Systemic Therapies for HER2-Positive Advanced Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. First-Line Therapy: Trastuzumab, Pertuzumab, and Cytotoxic Chemotherapy
3. Second-Line Therapy: Trastuzumab Deruxtecan (T-DXd)
4. Other Second-Line Therapy Options: Trastuzumab Emtansine (T-DM1)
5. Third Line Options: Tyrosine Kinase Inhibitors
5.1. Lapatinib
5.2. Neratinib
5.3. Tucatinib
5.4. Novel Tyrosine Kinase Inhibitors
5.4.1. Pyrotinib
5.4.2. Poziotinib
5.5. Trastuzumab Duocarmazine
5.6. ARX788
5.7. Other Therapies
5.7.1. Margetuximab (MGAH22)
5.7.2. Bispecific Antibodies
5.7.3. Bispecific Fusion Proteins
5.7.4. Immune Checkpoint Inhibitors
Anti-PDL1 Agents
Anti-PD1 Agents
5.7.5. CDK4/6 Inhibitors
5.7.6. PI3K/Akt/mTOR/AKT Inhibitors
5.7.7. mTOR Inhibitors
5.7.8. PI3K Inhibitors
5.7.9. AKT1 Inhibitors
| Trial | Phase | Setting | Arms | Previous Treatment | n | ORR (%) | Median PFS (Months) | Median OS (Months) | Safety Profile |
|---|---|---|---|---|---|---|---|---|---|
| EGF100151, Geyer et al. (2006) [47] | III | ≥2nd line | Lapatinib + capecitabine Capecitabine | Trastuzumab, anthracycline + taxane | 324 | 22 14 | 8.4 4.1 | 75.0 weeks 64.7 weeks | 12% vs. 11% G3 diarrhoea, 7 vs. 11% G3 PPE |
| Emilia, Verma et al. (2012) [38] | III | 2nd line | T-DM1 Lapatinib + capecitabine (lap/cape) | 100% prior trastuzumab + taxane No pertuzumab | 991 | 44 31 | 9.6 6.4 | 29.9 25.9 | 41% vs. 57% all G3/4 toxicities T-DM1 higher risk of thrombocytopenia + abnormal LFTs |
| Nala, Saura et al. (2020) [69] | III | 3rd line | Neratinib + capecitabine Lapatinib + capecitabine | 75% previous T-DM1 42.5% trastuzumab + pertuzumab | 621 | 32.8 26.7 | 5.6 5.5 | 24 22.2 | 24% vs. 12.5% G3 diarrhoea despite loperamide prophylaxis |
| HER2CLIMB, Murthy et al. (2020) [82] | II | 3rd line | Trastuzumab + capecitabine + tucatinib Trastuzumab + capecitabine + placebo | 100% previous T-DM1 + trastuzumab/ pertuzumab | 612 | 40.6 22.8 | 7.8 5.68 | 24.7 19.2 | 12.9% vs. 8.5% G3 diarrhoea with no loperamide prophylaxis 5% vs. 0.5% G3 transaminitis |
| Phoebe, Xu et al. (2021 INTERIM) [104] | III | 3rd line | Pyrotinib + capecitabine Lapatinib + capecitabine | 100% received trastuzumab + taxane Max 2 lines of chemo | 267 | 67.2 51.5 | 12.5 5.6 | NR NR | 30.6% vs. 8.3% G3 diarrhoea |
| EFG104900, Blackwell et al. (2010) [43] | III | >3rd line | Lapatinib + trastuzumab Lapatinib | 100% prior trastuzumab No T-DM1 or pertuzumab | 296 | 10.3 6.9 | 12.1 weeks 8.1 weeks | 14 9.5 | Asymptomatic cardiac events: 3.4% vs. 1.4% Symptomatic: 2% vs. 0.7% |
| Trial | Phase | Arms | Previous Treatment of Brain Mets | Previous Systemic Treatment | N with CNS Disease | CNS ORR (%) | CNS Median PFS (Months) | CNS Median OS (Months) |
|---|---|---|---|---|---|---|---|---|
| EGF105084, Lin et al. (2009) [56] | II | Lapatinib Lapatinib + capecitabine (expansion phase) | 100% prior cranial radiotherapy | 100% trastuzumab | 240 50 | 6 20 | 2.4 3.65 | 6.37 NR |
| EGF107671, Lin et al. (2011) [57] | II | Lapatinib + capecitabine Lapatinib + topetecan | 100% prior cranial radiotherapy | 100% trastuzumab | 22 | 38 0 | NR NR | NR NR |
| Landscape, Bachelot et al. (2013) [58] | II (single arm) | Lapatinib + capecitabine | Nil | 93% trastuzumab-based chemotherapy | 45 | 65.9 | 5.5 | 17 |
| TBCRC 022, Freedman et al. (2019) [74] | II (single arm) | Neratinib + capecitabine | 92% prior neurosurgery +/− radiotherapy | Cohort A: 50% exposed to lapatinib Cohort B: 50% lapatinib naïve | 49 | 49 33 | 5.5 3.1 | 13.3 15.1 |
| HER2CLIMB, Murthy et al. (2020) [82] | II | Tucatinib + capecitabine + trastuzumab Placebo + capecitabine + trastuzumab | 43% prior cranial radiotherapy | 100% trastuzumab, pertuzumab and T-DM1 | 291 | 47.3 20 | 9.9 4.2 | 18.1 12 |
| Kamilla, Montemuro et al., 2020, Wuerstlein et al. (2022) [38] | IIIb | T-DM1 | 46.8% prior cranial radiotherapy | 100% trastuzumab and taxane chemotherapy | 126 (AS) | 21.4% | 5.5 | 18.9 |
| DESTINY-BREAST 01, Modi et al., 2021 [22] | II | Trastuzumab Deruxtecan | 58.3% prior cranial radiotherapy 12.5% prior surgery and RT 4.2% prior surgery, RT and capecitabine | 100% trastuzumab and taxane chemotherapy | 24 | 58.3 | 18.1 | NR |
| TUXEDO 1 Bartsch et al., 2021 [27]) | II | Trastuzumab Deruxtecan | 60% prior cranial radiotherapy | 100% trastuzumab and taxane chemotherapy | 15 | 83.3% | 14 | NR |
