Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. EUS-Based Treatment Modalities
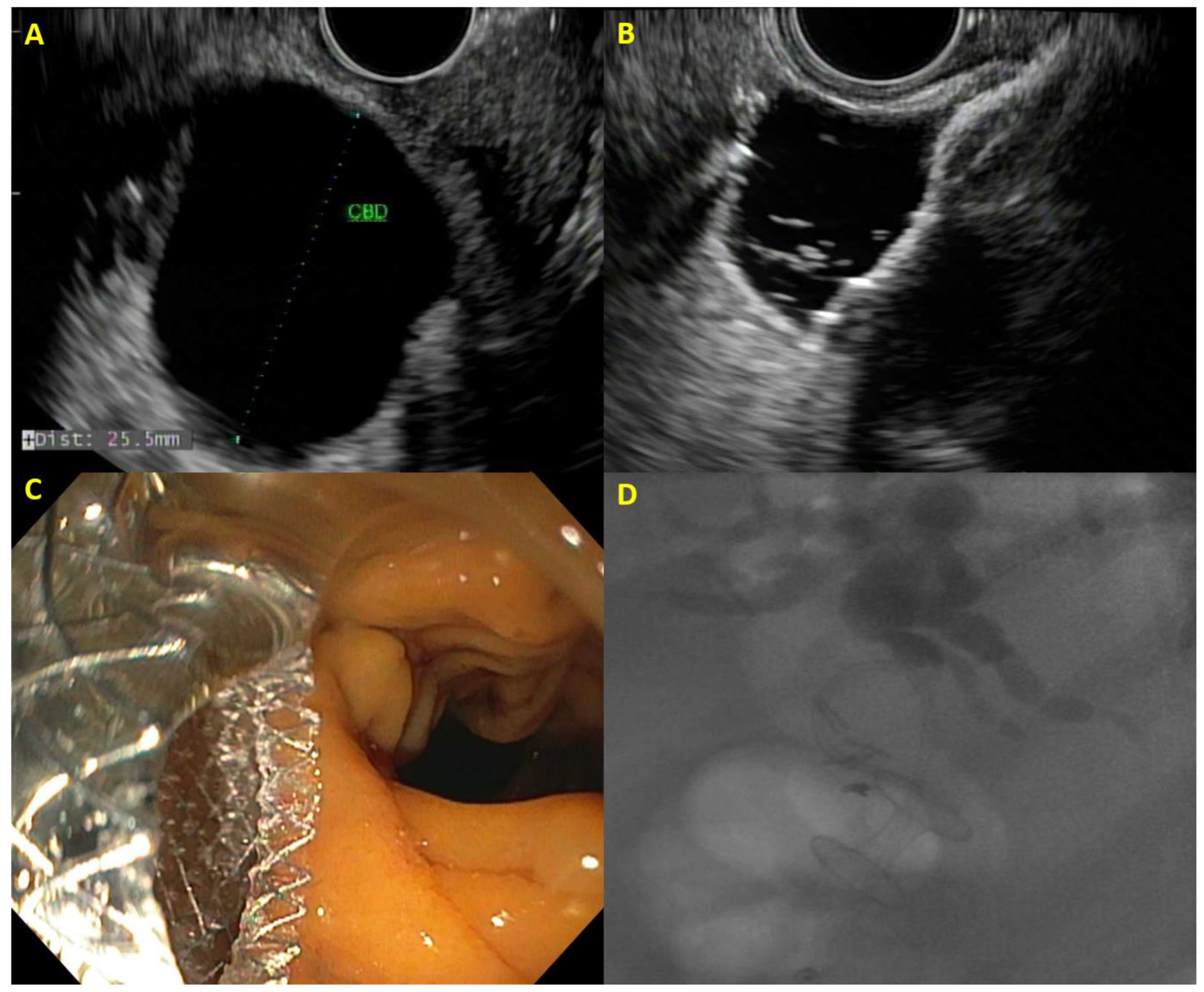
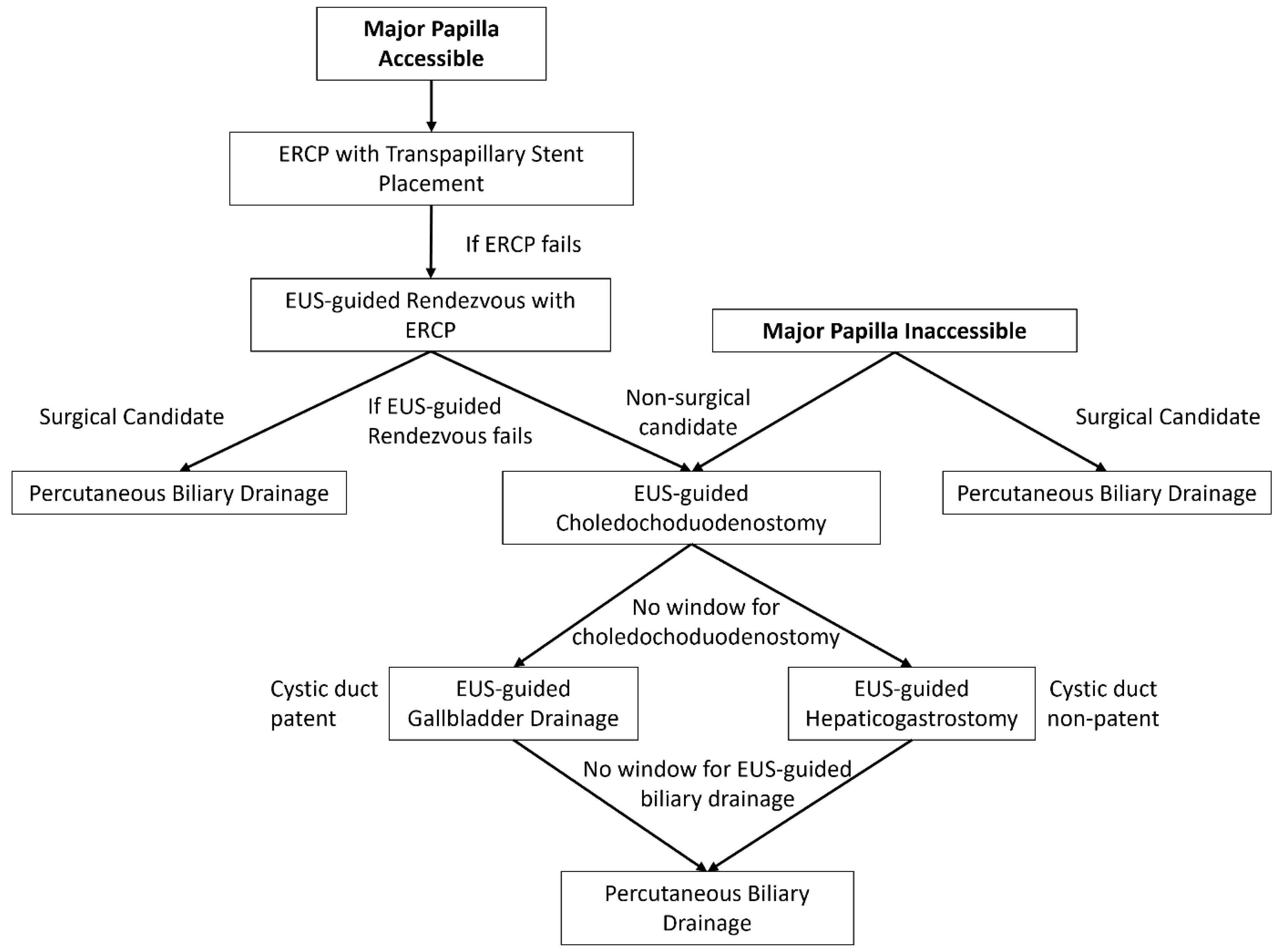
2.1. EUS-Guided Biliary Drainage
2.2. EUS-Guided Choledochoduodenostomy
2.3. EUS-Guided Hepaticogastrostomy
2.4. EUS-Guided Gallbladder Drainage
2.5. Comparison of EUS-Guided Biliary Drainage with Percutaneous Biliary Drainage
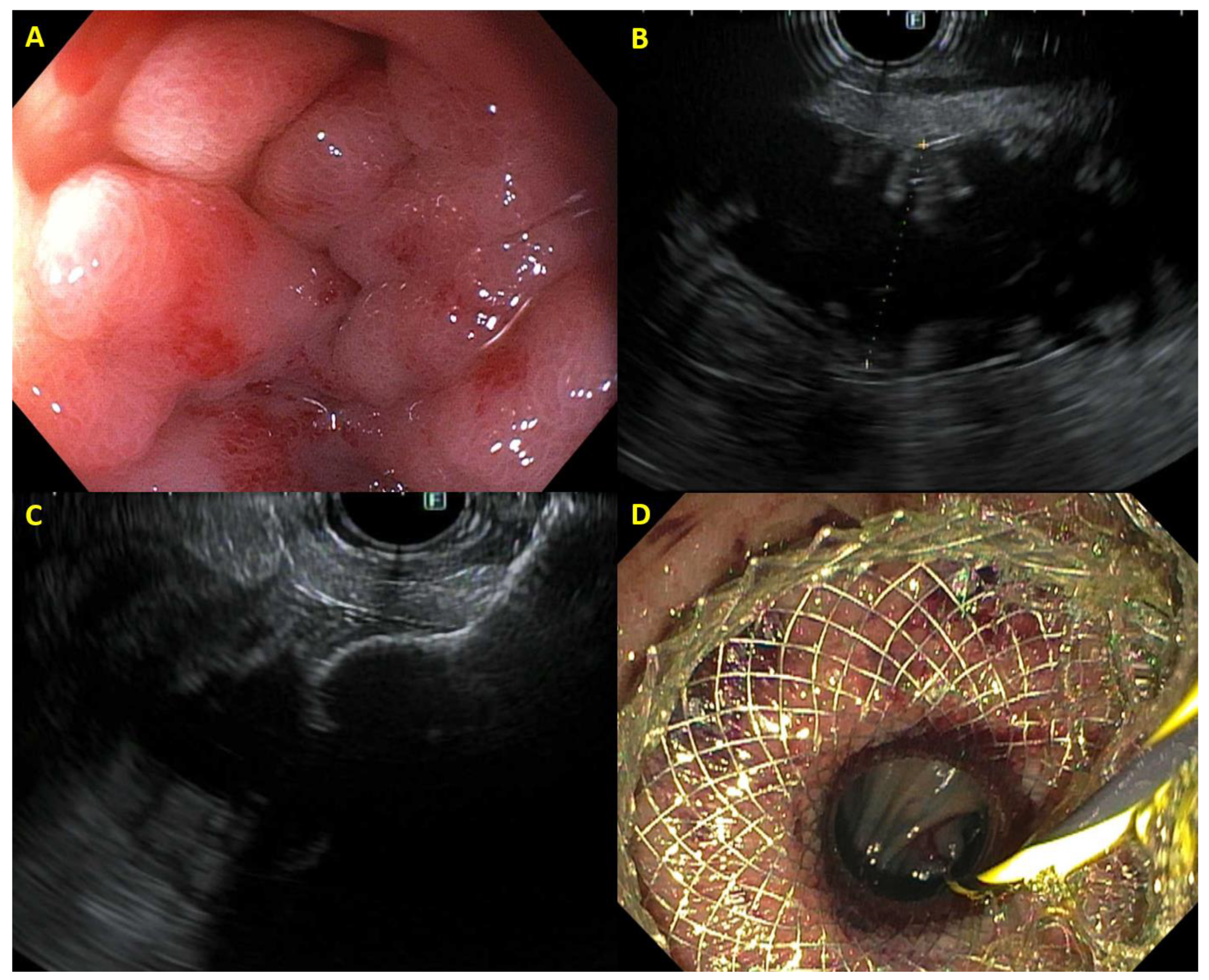
2.6. EUS-Guided Gastroenterostomy
2.7. Afferent Loop Syndrome
2.8. EUS-Guided Celiac Plexus Neurolysis
2.9. EUS-Guided Radiofrequency Ablation
3. Impact of Therapeutic EUS on Subsequent Surgery
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.; Al-Share, B.; Klapman, J.B.; Dam, A. The Role of Endoscopic Ultrasonography in the Diagnosis and Staging of Pancreatic Cancer. Cancers 2022, 14, 1373. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, M.; Yasukawa, S.; Fukutake, N.; Ogura, T.; Asada, M.; Shimokawa, T.; Inatomi, O.; Nakai, Y.; Shiomi, H.; Nebiki, H.; et al. Comparison of 22-gauge standard and Franseen needles in EUS-guided tissue acquisition for diagnosing solid pancreatic lesions: A multicenter randomized controlled trial. Gastrointest. Endosc. 2022, 96, 57–66.e52. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bhullar, F.; Alaber, O.; Kamal, A.; Hopson, P.; Kanthasamy, K.; Coughlin, S.; Archibugi, L.; Thiruvengadam, N.; Moreau, C.; et al. Comparative diagnostic accuracy of EUS needles in solid pancreatic masses: A network meta-analysis. Endosc. Int. Open 2021, 9, E853–E862. [Google Scholar] [CrossRef] [PubMed]
- Kloek, J.J.; Heger, M.; van der Gaag, N.A.; Beuers, U.; van Gulik, T.M.; Gouma, D.J.; Levi, M. Effect of preoperative biliary drainage on coagulation and fibrinolysis in severe obstructive cholestasis. J. Clin. Gastroenterol. 2010, 44, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.W.; Sherman, S.; Dua, K.S.; Slivka, A.; Roy, A.; Costamagna, G.; Deviere, J.; Peetermans, J.; Rousseau, M.; Nakai, Y.; et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: A randomized trial. Gastrointest. Endosc. 2019, 90, 602–612.e604. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Napoleon, B.; Kunda, R.; Arcidiacono, P.G.; Kongkam, P.; Larghi, A.; Van der Merwe, S.; Jacques, J.; Legros, R.; Thawee, R.E.; et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP with Covered Metallic Stents in Patients with Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology 2023, 165, 473–482.e2. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Sahai, A.; Donatelli, G.; Lam, E.; Forbes, N.; Mosko, J.; Paquin, S.C.; Donnellan, F.; Chatterjee, A.; Telford, J.; et al. Endoscopic ultrasound-guided biliary drainage of first intent with a lumen-apposing metal stent vs. endoscopic retrograde cholangiopancreatography in malignant distal biliary obstruction: A multicenter randomized controlled study (ELEMENT trial). Gastroenterology 2023, 165, 1249–1261.e5. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef]
