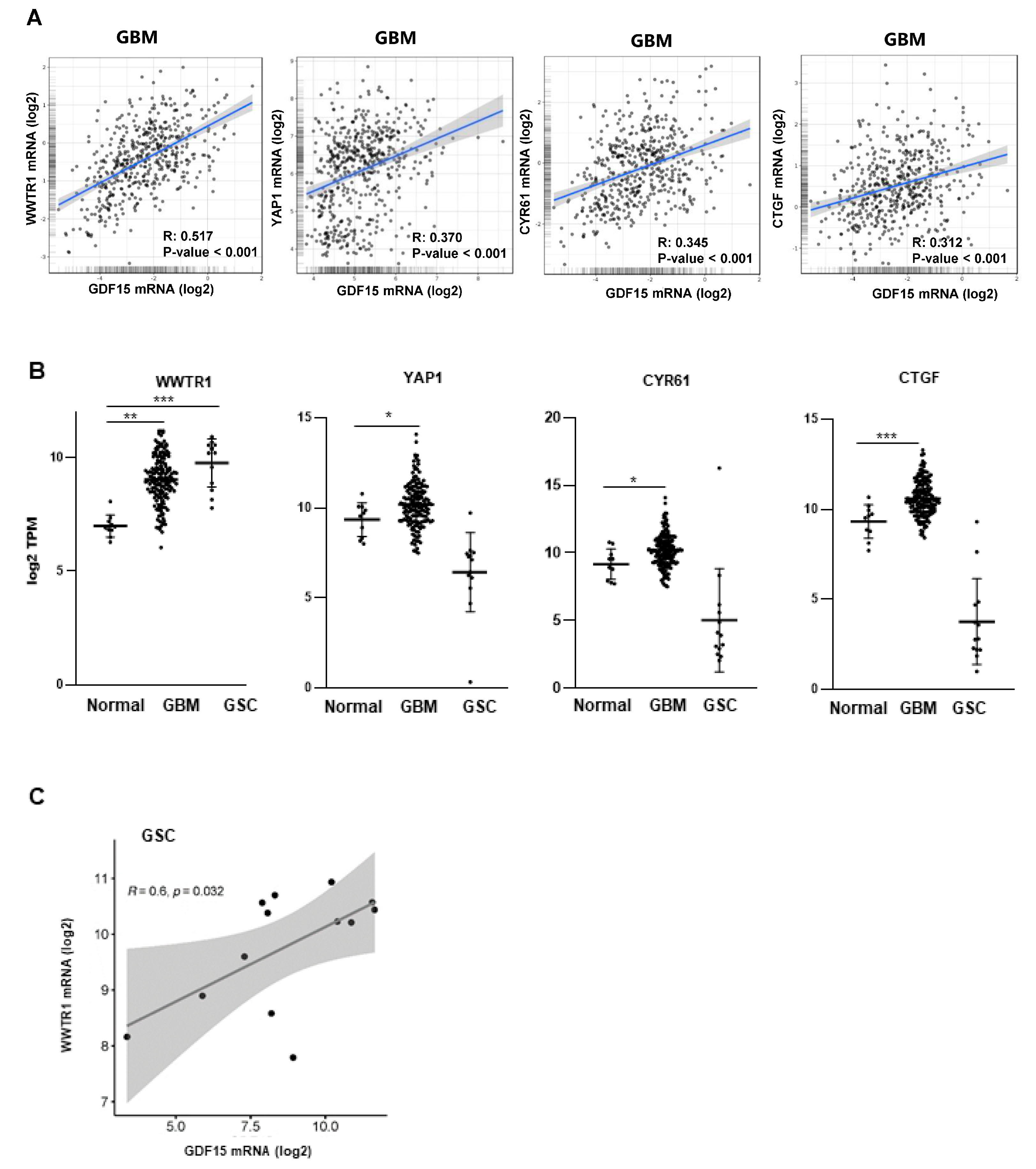
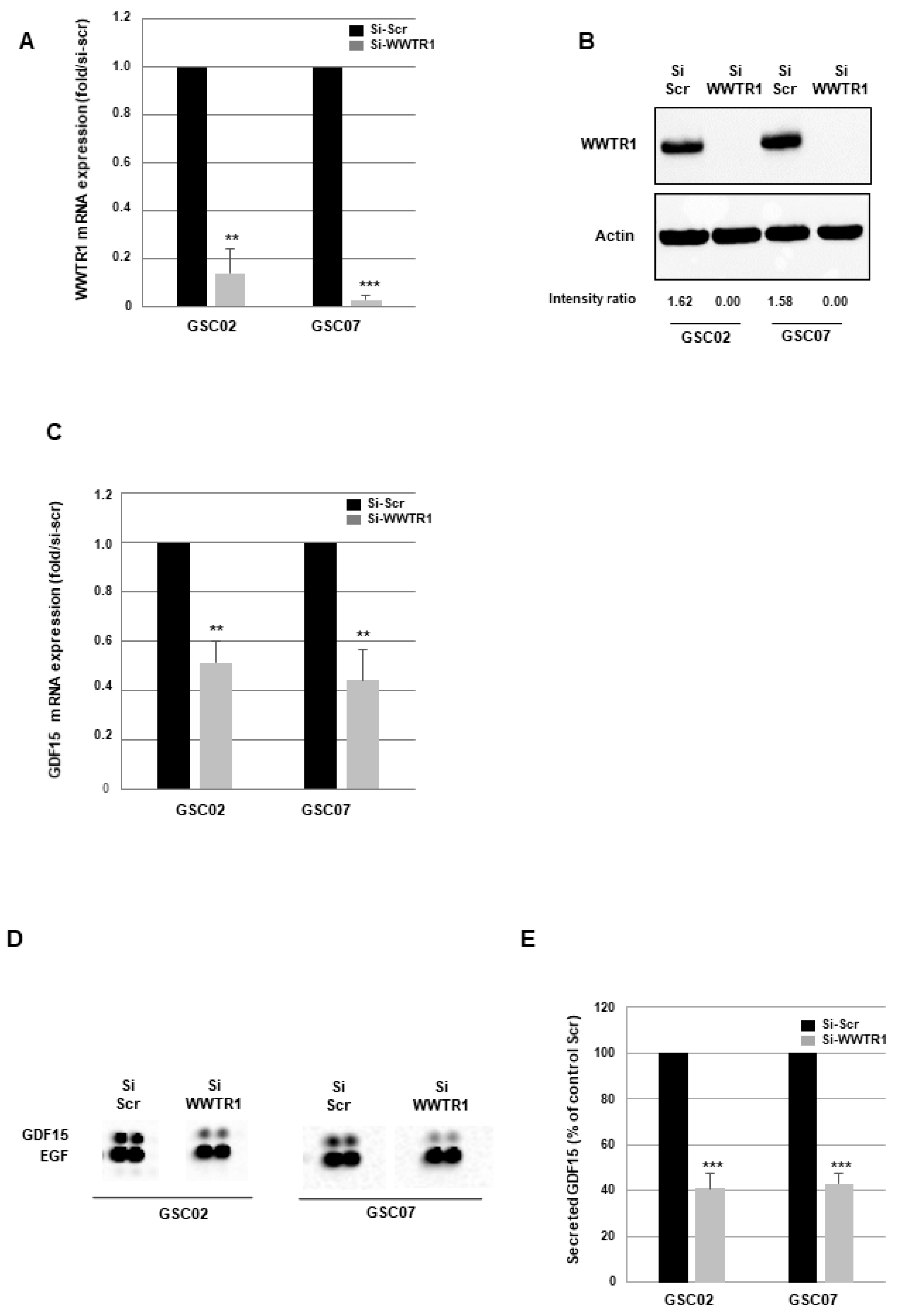
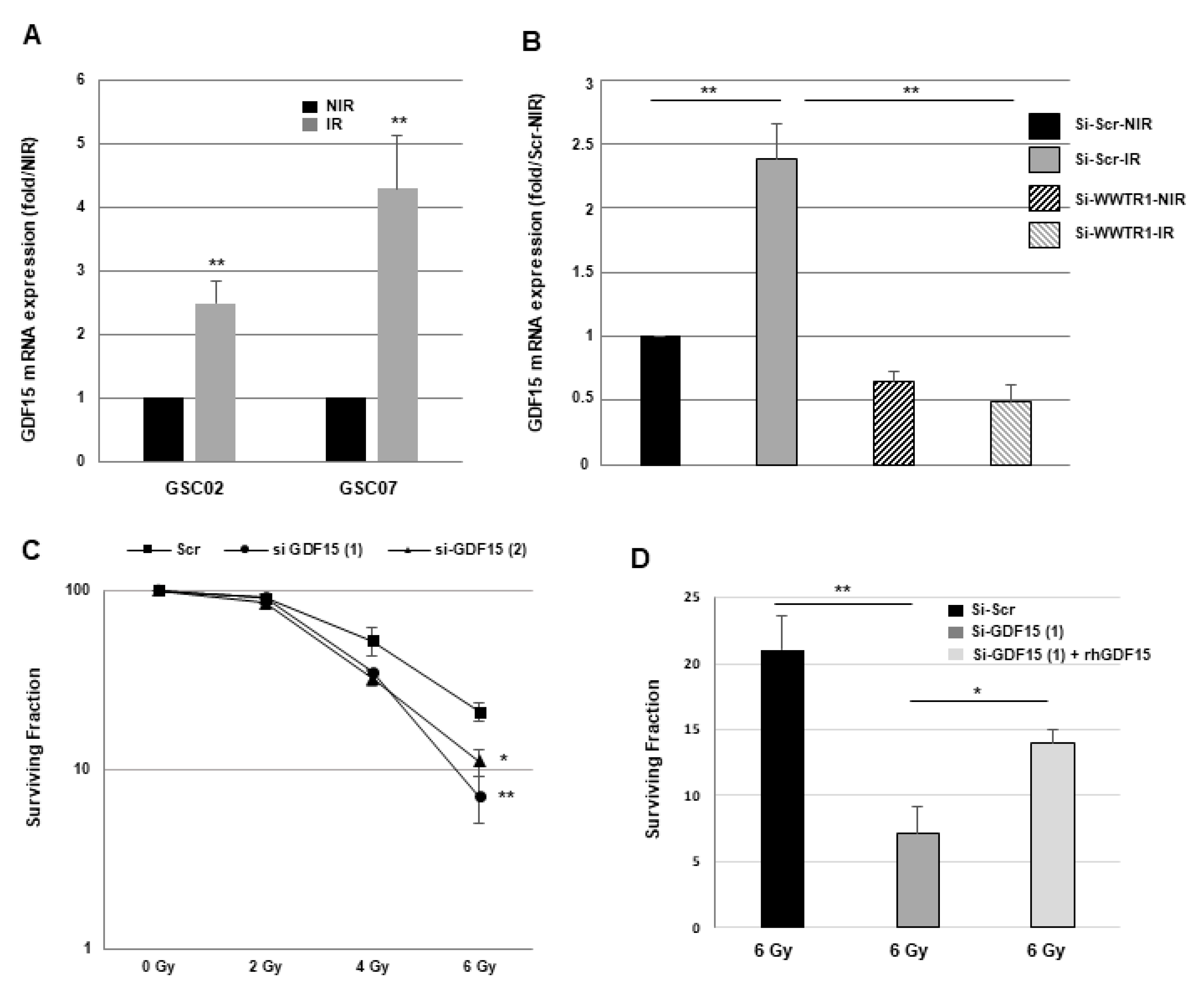
3.2. GDF15 Expression Is Regulated by the Transcription Factor WWTR1/TAZ in GSCs
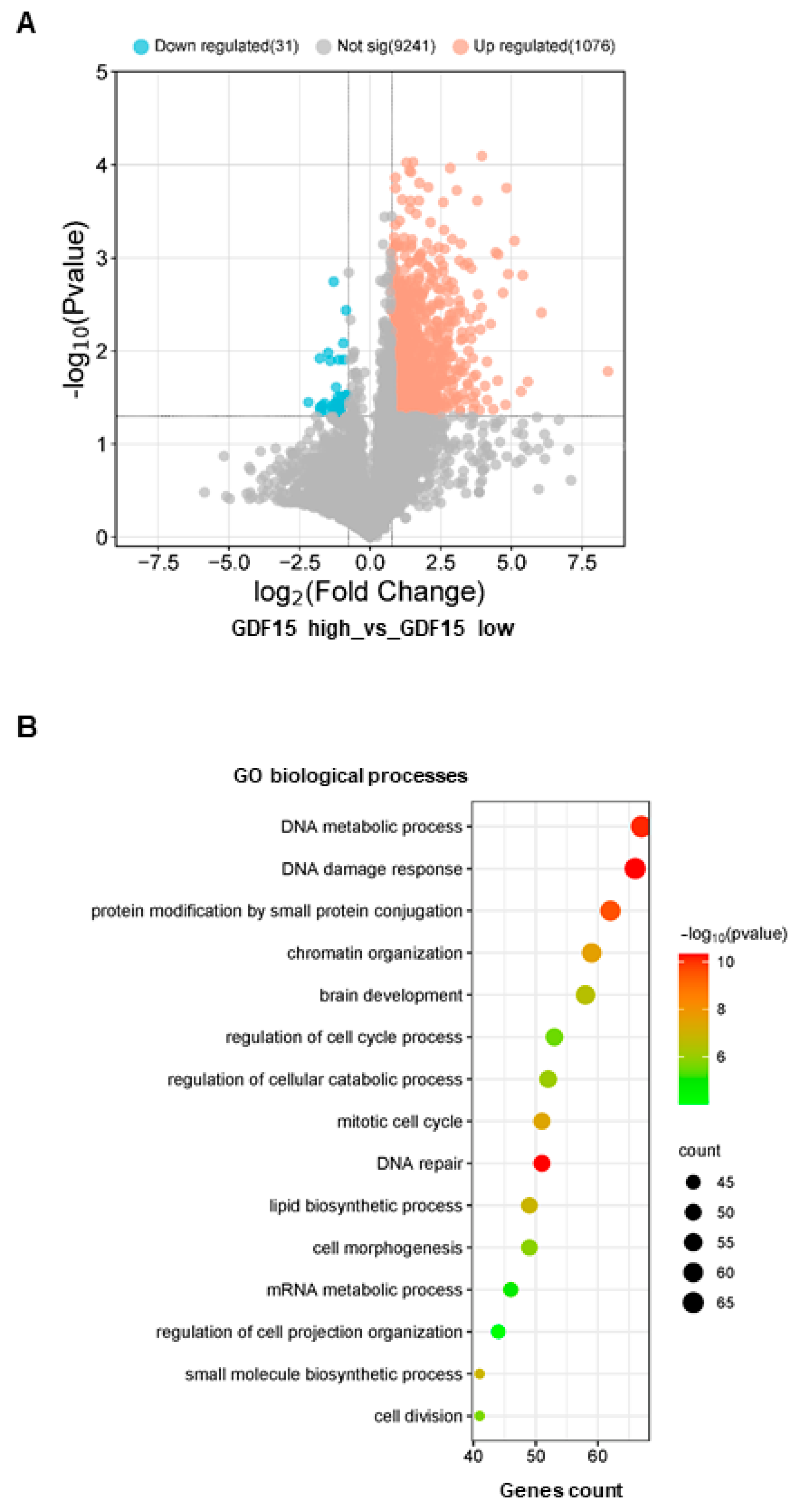
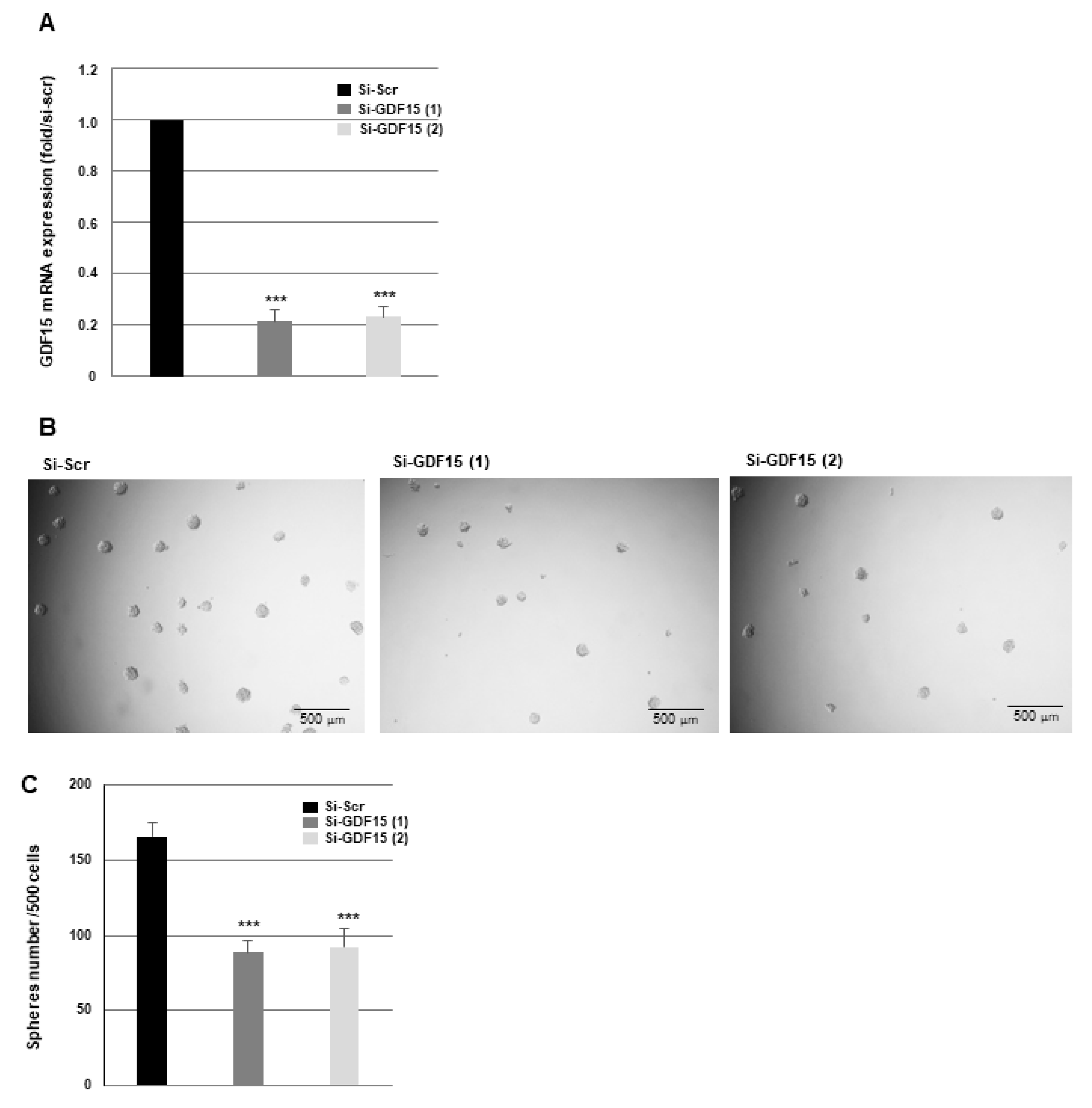
3.3. Down-Regulation of GDF15 Gene Expression Decreases Sphere-Forming Ability and Radiosensitizes GSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Ortensi, B.; Setti, M.; Osti, D.; Pelicci, G. Cancer stem cell contribution to glioblastoma invasiveness. Stem Cell Res. Ther. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Hebraud, B.; Bourin, P. Concise review: Growth differentiation factor 15 in pathology: A clinical role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Calvo, M.; Tarrio, N.; Reboredo, M.; Haz-Conde, M.; Garcia, J.; Quindos, M.; Figueroa, A.; Anton-Aparicio, L.; Calvo, L.; Valladares-Ayerbes, M. Circulating levels of GDF15, MMP7 and miR-200c as a poor prognostic signature in gastric cancer. Futur. Oncol. 2014, 10, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.M.; Levert-Mignon, A.J.; Lord, S.J.; Lee-Ng, K.K.; Botelho, N.K.; Falkenback, D.; Thomas, M.L.; Bobryshev, Y.V.; Whiteman, D.C.; Brown, D.A.; et al. MIC-1/GDF15 in Barrett’s oesophagus and oesophageal adenocarcinoma. Br. J. Cancer 2015, 112, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Chakraborty, S.; Baine, M.J.; Mallya, K.; Smith, L.M.; Sasson, A.; Brand, R.; Guha, S.; Jain, M.; Wittel, U.; et al. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS ONE 2013, 8, e55171. [Google Scholar] [CrossRef]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS ONE 2015, 10, e0127518. [Google Scholar] [CrossRef]