| DESTINY BREAST 03, Cortes et al., 2022 [24] | III | Trastuzumab Deruxtecan | N/A | 100% trastuzumab and taxane chemotherapy | 82 | 67.4% | 15 | NR |
| PATRICIA, Lin et al., 2021 [102] | II | High-dose trastuzumab/pertuzumab | 100% prior cranial radiotherapy | 100% trastuzumab and taxane chemotherapy | 39 | 11% | N/A | N/A |
| Trial Identifier | Phase | Treatment Setting | Population | Planned Sample Size | Treatment Arms | Primary Endpoint | Study Status |
|---|---|---|---|---|---|---|---|
| Tucatinib | |||||||
| NCT03975647 (HER2CLIMB-02) | III (RCT double blind) | 2nd line | HER2 + MBC | 460 | Tucatinib + T-DM1 vs. placebo and T-DM1 | PFS | Recruited, results awaited |
| NCT04539938 (HER2CLIMB-04) | II (single arm) | 3rd line | HER2 + MBC | 70 | Tucatinib + T-Dxd | ORR | Recruiting |
| NCT05132582 (HER2-CLIMB-05) | III | 2nd line | HER2 + MBC | 650 | Trastuzumab and pertuzumab and Placebo Vs | PFS | Recruiting |
| NCT03054363 | Ib/II (single arm) | ≥3rd line | HR + HER2 + MBC | 42 | Tucatinib, palbociclib + letrozole | Phase Ib: incidence of toxicity Phase II: PFS | Active, not recruiting |
| NCT03501979 | II (single arm) | No previous LMD specific therapy | HER2 + MBC with leptomeningeal disease | 30 | Tucatinib, trastuzumab + capecitabine | OS | Active, not recruiting |
| NCT04760431 (HER2BRAIN) | II (RCT double bind) | 2nd line (progression on/after trastuzumab) | HER2 + MBC with active brain metastases | 120 | Trastuzumab, taxane + pertuzumab vs. trastuzumab, taxane + TKI (Tucatinib, pyrotinib or neratinib) | CNS ORR | Not yet recruiting |
| Neratinib | |||||||
| NCT03377387 | Ib/II | Ib: any line II: ≥2nd line | HER2 + MBC | 48 | Capecitabine 7/7 + neratinib | Phase Ib: MTD | Active, not recruiting |
| NCT01494662 | II (4 cohorts, non-randomised) | Varied | HER2 + MBC with brain metastases | 168 | (1) Neratinib (2) Neratinib + surgical resection (3) Neratinib + capecitabine (4) Neratinib + T-DM1 | CNS ORR | Active, not recruiting |
| Pyrotinib | |||||||
| NCT03080805 (PHOEBE) | III (RCT) | ≥2nd line (must have had prior trastuzumab + taxane) | HER2 + MBC | 240 | Pyrotinib + capecitabine vs. Lapatinib + capecitabine | PFS | Unknown |
| Poziotinib | |||||||
| NCT02659514 | II (single arm) | 3rd line (must have had previous trastuzumab and T-DM1) | HER2 + MBC | 67 | Poziotinib monotherapy | ORR | Active, not recruiting |
| Antibody Drug Conjugates | |||||||
| NCT04538742 (DESTINY-BREAST07) | Ib/II | 1st line | HER2 + MBC including those with active brain metastases | 350 | 7 cohorts combining T-DXd with: (1) Durvalumab (2) Pertuzumab (3) Paclitaxel (4) Durvalumab/ paclitaxel (5) T-DXd alone (6) Tucatinib (7) Tucatinib in active brain mets (8) T-DXd alone in active brain mets | Phase Ib/II: incidence of toxicity | Active, not recruiting |
| NCT04739761 (DESTINY-BREAST12) | III | ≥2nd line | HER2 + MBC including those with active brain metastases | T-DXd | ORR, PFS | Active, not recruiting | |
| NCT04492488 | I/II | ≥2nd line | HER2 + advanced cancers | 129 | MRG002 | NTD, RP2D, ORR and Aes | Recruiting |
| NCT03944499 | I | ≥2nd line | HER2 + advanced cancers | 297 | FS-1502 | NTD, RP2D, ORR and Aes | Recruiting |
| NCT03953833 | I | ≥2nd line | HER2 + advanced cancers | 45 | B003 | NTD, RP2D, ORR and Aes | Active, not recruiting |
| NCT04450732 | I | ≥2nd line | HER2 + advanced cancers | 96 | GQ1001 | NTD, RP2D, ORR and Aes | Recruiting |
| Trial | Phase | Patient Number | Arms | Results/Status |
|---|---|---|---|---|
| PANACEA (NCT02129556) | Ib-II | n = 58 | Trastuzumab + pembrolizumab | ORR: 15% of PD-L1 + pts No ORs among PD-L1 − pts mPFS: 2.7 mos (90% CI 2.6–4.0) in PD-L1+ mPFS: 2.5 mos (90% CI 1.4–2.7) in PD-L1-ve |
| KATE-2 | II | n = 202 | TDM-1 + atezolizumab TDM-1 + placebo | mPFS in PDL1 + pts T-DM1+ atezo 8.5 m T-DM1+ placebo 4.1 m HR 0.60 (CI 0.32–1.11) |
| KATE-3 (NCT04740918) | III | n = 320 | TDM1+ atezolizumab/placebo | Active–not recruiting |
| NCT03125928 | II | n = 50 | Single arm Paclitaxel-trastuzumab-pertuzumab-atezolizumab | Recruiting |