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound 2018, 7, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Ogura, T.; Ishiwatari, H.; Nakai, Y.; Iwata, K.; Mukai, T.; Shimizu, M.; Isayama, H.; Yasuda, I.; Itoi, T. Utility of dedicated bougie dilator for a 0.018-inch guidewire during EUS-guided biliary drainage: A multi-center retrospective cohort study. J. Hepatobiliary Pancreat. Sci. 2022, 29, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Itoi, T. Technical tips and recent development of endoscopic ultrasound-guided choledochoduodenostomy. DEN Open 2021, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Chandrasekhara, V.; Bhatt, A.; Bucobo, J.C.; Copland, A.P.; Krishnan, K.; Kumta, N.A.; Pannala, R.; Parsi, M.A.; Rahimi, E.F.; et al. Lumen-apposing metal stents (with videos). Gastrointest. Endosc. 2021, 94, 457–470. [Google Scholar] [CrossRef]
- Fugazza, A.; Fabbri, C.; Di Mitri, R.; Petrone, M.C.; Colombo, M.; Cugia, L.; Amato, A.; Forti, E.; Binda, C.; Maida, M.; et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after failed ERCP: A retrospective nationwide analysis. Gastrointest. Endosc. 2022, 95, 896–904.e891. [Google Scholar] [CrossRef] [PubMed]
- Jacques, J.; Privat, J.; Pinard, F.; Fumex, F.; Valats, J.C.; Chaoui, A.; Cholet, F.; Godard, B.; Grandval, P.; Legros, R.; et al. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: A retrospective analysis. Endoscopy 2019, 51, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, J.A.; Fockens, P.; Besselink, M.G.; Busch, O.; Daams, F.; Montazeri, N.S.M.; Wilmink, J.W.; Voermans, R.P.; van Wanrooij, R.L.J. EUS-guided choledochoduodenostomy using single step lumen-apposing metal stents for primary drainage of malignant distal biliary obstruction (SCORPION-p): A prospective pilot study. Endoscopy 2023, 97, AB864. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Khashab, M.A.; Van der Merwe, S.; Kunda, R.; El Zein, M.H.; Teoh, A.Y.; Marson, F.P.; Fabbri, C.; Tarantino, I.; Varadarajulu, S.; Modayil, R.J.; et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc. Int. Open 2016, 4, E487–E496. [Google Scholar] [CrossRef]
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest. Endosc. 2020, 92, 623–631.e621. [Google Scholar] [CrossRef] [PubMed]