- Mehta, R.S.; Chong, D.Q.; Song, M.; Meyerhardt, J.A.; Ng, K.; Nishihara, R.; Qian, Z.; Morikawa, T.; Wu, K.; Giovannucci, E.L.; et al. Association Between Plasma Levels of Macrophage Inhibitory Cytokine-1 Before Diagnosis of Colorectal Cancer and Mortality. Gastroenterology 2015, 149, 614–622. [Google Scholar] [CrossRef]
- Zhang, Y.; Hua, W.; Niu, L.C.; Li, S.M.; Wang, Y.M.; Shang, L.; Zhang, C.; Li, W.N.; Wang, R.; Chen, B.L.; et al. Erratum to: Elevated growth differentiation factor 15 expression predicts poor prognosis in epithelial ovarian cancer patients. Tumour Biol. 2016, 37, 8465. [Google Scholar] [CrossRef]
- Park, H.; Nam, K.S.; Lee, H.J.; Kim, K.S. Ionizing Radiation-Induced GDF15 Promotes Angiogenesis in Human Glioblastoma Models by Promoting VEGFA Expression Through p-MAPK1/SP1 Signaling. Front. Oncol. 2022, 12, 801230. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, N.; Utsunomiya, S.; Nishio, M.; Shigeoka, M.; Takase, N.; Arai, N.; Kakeji, Y.; Koma, Y.; Yokozaki, H. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab. Investig. 2015, 95, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, Y.; Guo, T.; Liu, F.; Xu, Y.; Cai, S. Hypoxia Induces Growth Differentiation Factor 15 to Promote the Metastasis of Colorectal Cancer via PERK-eIF2alpha Signaling. BioMed Res. Int. 2020, 2020, 5958272. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Hu, S.; Gao, L.; Tang, N.; Liu, R.; Qin, Y.; Ren, C.; Du, S. GDF15 expression in glioma is associated with malignant progression, immune microenvironment, and serves as a prognostic factor. CNS Neurosci. Ther. 2022, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Scrideli, C.A.; Carlotti, C.G., Jr.; Okamoto, O.K.; Andrade, V.S.; Cortez, M.A.; Motta, F.J.; Lucio-Eterovic, A.K.; Neder, L.; Rosemberg, S.; Oba-Shinjo, S.M.; et al. Gene expression profile analysis of primary glioblastomas and non-neoplastic brain tissue: Identification of potential target genes by oligonucleotide microarray and real-time quantitative PCR. J. Neurooncol. 2008, 88, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Shnaper, S.; Desbaillets, I.; Brown, D.A.; Murat, A.; Migliavacca, E.; Schluep, M.; Ostermann, S.; Hamou, M.F.; Stupp, R.; Breit, S.N.; et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int. J. Cancer 2009, 125, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Codo, P.; Weller, M.; Kaulich, K.; Schraivogel, D.; Silginer, M.; Reifenberger, G.; Meister, G.; Roth, P. Control of glioma cell migration and invasiveness by GDF-15. Oncotarget 2016, 7, 7732–7746. [Google Scholar] [CrossRef]
- Louca, M.; Gkretsi, V.; Stylianopoulos, T. Coordinated Expression of Ras Suppressor 1 (RSU-1) and Growth Differentiation Factor 15 (GDF15) Affects Glioma Cell Invasion. Cancers 2019, 11, 1159. [Google Scholar] [CrossRef]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef]