| NRG-BR004 (NCT03199885) | III | n = 600 | Paclitaxel-trastuzumab-pertuzumab-atezolizumab/placebo | Active–not recruiting |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, C.R.; Kraus, M.H.; Aaronson, S.A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985, 229, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Luque-Cabal, M.; García-Teijido, P.; Fernández-Pérez, Y.; Sánchez-Lorenzo, L.; Palacio-Vázquez, I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin. Med. Insights Oncol. 2016, 10, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U. Better treatments needed for breast cancer brain metastases. Lancet Oncol. 2015, 16, 1583–1584. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; O’Shaughnessy, J.; Mason, G.; Yardley, D.A.; Jahanzeb, M.; Brufsky, A.; Rugo, H.S.; Swain, S.M.; Kaufman, P.A.; Tripathy, D.; et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: Patient characteristics, treatment, and survival from systhers. Clin. Cancer Res. 2019, 25, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Adams, C.W.; Allison, D.E.; Flagella, K.; Presta, L.; Clarke, J.; Dybdal, N.; McKeever, K.; Sliwkowski, M.X. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol. Immunother. 2006, 55, 717–727. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Monturus, E.; Clark, E.; et al. End-of-study analysis from the phase III, randomized, double-blind, placebo (Pla)-controlled CLEOPATRA study of first-line (1L) pertuzumab (P), trastuzumab (H), and docetaxel (D) in patients (pts) with HER2-positive metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, 1020. [Google Scholar] [CrossRef]
- Bachelot, T.; Ciruelos, E.; Schneeweiss, A.; Puglisi, F.; Peretz-Yablonski, T.; Bondarenko, I.; Paluch-Shimon, S.; Wardley, A.; Merot, J.L.; Du Toit, Y.; et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann. Oncol. 2019, 30, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Rizwanullah, M.; Im, S.-A.; Sánchez-Ruiz, A.C.; Lang, I.; Tomasello, G.; Douthwaite, H.; Badovinac Crnjevic, T.; Heeson, S.; Eng-Wong, J.; et al. PHEREXA: A phase III study of trastuzumab (H) + capecitabine (X) ± pertuzumab (P) for patients (pts) who progressed during/after one line of H-based therapy in the HER2-positive metastatic breast cancer (MBC) setting. J. Clin. Oncol. 2016, 34, 504. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)†. Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.; Trudeau, M.; Awada, A.; Blackwell, K.; Bachelot, T.; Salazar, V.; DeSilvio, M.; Westlund, R.; Zaks, T.; Spector, N.; et al. Lapatinib monotherapy in patients with HER2-overexpressing relapsed or refractory inflammatory breast cancer: Final results and survival of the expanded HER2+ cohort in EGF103009, a phase II study. Lancet Oncol. 2009, 10, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Pippen, J.; Pivot, X.; Lichinitser, M.; Sadeghi, S.; Dieras, V.; Gomez, H.L.; Romieu, G.; Manikhas, A.; Kennedy, M.J.; et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-Positive metastatic breast cancer. J. Clin. Oncol. 2009, 27, 5538–5546. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Hegg, R.; Im, S.A.; Park, I.H.; Burdaeva, O.; Kurteva, G.; Press, M.F.; Tjulandin, S.; Iwata, H.; Simon, S.D.; et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor–positive metastatic breast. J. Clin. Oncol. 2021, 39, 79–89. [Google Scholar] [CrossRef]
- Arpino, G.; de la Haba Rodriguez, J.; Ferrero, J.-M.; De Placido, S.; Klingbiel, D.; Revelant, V.; Wohlfarth, C.; Poppe, R.; Rimawi, M.F. PD3-02 Final analysis of PERTAIN: A randomised, two-arm, open-label multicentre phase II trial assessing the efficacy and safety and safety of first-line pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in patients with HER2-po. In Proceedings of the Abstracts: 2020 San Antonio Breast Cancer Virtual Symposium, San Antonio, TX, USA, 8–11 December 2020. [Google Scholar]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Wing, J.; Chiu, Y.; Xu, B.; Hamilton, E.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2022, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Chen, S.; Cathcart, J.; Lee, C.; Perrin, C. 138O CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Ann. Oncol. 2020, 31, S63–S64. [Google Scholar] [CrossRef]
- Bartsch, R.; Sophie Berghoff, A.; Furtner, J.; Marhold, M.; Sophie Bergen, E.; Roider-Schur, S.; Martina Starzer, A.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04739761 (accessed on 21 May 2021).