- Shibuki, T.; Okumura, K.; Sekine, M.; Kobori, I.; Miyagaki, A.; Sasaki, Y.; Takano, Y.; Hashimoto, Y. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Clin. Endosc. 2023, 56, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Kitano, M.; Takenaka, M.; Okuda, A.; Minaga, K.; Yamao, K.; Yamashita, Y.; Hatamaru, K.; Noguchi, C.; Gotoh, Y.; et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video). Dig. Endosc. 2018, 30, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kitano, R.; Ibusuki, M.; Sakamoto, K.; Kimoto, S.; Kobayashi, Y.; Sumida, Y.; Nakade, Y.; Ito, K.; Yoneda, M. Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting without Dilation Device Application for Malignant Distal Biliary Obstruction in Pancreatic Cancer. Dig. Dis. Sci. 2023, 68, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Chiba, Y.; Masuda, D.; Kitano, M.; Sano, T.; Saori, O.; Yamamoto, K.; Imaoka, H.; Imoto, A.; Takeuchi, T.; et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy 2016, 48, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Kitano, M.; Itoi, T.; Pérez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091. [Google Scholar] [CrossRef]
- Binda, C.; Anderloni, A.; Fugazza, A.; Amato, A.; de Nucci, G.; Redaelli, A.; Di Mitri, R.; Cugia, L.; Pollino, V.; Macchiarelli, R.; et al. Eus-guided gallbladder drainage using a lumen-apposing metal stent as rescue treatment for malignant distal biliary obstruction: A large multicenter experience. Gastrointest. Endosc. 2023, 98, 765–773. [Google Scholar] [CrossRef]
- Issa, D.; Irani, S.; Law, R.; Shah, S.; Bhalla, S.; Mahadev, S.; Hajifathalian, K.; Sampath, K.; Mukewar, S.; Carr-Locke, D.L.; et al. Endoscopic ultrasound-guided gallbladder drainage as a rescue therapy for unresectable malignant biliary obstruction: A multicenter experience. Endoscopy 2021, 53, 827–831. [Google Scholar] [CrossRef]
- Imai, H.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Miyata, T.; Yamao, K.; Sakamoto, H.; Harwani, Y.; Kudo, M. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest. Endosc. 2016, 84, 147–151. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, S.W.; Hyun, B.; Lee, J.; Koh, D.H.; Chung, D. Identification of risk factors for obstructive cholecystitis following placement of biliary stent in unresectable malignant biliary obstruction: A 5-year retrospective analysis in single center. Surg. Endosc. 2021, 35, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, H.S.; Kim, J.W.; Baik, S.K.; Kwon, S.O.; Kim, H.G.; Lee, D.H.; Yoo, B.M.; Kim, J.H.; Moon, Y.S.; et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest. Endosc. 