- Peng, H.; Li, Z.; Fu, J.; Zhou, R. Growth and differentiation factor 15 regulates PD-L1 expression in glioblastoma. Cancer Manag. Res. 2019, 11, 2653–2661. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, N.; Guan, Y.; Wang, X.; Zang, G.; Lv, X.; Deng, S.; Wang, W.; Li, T.; Chen, J. GDF15 promotes glioma stem cell-like phenotype via regulation of ERK1/2-c-Fos-LIF signaling. Cell Death Discov. 2021, 7, 3. [Google Scholar] [CrossRef]
- Dahan, P.; Martinez Gala, J.; Delmas, C.; Monferran, S.; Malric, L.; Zentkowski, D.; Lubrano, V.; Toulas, C.; Cohen-Jonathan Moyal, E.; Lemarie, A. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: Possible involvement in radioresistance. Cell Death Dis. 2014, 5, e1543. [Google Scholar] [CrossRef] [PubMed]
- Deshors, P.; Toulas, C.; Arnauduc, F.; Malric, L.; Siegfried, A.; Nicaise, Y.; Lemarie, A.; Larrieu, D.; Tosolini, M.; Cohen-Jonathan Moyal, E.; et al. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019, 10, 816. [Google Scholar] [CrossRef]
- Lemarie, A.; Lubrano, V.; Delmas, C.; Lusque, A.; Cerapio, J.P.; Perrier, M.; Siegfried, A.; Arnauduc, F.; Nicaise, Y.; Dahan, P.; et al. The STEMRI trial: Magnetic resonance spectroscopy imaging can define tumor areas enriched in glioblastoma stem-like cells. Sci. Adv. 2023, 9, eadi0114. [Google Scholar] [CrossRef] [PubMed]
- Kowalski-Chauvel, A.; Modesto, A.; Gouaze-Andersson, V.; Baricault, L.; Gilhodes, J.; Delmas, C.; Lemarie, A.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. Alpha-6 integrin promotes radioresistance of glioblastoma by modulating DNA damage response and the transcription factor Zeb1. Cell Death Dis. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Kowalski-Chauvel, A.; Lacore, M.G.; Arnauduc, F.; Delmas, C.