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro. Oncol. 2023, 25, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Alder, L.; Trapani, D.; Bradbury, C.; Van Swearingen, A.E.D.; Tolaney, S.M.; Khasraw, M.; Anders, C.K.; Lascola, C.D.; Hsu, L.; Lin, N.U.; et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. NPJ Breast Cancer 2023, 9, 19. [Google Scholar] [CrossRef]
- Issell, B.F.; Crooke, S.T. Maytansine. Cancer Treat. Rev. 1978, 5, 199–207. [Google Scholar] [CrossRef]
- Perez, E.A.; Hurvitz, S.A.; Amler, L.C.; Mundt, K.E.; Ng, V.; Guardino, E.; Gianni, L. Relationship between HER2 expression and efficacy with first-line trastuzumab emtansine compared with trastuzumab plus docetaxel in TDM4450g: A randomized phase II study of patients with previously untreated HER2-positive metastatic breast cancer. Breast Cancer Res. 2014, 16, R50. [Google Scholar] [CrossRef]
- Krop, I.E.; Beeram, M.; Modi, S.; Jones, S.F.; Holden, S.N.; Yu, W.; Girish, S.; Tibbitts, J.; Yi, J.H.; Sliwkowski, M.X.; et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J. Clin. Oncol. 2010, 28, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; LoRusso, P.; Miller, K.D.; Modi, S.; Yardley, D.; Rodriguez, G.; Guardino, E.; Lu, M.; Zheng, M.; Girish, S.; et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 2012, 30, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Dirix, L.; Kocsis, J.; Bianchi, G.V.; Lu, J.; Vinholes, J.; Guardino, E.; Song, C.; Tong, B.; Ng, V.; et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2013, 31, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H.; et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: Primary results from the phase III MARIANNE study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Konecny, G.E.; Pegram, M.D.; Venkatesan, N.; Finn, R.; Yang, G.; Rahmeh, M.; Untch, M.; Rusnak, D.W.; Spehar, G.; Mullin, R.J.; et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.; Sledge, G.; Koehler, M.; Ellis, C.; Casey, M.; Vukelja, S.; Bischoff, J.; et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1124–1130. [Google Scholar] [CrossRef]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.S.; Sledge, G.; Aktan, G.; Ellis, C.; Florance, A.; Vukelja, S.; Bischoff, J.; et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 study. J. Clin. Oncol. 2012, 30, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743, Erratum in N. Engl. J. Med. 2007, 356, 1487. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Diéras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.J.; Roché, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.; Ashley, S.; Miles, D.; Chan, S.; Wardley, A.; Davidson, N.; Bhatti, R.; Shehata, M.; Nouras, H.; Camburn, T.; et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases-the UK experience. Br. J. Cancer 2010, 102, 995–1002. [Google Scholar] [CrossRef]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.Y.; Gonçalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Manikhas, A.; Zurawski, B.; Chmielowska, E.; Karaszewska, B.; Allerton, R.; Chan, S.; Fabi, A.; Bidoli, P.; Gori, S.; et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2015, 33, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Gijsen, M.; Mullooly, M.; Bennett, R.; Bouguern, N.; Pedersen, K.; O’Brien, N.A.; Roxanis, I.; Li, J.L.; Bridge, E.; et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 2013, 4, 1592–1605. [Google Scholar] [CrossRef]
- Awada, A.; Colomer, R.; Inoue, K.; Bondarenko, I.; Badwe, R.A.; Demetriou, G.; Lee, S.C.; Mehta, A.O.; Kim, S.B.; Bachelot, T.; et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer the NEfERT-T randomized clinical trial. JAMA Oncol. 2016, 2, 1557–1564. [Google Scholar] [CrossRef]
- Awada, A.H.; Brufsky, A.M.; Saura, C.; Freedman, R.A.; Lin, N.U.; Bebchuk, J.; Xu, F.; Hurvitz, S. Abstract P2-20-01: Impact of neratinib on development and progression of central nervous system (CNS) metastases in patients with HER2-positive metastatic breast cancer (MBC): Findings from the NALA, NEfERT-T, and TBCRC 022 trials. Cancer Res. 2020, 80 (Suppl. S4), P2-20-01. [Google Scholar] [CrossRef]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.J.; Masuda, N.; et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J. Clin. Oncol. 2019, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Saura, C.; Oliveira, M.; Trudeau, M.E.; Moy, B.; Delaloge, S.; Gradishar, W.; Kim, S.B.; Haley, B.; Ryvo, L.; et al. Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with CNS Involvement from the NALA trial. Oncologist 2021, 26, e1327–e1338. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Puhalla, S.; Sikov, W.M.; Montero, A.J.; Salkeni, M.A.; Razaq, W.; Beumer, J.H.; Kiesel, B.; Buyse, M.E.; Adamson, L.M.; et al. NSABP FB-10: Phase Ib dose-escalation trial evaluating trastuzumab emtansine (T-DM1) with neratinib (N) in women with metastatic HER2+ breast cancer (MBC). J. Clin. Oncol. 2018, 36, 1027. [Google Scholar] [CrossRef]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; De Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, V.; Anderson, D.; Winski, S.; Winkler, J.; Koch, K.; Lee, P.A. Abstract 852: ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice. Cancer Res. 2012, 72 (Suppl. S8), 852. [Google Scholar] [CrossRef]
- Metzger Filho, O.; Leone, J.P.; Li, T.; Tan-Wasielewski, Z.; Trippa, L.; Barry, W.T.; Younger, J.; Lawler, E.; Walker, L.; Freedman, R.A.; et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann. Oncol. 2020, 31, 1231–1239. [Google Scholar] [CrossRef]