2006, 64, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Robles-Medranda, C.; Oleas, R.; Puga-Tejada, M.; Alcivar-Vasquez, J.; Del Valle, R.; Olmos, J.; Arevalo-Mora, M.; Egas-Izquierdo, M.; Tabacelia, D.; Baquerizo-Burgos, J.; et al. Prophylactic EUS-guided gallbladder drainage prevents acute cholecystitis in patients with malignant biliary obstruction and cystic duct orifice involvement: A randomized trial (with video). Gastrointest. Endosc. 2023, 97, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Choi, J.H.; Park, D.H.; Song, T.J.; Kim, D.U.; Paik, W.H.; Hwangbo, Y.; Lee, S.S.; Seo, D.W.; Lee, S.K.; et al. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin. Gastroenterol. Hepatol. 2016, 14, 1011–1019.e1013. [Google Scholar] [CrossRef] [PubMed]
- Ginestet, C.; Sanglier, F.; Hummel, V.; Rouchaud, A.; Legros, R.; Lepetit, H.; Dahan, M.; Carrier, P.; Loustaud-Ratti, V.; Sautereau, D.; et al. EUS-guided biliary drainage with electrocautery-enhanced lumen-apposing metal stent placement should replace PTBD after ERCP failure in patients with distal tumoral biliary obstruction: A large real-life study. Surg. Endosc. 2022, 36, 3365–3373. [Google Scholar] [CrossRef]
- Sawas, T.; Bailey, N.J.; Yeung, K.; James, T.W.; Reddy, S.; Fleming, C.J.; Marya, N.B.; Storm, A.C.; Abu Dayyeh, B.K.; Petersen, B.T.; et al. Comparison of EUS-guided choledochoduodenostomy and percutaneous drainage for distal biliary obstruction: A multicenter cohort study. Endosc. Ultrasound 2022, 11, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mangiavillano, B.; Paduano, D.; Binda, C.; Crinò, S.F.; Gkolfakis, P.; Ramai, D.; Fugazza, A.; Tarantino, I.; Lisotti, A.; et al. Methods for Drainage of Distal Malignant Biliary Obstruction after ERCP Failure: A Systematic Review and Network Meta-Analysis. Cancers 2022, 14, 3291. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.; Lillemoe, K.D. Surgical palliation of pancreatic cancer. Cancer J. 2012, 18, 577–583. [Google Scholar] [CrossRef]
- Garcia-Alonso, F.J.; Chavarria, C.; Subtil, J.C.; Aparicio, J.R.; Busto Bea, V.; Martinez-Moreno, B.; Vila, J.J.; Martín-Álvarez, V.; Sanchez-Delgado, L.; de la Serna-Higuera, C.; et al. Prospective multicenter assessment of the impact of EUS-guided gastroenterostomy on patient quality of life in unresectable malignant gastric outlet obstruction. Gastrointest. Endosc. 2023, 98, 28–35. [Google Scholar] [CrossRef]
- Vanella, G.; Dell’Anna, G.; Capurso, G.; Maisonneuve, P.; Bronswijk, M.; Crippa, S.; Tamburrino, D.; Macchini, M.; Orsi, G.; Casadei-Gardini, A.; et al. EUS-guided gastroenterostomy for management of malignant gastric outlet obstruction: A prospective cohort study with matched comparison with enteral stenting. Gastrointest. Endosc. 2023, 98, 337–347.e5. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Lakhtakia, S.; Tarantino, I.; Perez-Miranda, M.; Kunda, R.; Maluf-Filho, F.; Dhir, V.; Basha, J.; Chan, S.M.; Ligresti, D.; et al. Endoscopic ultrasonography-guided gastroenterostomy versus uncovered duodenal metal stenting for unresectable malignant gastric outlet obstruction (DRA-GOO): A multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aldehuelo, R.; Subtil Iñigo, J.C.; Martínez Moreno, B.; Gornals, J.; Guarner-Argente, C.; Repiso Ortega, A.; Peralta Herce, S.; Aparicio, J.R.; Rodríguez de Santiago, E.; Bazaga, S.; et al. EUS-guided gastroenterostomy versus duodenal self-expandable metal stent for malignant gastric outlet obstruction: Results from a nationwide multicenter retrospective study (with video). Gastrointest. Endosc. 2022, 96, 1012–1020.e1013. [Google Scholar] [CrossRef] [PubMed]
- Chandan, S.; Khan, S.R.; Mohan, B.P.; Shah, A.R.; Bilal, M.; Ramai, D.; Bhogal, N.; Dhindsa, B.; Kassab, L.L.; Singh, S.; et al. EUS-guided gastroenterostomy versus enteral stenting for gastric outlet obstruction: Systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E496–E504. [Google Scholar] [CrossRef] [PubMed]
- Bronswijk, M.; Vanella, G.; van Malenstein, H.; Laleman, W.; Jaekers, J.; Topal, B.; Daams, F.; Besselink, M.G.; Arcidiacono, P.G.; Voermans, R.P.; et al. Laparoscopic versus EUS-guided gastroenterostomy for gastric outlet obstruction: An international multicenter propensity score-matched comparison (with video). Gastrointest. Endosc. 2021, 94, 526–536.e522. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandan, S.; Mohan, B.P.; Atla, P.R.; McCabe, E.J.; Robbins, D.H.; Trindade, A.J.; Benias, P.C. EUS-guided gastroenterostomy versus surgical gastroenterostomy for the management of gastric outlet obstruction: A systematic review and meta-analysis. Endosc. Int. Open 2022, 10, E448–E458. [Google Scholar] [CrossRef] [PubMed]
- Bronswijk, M.; Vanella, G.; van Wanrooij, R.L.J.; Samanta, J.; Lauwereys, J.; Pérez-Cuadrado-Robles, E.; Dell’Anna, G.; Dhar, J.; Gupta, V.; van Malenstein, H.; et al. Same-session Double EUS-bypass versus Surgical Gastroenterostomy and Hepaticojejunostomy: An International Multicenter Comparison. Gastrointest. Endosc. 2023, 98, 225–236.e1. [Google Scholar] [CrossRef] [PubMed]
- Vanella, G.; Bronswijk, M.; van Wanrooij, R.L.; Dell’Anna, G.; Laleman, W.; van Malenstein, H.; Voermans, R.P.; Fockens, P.; Van der Merwe, S.; Arcidiacono, P.G. Combined endoscopic mAnagement of BiliaRy and gastrIc OutLET obstruction (CABRIOLET Study): A multicenter retrospective analysis. DEN Open 2023, 3, e132. [Google Scholar] [CrossRef] [PubMed]