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. The m6A RNA Demethylase ALKBH5 Promotes Radioresistance and Invasion Capability of Glioma Stem Cells. Cancers 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.; Salazar, K.L.; Balasubramaniyan, V.; Wani, K.; Heathcock, L.; Hollingsworth, F.; James, J.D.; Gumin, J.; Diefes, K.L.; Kim, S.H.; et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011, 25, 2594–2609. [Google Scholar] [CrossRef]
- Gao, M.; Fu, Y.; Zhou, W.; Gui, G.; Lal, B.; Li, Y.; Xia, S.; Ji, H.; Eberhart, C.G.; Laterra, J.; et al. EGFR Activates a TAZ-Driven Oncogenic Program in Glioblastoma. Cancer Res. 2021, 81, 3580–3592. [Google Scholar] [CrossRef]
- Minata, M.; Audia, A.; Shi, J.; Lu, S.; Bernstock, J.; Pavlyukov, M.S.; Das, A.; Kim, S.H.; Shin, Y.J.; Lee, Y.; et al. Phenotypic Plasticity of Invasive Edge Glioma Stem-like Cells in Response to Ionizing Radiation. Cell Rep. 2019, 26, 1893–1905.e1897. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, F.; Wei, Y.; Zhang, L.; Guo, D.; Wang, B.; Li, W. Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene 2019, 38, 2788–2799. [Google Scholar] [CrossRef]
- Thrash, H.L.; Pendergast, A.M. Multi-Functional Regulation by YAP/TAZ Signaling Networks in Tumor Progression and Metastasis. Cancers 2023, 15, 4701. [Google Scholar] [CrossRef] [PubMed]
- Marquard, S.; Thomann, S.; Weiler, S.M.E.; Sticht, C.; Gretz, N.; Schirmacher, P.; Breuhahn, K. [Nonautonomous effects of oncogenic YAP in hepatocarcinogenesis]. Pathologe 2017, 38, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Mao, B.; Cheng, C.; Zou, Z.; Gao, J.; Yang, Y.; Lei, T.; Qi, X.; Yuan, Z.; Xu, W.; et al. YAP promotes breast cancer metastasis by repressing growth differentiation factor-15. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Schiegnitz, E.; Kammerer, P.W.; Rode, K.; Schorn, T.; Brieger, J.; Al-Nawas, B. Growth differentiation factor 15 as a radiation-induced marker in oral carcinoma increasing radiation resistance. J. Oral Pathol. Med. 2016, 45, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Chang, J.T.; Lee, L.Y.; Fan, K.H.; Lu, Y.C.; Li, Y.C.; Chiang, C.H.; You, G.R.; Chen, H.Y.; Cheng, A.J. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget 2017, 8, 1508–1528. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Chang, J.C.; Chen, H.H.; Hsu, C.Y.; Hsu, H.C.; Wu, K.L. Carcinoembryonic antigen as a marker of radioresistance in colorectal cancer: A potential role of macrophages. BMC Cancer 2018, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Sandor, N.; Schilling-Toth, B.; Kis, E.; Benedek, A.; Lumniczky, K.; Safrany, G.; Hegyesi, H. Growth Differentiation Factor-15 (GDF-15) is a potential marker of radiation response and radiation sensitivity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 142–149. [Google Scholar] [CrossRef]
- Chang, J.T.; Chan, S.H.; Lin, C.Y.; Lin, T.Y.; Wang, H.M.; Liao, C.T.; Wang, T.H.; Lee, L.Y.; Cheng, A.J. Differentially expressed genes in radioresistant nasopharyngeal cancer cells: gp96 and GDF15. Mol. Cancer Ther. 2007, 6, 2271–2279. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chang, J.T.; Wang, H.M.; Chan, S.H.; Chiu, C.C.; Lin, C.Y.; Fan, K.H.; Liao, C.T.; Chen, I.H.; Liu, T.Z.; et al. Proteomics of the radioresistant phenotype in head-and-neck cancer: Gp96 as a novel prediction marker and sensitizing target for radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 246–256. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Hu, S.; Pan, Y.; Zhang, J.; Tai, G.; Shao, C. GDF15 Contributes to Radioresistance by Mediating the EMT and Stemness of Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10911. [Google Scholar] [CrossRef]
- Gopal, U.; Mowery, Y.; Young, K.; Pizzo, S.V. Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J. Biol. Chem. 2019, 294, 13939–13952. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, Q.; Wang, Y.; Tian, C.; Yang, Q.; Zhao, Y.; Liu, L.; Wu, G.; Xu, S. CDK5 Activates Hippo Signaling to Confer Resistance to Radiation Therapy Via Upregulating TAZ in Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, L.; Chen, P.; Li, S.; Cheng, Y. Thymine DNA glycosylase-regulated TAZ promotes radioresistance by targeting nonhomologous end joining and tumor progression in esophageal cancer. Cancer Sci. 2020, 111, 3613–3625. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bian, Y.; Wang, Y.; Chen, L.; Yu, A.; Sun, X. S100A4 influences cancer stem cell-like properties of MGC803 gastric cancer cells by regulating GDF15 expression. Int. J. Oncol. 2016, 49, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, A.; Tominaga, K.; Nishimura, T.; Yano, M.; Kiyokawa, E.; Noguchi, M.; Noguchi, M.; Kanauchi, H.; Ogawa, T.; Minato, H.; et al. An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stem-like cells. Oncotarget 2017, 8, 24869–24881. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xu, H.X.; Li, J.P.; Wang, S.; Fu, Z.; Jia, J.; Wang, L.; Zhu, Z.F.; Lu, R.; Yao, Z. Growth differentiation factor 15 induces growth and metastasis of human liver cancer stem-like cells via AKT/GSK-3beta/beta-catenin signaling. Oncotarget 2017, 8, 16972–16987. [Google Scholar] [CrossRef] [PubMed]
- Tanno, T.; Lim, Y.; Wang, Q.; Chesi, M.; Bergsagel, P.L.; Matthews, G.; Johnstone, R.W.; Ghosh, N.; Borrello, I.; Huff, C.A.; et al. Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood 2014, 123, 725–733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentaberry-Rosa, A.; Nicaise, Y.; Delmas, C.; Gouazé-Andersson, V.; Cohen-Jonathan-Moyal, E.; Seva, C. Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance. Cancers 2024, 16, 27. https://doi.org/10.3390/cancers16010027
Bentaberry-Rosa A, Nicaise Y, Delmas C, Gouazé-Andersson V, Cohen-Jonathan-Moyal E, Seva C. Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance. Cancers. 2024; 16(1):27. https://doi.org/10.3390/cancers16010027
Chicago/Turabian StyleBentaberry-Rosa, Alexandre, Yvan Nicaise, Caroline Delmas, Valérie Gouazé-Andersson, Elizabeth Cohen-Jonathan-Moyal, and Catherine Seva. 2024. "Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance" Cancers 16, no. 1: 27. https://doi.org/10.3390/cancers16010027
APA StyleBentaberry-Rosa, A., Nicaise, Y., Delmas, C., Gouazé-Andersson, V., Cohen-Jonathan-Moyal, E., & Seva, C. (2024). Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance. Cancers, 16(1), 27. https://doi.org/10.3390/cancers16010027