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.I.; Khan, Q.J.; Krop, I.E.; Welch, S.; Bedard, P.L.; Conlin, A.K.; Chaves, J.; et al. Efficacy results of a phase 1b study of ONT-380, a CNS-penetrant TKI, in combination with T-DM1 in HER2+ metastatic breast cancer (MBC), including patients (pts) with brain metastases. J. Clin. Oncol. 2016, 34, 513. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Okines, A.F.C.; Paplomata, E.; Wahl, T.A.; Wright, G.L.S.; Sutherland, S.; Jakobsen, E.; Valdes, F.; Chan, A.; Clark, A.S.; Conlin, A.K.; et al. Management of adverse events in patients with HER2+ metastatic breast cancer treated with tucatinib, trastuzumab, and capecitabine (HER2CLIMB). J. Clin. Oncol. 2020, 38, 1043. [Google Scholar] [CrossRef]
- Stringer-Reasor, E.M.; O’Brien, B.J.; Topletz-Erickson, A.; White, J.B.; Lobbous, M.; Riley, K.; Childress, J.; LaMaster, K.; Melisko, M.E.; Morikawa, A.; et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J. Clin. Oncol. 2021, 39, 1044. [Google Scholar] [CrossRef]
- Murthy, R.K.; O’Brien, B.; Berry, D.A.; Singareeka-Raghavendra, A.; Monroe, M.G.; Johnson, J.; White, J.; Childress, J.; Sanford, J.; Schwartz-Gomez, J.; et al. Abstract PD4-02: Safety and efficacy of a tucatinib-trastuzumab-capecitabine regimen for treatment of leptomeningeal metastasis (LM) in HER2-positive breast cancer: Results from TBCRC049, a phase 2 non-randomized study. Cancer Res. 2022, 82, PD4-02. [Google Scholar] [CrossRef]
- Hurvitz, S.; Loi, S.; O’Shaughnessy, J.; Okines, A.; Tolaney, S.; Sohn, J.H.; Saura, C.; Zhu, X.; Cameron, D.; Bachelot, T.; et al. HER2CLIMB-02: Randomized, Double-Blind Phase 3 Trial of Tucatinib and Trastuzumab Emtansine for Previously Treated HER2-Positive Metastatic Breast Cancer. Abstract GS1-10. SABCS 2023.
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.; Khan, Q.; Krop, I.; Welch, S.; Conlin, A.; Chaves, J.; Bedard, P.L.; et al. Tucatinib combined with ado-Trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2018, 4, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Chen, S.; Zhu, W.; Fan, Y.; Wang, J.; Luo, Y.; Xing, P.; Lan, B.; Li, M.; et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ERBB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J. Clin. Oncol. 2017, 35, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or lapatinib combined with capecitabine in HER2–positive metastatic breast cancer with prior taxanes, anthracyclines, and/ or trastuzumab: A randomized, phase II study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Bian, L.; Hu, X.; Zhang, Q.; Ouyang, Q.; Feng, J.; Yin, Y.; Sun, T.; Tong, Z.; Wang, X.; et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): A randomized, double-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 2020, 1, 13. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Ouyang, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z. Pyrotinib plus capecitabine for HER2-positive metastatic breast cancer patients with brain metastases (PERMEATE): A multicenter, single-arm phase II study. J. Clin. Oncol. 2021, 39, 1037. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, K.H.; Sohn, J.H.; Lee, K.S.; Jung, K.H.; Kim, J.H.; Lee, K.H.; Ahn, J.S.; Kim, T.Y.; Kim, G.M.; et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: Results of the NOV120101-203 trial. Int. J. Cancer 2018, 143, 3240–3247. [Google Scholar] [CrossRef]
- Brufsky, A.; Zulfiqar, M.; Peguero, J.; Lathrop, K.; Bhat, G.; Lebel, F. PD1-07 A Phase 2 study of poziotinib in patients with HER2-positive metastatic breast cancer heavily pre-treated with HER2-targeted therapy. In Proceedings of the San Antonio Breast Cancer Symposium. Cancer Res. 2021, 81 (Suppl. S4), PD1-07. [Google Scholar] [CrossRef]
- Veeraraghavan, J.; Mistry, R.; Nanda, S.; Sethunath, V.; Shea, M.; Mitchell, T.; Anurag, M. HER2 L755S mutation is acquired upon resistance to lapatinib and neratinib and confers cross-resistance to tucatinib and trastuzumab in HER2-positive breast cancer cell models. In Proceedings of the San Antonio Breast Cancer Symposium. Cancer Res. 2020, 81, PD3-09. [Google Scholar]
- Van Herpen, C.M.; Banerji, U.; Mommers, E.C.; Koper, N.P.; Goedings, P.; Lopez, J.; Awada, A.; Fiebrich, H.B.; Aftimos, P.G. 333 Phase I dose-escalation trial with the DNA-alkylating anti-HER2 antibody-drug conjugate SYD985. Eur. J. Cancer 2015, 51, S65. [Google Scholar] [CrossRef]
- Saura, C.; Thistlethwaite, F.; Banerji, U.; Lord, S.; Moreno, V.; MacPherson, I.; Boni, V.; Rolfo, C.D.; de Vries, E.G.E.; Van Herpen, C.M.L.; et al. A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. J. Clin. Oncol. 2018, 36, 1014. [Google Scholar] [CrossRef]
- Aftimos, P.; van Herpen, C.; Mommers, E.; Koper, N.; Goedings, P.; Oesterholt, M.; Awada, A.; Desar, I.; Lim, J.; Dean, E.; et al. Abstract P6-12-02: SYD985, a novel anti-HER2 ADC, shows promising activity in patients with HER2-positive and HER2-negative metastatic breast cancer. Cancer Res. 2017, 77, P6-12-02. [Google Scholar] [CrossRef]
- ESMO Congress 2021|OncologyPRO [Internet]. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/primary-outcome-of-the-phase-iii-syd985.002-tulip-trial-comparing-vic-trastuzumab-duocarmazine-to-physician-s-choice-treatment-in-patients-with-p (accessed on 3 October 2023).