- Canakis, A.; Hathorn, K.E.; Irani, S.S.; Baron, T.H. Single session endoscopic ultrasound-guided double bypass (hepaticogastrostomy and gastrojejunostomy) for concomitant duodenal and biliary obstruction: A case series. J. Hepatobiliary Pancreat. Sci. 2022, 29, 941–949. [Google Scholar] [CrossRef]
- Pérez-Cuadrado-Robles, E.; Bronswijk, M.; Prat, F.; Barthet, M.; Palazzo, M.; Arcidiacono, P.; Schaefer, M.; Devière, J.; van Wanrooij, R.L.J.; Tarantino, I.; et al. Endoscopic ultrasound-guided drainage using lumen-apposing metal stent of malignant afferent limb syndrome in patients with previous Whipple surgery: Multicenter study (with video). Dig. Endosc. 2022, 34, 1433–1439. [Google Scholar] [CrossRef]
- Brewer Gutierrez, O.I.; Irani, S.S.; Ngamruengphong, S.; Aridi, H.D.; Kunda, R.; Siddiqui, A.; Dollhopf, M.; Nieto, J.; Chen, Y.I.; Sahar, N.; et al. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome: A multicenter experience. Endoscopy 2018, 50, 891–895. [Google Scholar] [CrossRef]
- De Bie, C.; Bronswijk, M.; Vanella, G.; Pérez-Cuadrado-Robles, E.; van Malenstein, H.; Laleman, W.; Van der Merwe, S. EUS-guided hepaticogastrostomy for patients with afferent loop syndrome: A comparison with EUS-guided gastroenterostomy or percutaneous drainage. Surg. Endosc. 2022, 36, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Koshita, S.; Masu, K.; Ogawa, T.; Kusunose, H.; Murabayashi, T.; Sakai, T.; Kozakai, F.; Ito, K. Efficacy of EUS-guided celiac plexus neurolysis compared with medication alone for unresectable pancreatic cancer in the oxycodone/fentanyl era: A prospective randomized control study. Gastrointest. Endosc. 2020, 92, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Gleeson, F.C.; Topazian, M.D.; Fujii-Lau, L.L.; Enders, F.T.; Larson, J.J.; Mara, K.; Abu Dayyeh, B.K.; Alberts, S.R.; Hallemeier, C.L.; et al. Combined Celiac Ganglia and Plexus Neurolysis Shortens Survival, without Benefit, vs Plexus Neurolysis Alone. Clin. Gastroenterol. Hepatol. 2019, 17, 728–738.e729. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Yasuda, I.; Kawakami, H.; Hayashi, T.; Hisai, H.; Irisawa, A.; Mukai, T.; Katanuma, A.; Kubota, K.; Ohnishi, T.; et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: A randomized multicenter trial. Endoscopy 2013, 45, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wyse, J.M.; Carone, M.; Paquin, S.C.; Usatii, M.; Sahai, A.V. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J. Clin. Oncol. 2011, 29, 3541–3546. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Sutton, B.; Hawes, R.H.; Varadarajulu, S. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: A randomized controlled trial (with videos). Gastrointest. Endosc. 2019, 89, 58–66.e53. [Google Scholar] [CrossRef]