- Hurvitz, S.A.; Park, H.; Frentzas, S.; Shannon, C.M.; Cuff, K.; Eek, R.W.; Budd, G.T.; McCartney, A.; O’Shaughnessy, J.; Lu, J.M.; et al. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpresing solid tumors: Results from two phase 1 clinical trials. J. Clin. Oncol. 2021, 39, 1038. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, D.; Shen, W.; Xiao, Q.; Gu, Y.; O’Shaughnessy, J.; Hu, X. Phase I Trial of a Novel Anti-HER2 Antibody-Drug Conjugate, ARX788, for the Treatment of HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2022, 28, 4212–4221. [Google Scholar] [CrossRef]
- ACE-Breast-02 Pivotal Phase 3 Study of Ambrx’s ARX788 for the Treatment of HER2 Positive Metastatic Breast Cancer Achieves Positive Results. Available online: https://ir.ambrx.com/news/news-details/2023/ACE-Breast-02-Pivotal-Phase-3-Study-of-Ambrxs-ARX788-for-the-Treatment-of-HER2-Positive-Metastatic-Breast-Cancer-Achieves-Positive-Results/default.aspx (accessed on 2 October 2023).
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.-A.; Wright, G.L.S.; Escriva-de-Romani, S.; DeLaurentiis, M.; Cortes, J.; Bahadur, S.W.; Haley, B.B.; Oyola, R.H.; Riseberg, D.A.; et al. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J. Clin. Oncol. 2019, 37, 1000. [Google Scholar] [CrossRef]
- Geuijen, C.; Rovers, E.; Gallenne, T.; Maussang-Detaille, D.; Kramer, A.; Nieuwenhuizen, N.; Clements, C.; van Zoest, K.; Nijhuis, R.; Visser, T.; et al. Abstract LB-261: Mechanism of action of MCLA-128, a humanized bispecific IgG1 antibody targeting the HER2:HER3 heterodimer. Cancer Res. 2015, 75 (Suppl. S15), LB-261. [Google Scholar] [CrossRef]
- Alsina, M.; Varga, A.; Amatu, A.; Schellens, J.H.M.; Witteveen, P.O.; Boni, V.; Moreno, V.; Bol, K.; Lourbakos, A.; Magin Ferrer, M.; et al. Phase I/II study of single agent MCLA-128, a full length IgG1 bispecific antibody targeting the HER3 pathway: Overall safety at the recommended phase II dose (R2PD) and preliminary activity in HER2+ metastatic gastric/gastroesophageal junction cancer (GC/GEJ). Ann. Oncol. 2018, 29, viii223–viii224. [Google Scholar] [CrossRef]
- Weisser, N.; Wickman, G.; Davies, R.; Rowse, G. Abstract 31: Preclinical development of a novel biparatopic HER2 antibody with activity in low to high HER2 expressing cancers. Cancer Res. 2017, 77, 31. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Mayordomo, J.I.; Hanna, D.L.; Ajani, J.A.; Blum Murphy, M.A.; Murthy, R.K.; Piha-Paul, S.A.; Bauer, T.M.; Bendell, J.C.; et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J. Clin. Oncol. 2018, 36, 2500. [Google Scholar] [CrossRef]
- Escriva-de Romani, S.; Cejalvo, J.M.; Alba, E.; Friedman, J.; Rodriguez-Lescure, A.; Savard, M.-F.; Pezo, R.C.; Gion, M.; Ruiz-Borrego, M.; Hamilton, E.; et al. Primary results from a phase 2a study of zanidatamab (zani) + palbociclib (palbo) + fulvestrant (fulv) in HER2+/HR+ metastatic breast cancer (mBC). Abstract LB01-04. SABCS 2023.
- Hinner, M.J.; Aiba, R.-S.B.; Wiedenmann, A.; Schlosser, C.; Allersdorfer, A.; Matschiner, G.; Rothe, C.; Moebius, U.; Kohrt, H.E.; Olwill, S.A. Costimulatory T cell engagement via a novel bispecific anti-CD137 /anti-HER2 protein. J. Immunother. Cancer 2015, 3, P187. [Google Scholar] [CrossRef]
- Piha-Paul, S.; Bendell, J.; Tolcher, A.; Hurvitz, S.; Patnaik, A.; Shroff, R.; Pohlmann, P.; Zettl, M.; Hahn, N.; Krishnamurthy, A.; et al. O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J. Immunother. Cancer 2020, 8 (Suppl. S1), A1–A12. [Google Scholar] [CrossRef]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Lustberg, M.; Wesolowski, R.; Ramaswamy, B.; Parwani, A.V.; Li, Z. PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J. 2018, 24, 911–919. [Google Scholar] [CrossRef]
- Park, S.G.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef]
- Dirix, L.; Takacs, I.; Nikolinakos, P.; Jerusalem, G.; Arkenau, H.-T.; Hamilton, E.; von Heydebreck, A.; Grote, H.-J.; Chin, K.; Lippman, M. Abstract S1-04: Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase Ib JAVELIN solid tumor trial. Cancer Res. 2016, 76, S1-04. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.B.; Im, S.A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Hamilton, E.P. PD3-07 Trastuzumab-deruxtecan (T-DXd; DS-8201 with nivolumab in patients with HER2-expressing advanced breast cancer: A 2-part phase 1b, multicentre, open label study. In Proceedings of the San Antonio Breast Cancer Symposium, Virtual, 8–11 December 2020. [Google Scholar]
- Agostinetto, E.; Montemurro, F.; Puglisi, F.; Criscitiello, C.; Bianchini, G.; Del Mastro, L.; Introna, M.; Tondini, C.; Santoro, A.; Zambelli, A. Immunotherapy for HER2-Positive Breast Cancer: Clinical Evidence and Future Perspectives. Cancers 2022, 14, 2136. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Wang, Q.; Watt, A.C.; Tolaney, S.M.; Dillon, D.A.; Li, W.; Ramm, S.; Palmer, A.C.; Yuzugullu, H.; Varadan, V.; et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016, 29, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Kabos, P.; Yardley, D.; Dieras, V.; Costigan, T.; Klise, S.; Awada, A. Abstract P1-17-03: Abemaciclib for the treatment of brain metastases secondary to hormone receptor positive breast cancer. Cancer Res. 2018, 78, P1-17-03. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.; Troso-Sandoval, T.; Ricci, F.; Im, S.-A.; Kim, S.-B.; Johnston, S.R.D.; Chan, A.; et al. MonarcHER: A randomized phase II study of abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with HR+, HER2+ advanced breast cancer (ABC). Ann. Oncol. 2019, 30, v861–v862. [Google Scholar] [CrossRef]
- Prat, A.; Brase, J.C.; Cheng, Y.; Nuciforo, P.; Paré, L.; Pascual, T.; Martinez, D.; Galvan, P.; Vidal, M.; Adamo, B.; et al. PAM50 intrinsic subtype in hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) treated with exemestane (EXE) in combination with everolimus (EVE) or placebo (PBO): A correlative analysis o. Eur. J. Cancer 2018, 92, S117–S118. [Google Scholar] [CrossRef]
- Shagisultanova, E.; Chalasani, P.; Brown-Glaberman, U.A.; Gradishar, W.J.; Brenner, A.J.; Stopeck, A.; Gao, D.; McSpadden, T.; Kabos, P.; Borges, V.F. Tucatinib, palbociclib, and letrozole in HR+/HER2+ metastatic breast cancer: Report of phase IB safety cohort. J. Clin. Oncol. 2019, 37, 1029. [Google Scholar] [CrossRef]
- Nagata, Y.; Lan, K.H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef]