- LeBlanc, J.K.; Al-Haddad, M.; McHenry, L.; Sherman, S.; Juan, M.; McGreevy, K.; Johnson, C.; Howard, T.J.; Lillemoe, K.D.; DeWitt, J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: One injection or two? Gastrointest. Endosc. 2011, 74, 1300–1307. [Google Scholar] [CrossRef]
- Koulouris, A.I.; Alexandre, L.; Hart, A.R.; Clark, A. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology 2021, 21, 434–442. [Google Scholar] [CrossRef]
- Thosani, N.; Cen, P.; Rowe, J.; Guha, S.; Bailey-Lundberg, J.M.; Bhakta, D.; Patil, P.; Wray, C.J. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) for advanced pancreatic and periampullary adenocarcinoma. Sci. Rep. 2022, 12, 16516. [Google Scholar] [CrossRef]
- Faraoni, E.Y.; O’Brien, B.J.; Strickland, L.N.; Osborn, B.K.; Mota, V.; Chaney, J.; Atkins, C.L.; Cen, P.; Rowe, J.; Cardenas, J.; et al. Radiofrequency Ablation Remodels the Tumor Microenvironment and Promotes Neutrophil-Mediated Abscopal Immunomodulation in Pancreatic Cancer. Cancer Immunol. Res. 2023, 11, 4–12. [Google Scholar] [CrossRef]
- Fegrachi, S.; Walma, M.S.; de Vries, J.J.J.; van Santvoort, H.C.; Besselink, M.G.; von Asmuth, E.G.; van Leeuwen, M.S.; Borel Rinkes, I.H.; Bruijnen, R.C.; de Hingh, I.H.; et al. Safety of radiofrequency ablation in patients with locally advanced, unresectable pancreatic cancer: A phase II study. Eur. J. Surg. Oncol. 2019, 45, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided Radiofrequency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J. Gastrointestin Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, F.; Pea, A.; Conigliaro, R.; Butturini, G.; Frigerio, I.; Regi, P.; Giardino, A.; Bertani, H.; Paini, M.; Pederzoli, P.; et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg. Endosc. 2018, 32, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Napoleon, B.; Facciorusso, A.; Lakhtakia, S.; Borbath, I.; Caillol, F.; Do-Cong Pham, K.; Rizzatti, G.; Forti, E.; Palazzo, L.; et al. Endoscopic Ultrasound-guided Radiofrequency Ablation Versus Surgical Resection for Treatment of Pancreatic Insulinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 2834–2843.e2832. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Ferreira, M.; Garces-Duran, R.; Eisendrath, P.; Devière, J.; Deprez, P.; Monino, L.; Van Laethem, J.L.; Borbath, I. EUS-guided radiofrequency ablation of pancreatic/peripancreatic tumors and oligometastatic disease: An observational prospective multicenter study. Endosc. Int. Open 2022, 10, E1380–E1385. [Google Scholar] [CrossRef]
- Tyberg, A.; Sarkar, A.; Shahid, H.M.; Shah-Khan, S.M.; Gaidhane, M.; Simon, A.; Eisenberg, I.A.; Lajin, M.; Karagyozov, P.; Liao, K.; et al. EUS-Guided Biliary Drainage Versus ERCP in Malignant Biliary Obstruction Before Hepatobiliary Surgery: An International Multicenter Comparative Study. J. Clin. Gastroenterol. 2022, 57, 962–966. [Google Scholar] [CrossRef]