- Serra, V.; Scaltriti, M.; Prudkin, L.; Eichhorn, P.J.A.; Ibrahim, Y.H.; Chandarlapaty, S.; Markman, B.; Rodriguez, O.; Guzman, M.; Rodriguez, S.; et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 2011, 30, 2547–2557. [Google Scholar] [CrossRef]
- Andre, F.; Campone, M.; O’Regan, R.; Manlius, C.; Massacesi, C.; Sahmoud, T.; Mukhopadhyay, P.; Soria, J.C.; Naughton, M.; Hurvitz, S.A. Phase I Study of Everolimus Plus Weekly Paclitaxel and Trastuzumab in Patients with Metastatic Breast Cancer Pretreated with Trastuzumab. J. Clin. Oncol. 2010, 28, 5110–5115. [Google Scholar] [CrossRef]
- Jerusalem, G.; Fasolo, A.; Dieras, V.; Cardoso, F.; Bergh, J.; Vittori, L.; Zhang, Y.; Massacesi, C.; Sahmoud, T.; Gianni, L. Phase i trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res. Treat. 2011, 125, 447–455. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef] [PubMed]
- André, F.; O’Regan, R.; Ozguroglu, M.; Toi, M.; Xu, B.; Jerusalem, G.; Masuda, N.; Wilks, S.; Arena, F.; Isaacs, C.; et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014, 15, 580–591. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Hurvitz, S.; Fasolo, A.; Tseng, L.M.; Jerusalem, G.; Wilks, S.; O’Regan, R.; Isaacs, C.; Toi, M.; Burris, H.; et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: Combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J. Clin. Oncol. 2016, 34, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Shah, A.N.; Santa-Maria, C.A.; Siziopikou, K.; Rademaker, A.; Helenowski, I.; Cristofanilli, M.; Gradishar, W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018, 171, 371–381. [Google Scholar] [CrossRef]
- Baselga, J.; Phillips, G.D.L.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; De Haas, S.L.; Pegram, M.D. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-Positive metastatic breast cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
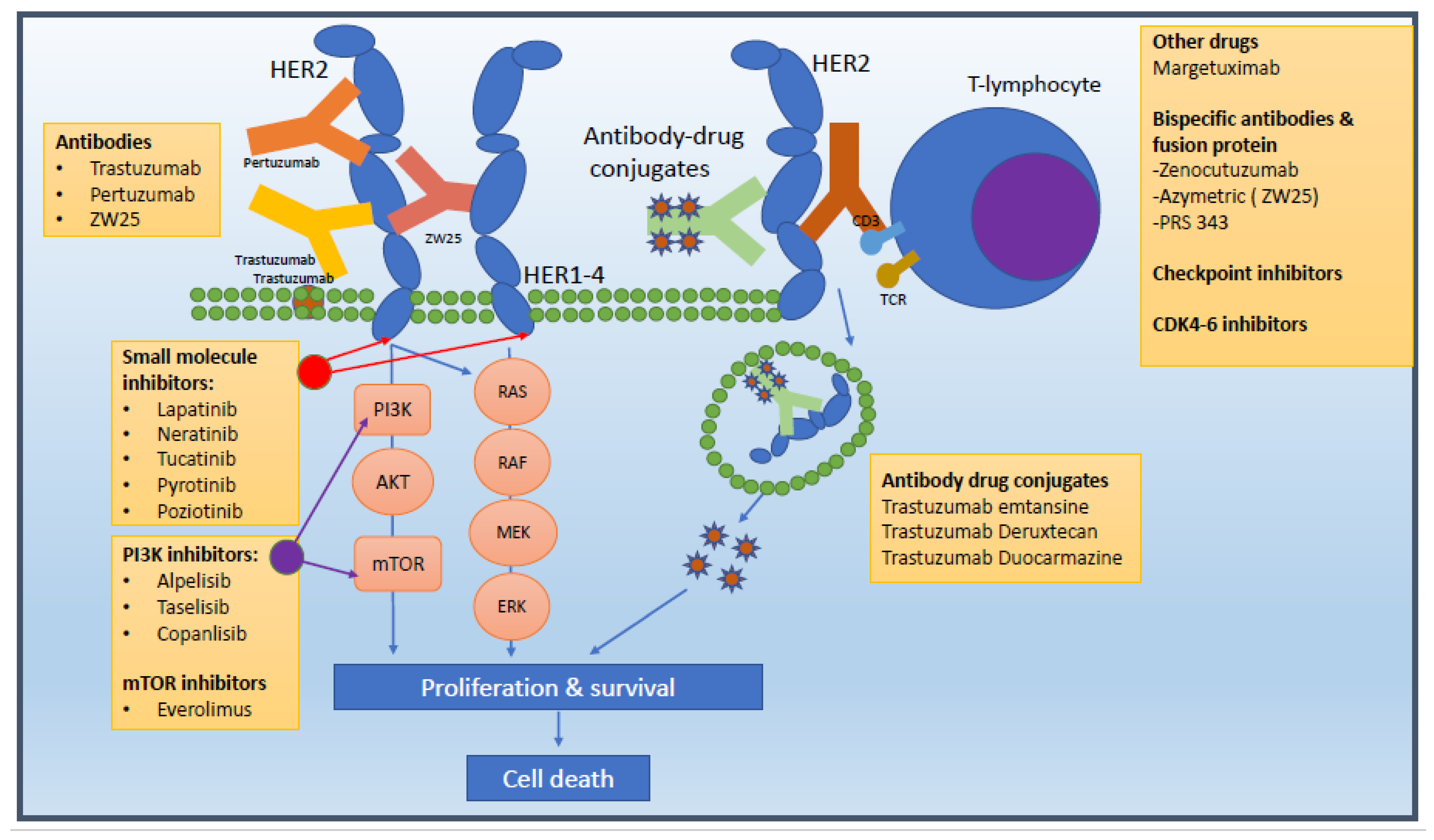
| Trial | Phase | Patient Number /Line | Arms | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|
| Cleopatra | III | n = 808 1st line | Docetaxel + trastuzumab + pertuzumab Docetaxel + trastuzumab + placebo | 18.5 12.4 | 57.1 40.8 |
| Peruse | IIIb | n = 1436 1st line | Docetaxel + pertuzumab + trastuzumab Paclitaxel + pertuzumab + trastuzumab Nab-paclitaxel + pertuzumab + trastuzumab | 19.4 23.2 19.2 | 66.5 64 70.9 |
| Pertain | II | n = 129 1st line | Aromatase inhibitor or taxane + Trastuzumab + pertuzumab Aromatase inhibitor or taxane + Trastuzumab | 21 16 | 60 57 |
| Marianne | III | n = 1095 First line | Taxane + trastuzumab TDM-1 TDM-1 + pertuzumab | 13.7 14.1 15.2 | 50.9 53.7 51.8 |
| Emilia | III | n = 991 Second line | TDM-1 Capecitabine + lapatinib | 9.6 6.4 | 29.9 25.9 |
| Destiny Breast 03 | III | n = 699 Second line | Trastuzumab deruxtecan T-DM1 | 28.8 6.8 | NR NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelis, V.; Okines, A.F.C. Systemic Therapies for HER2-Positive Advanced Breast Cancer. Cancers 2024, 16, 23. https://doi.org/10.3390/cancers16010023
Angelis V, Okines AFC. Systemic Therapies for HER2-Positive Advanced Breast Cancer. Cancers. 2024; 16(1):23. https://doi.org/10.3390/cancers16010023
Chicago/Turabian StyleAngelis, Vasileios, and Alicia F. C. Okines. 2024. "Systemic Therapies for HER2-Positive Advanced Breast Cancer" Cancers 16, no. 1: 23. https://doi.org/10.3390/cancers16010023
APA StyleAngelis, V., & Okines, A. F. C. (2024). Systemic Therapies for HER2-Positive Advanced Breast Cancer. Cancers, 16(1), 23. https://doi.org/10.3390/cancers16010023