- Janet, J.; Albouys, J.; Napoleon, B.; Jacques, J.; Mathonnet, M.; Magne, J.; Fontaine, M.; de Ponthaud, C.; Durand Fontanier, S.; Bardet, S.S.M.; et al. Pancreatoduodenectomy Following Preoperative Biliary Drainage Using Endoscopic Ultrasound-Guided Choledochoduodenostomy Versus a Transpapillary Stent: A Multicenter Comparative Cohort Study of the ACHBT-FRENCH-SFED Intergroup. Ann. Surg. Oncol. 2023, 30, 5036–5046. [Google Scholar] [CrossRef]
- Vanella, G.; Tamburrino, D.; Dell’Anna, G.; Petrone, M.C.; Crippa, S.; Falconi, M.; Arcidiacono, P.G. Endoscopic ultrasound-guided gastrojejunostomy does not prevent pancreaticoduodenectomy after long-term symptom-free neoadjuvant treatment. Endoscopy 2022, 54, E143–E145. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Moon, J.H.; Crinò, S.F.; Larghi, A.; Pham, K.D.; Teoh, A.Y.B.; Paduano, D.; Lee, Y.N.; Yoo, H.W.; Shin, I.S.; et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest. Endosc. 2022, 95, 115–122. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Moon, J.H.; Facciorusso, A.; Vargas-Madrigal, J.; Di Matteo, F.; Rizzatti, G.; De Luca, L.; Forti, E.; Mutignani, M.; Al-Lehibi, A.; et al. Endoscopic ultrasound-guided gallbladder drainage as a first approach for jaundice palliation in unresectable malignant distal biliary obstruction: Prospective study. Dig. Endosc. 2023. [Google Scholar] [CrossRef]
| Disease Complication | Therapeutic EUS Considerations | Adverse Events | Survival Post-Treatment |
|---|---|---|---|
| Biliary obstruction |
|
| 144–232 days (median) |
| Gastric outlet obstruction | EUS-guided Gastroenterostomy |
| 84–120 days (median) |
| Afferent Loop Syndrome | EUS-guided Enteroenterostomy |
| 110–120 days (median) |
| Pain | EUS-guided celiac plexus neurolysis |
| 168–314 days (median) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Papachristou, G.I. Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer. Cancers 2024, 16, 29. https://doi.org/10.3390/cancers16010029
Han S, Papachristou GI. Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer. Cancers. 2024; 16(1):29. https://doi.org/10.3390/cancers16010029
Chicago/Turabian StyleHan, Samuel, and Georgios I. Papachristou. 2024. "Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer" Cancers 16, no. 1: 29. https://doi.org/10.3390/cancers16010029
APA StyleHan, S., & Papachristou, G. I. (2024). Therapeutic Endoscopic Ultrasound for Complications of Pancreatic Cancer. Cancers, 16(1), 29. https://doi.org/10.3390/cancers16010029




