Optimized Modeling of Metastatic Triple-Negative Invasive Lobular Breast Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
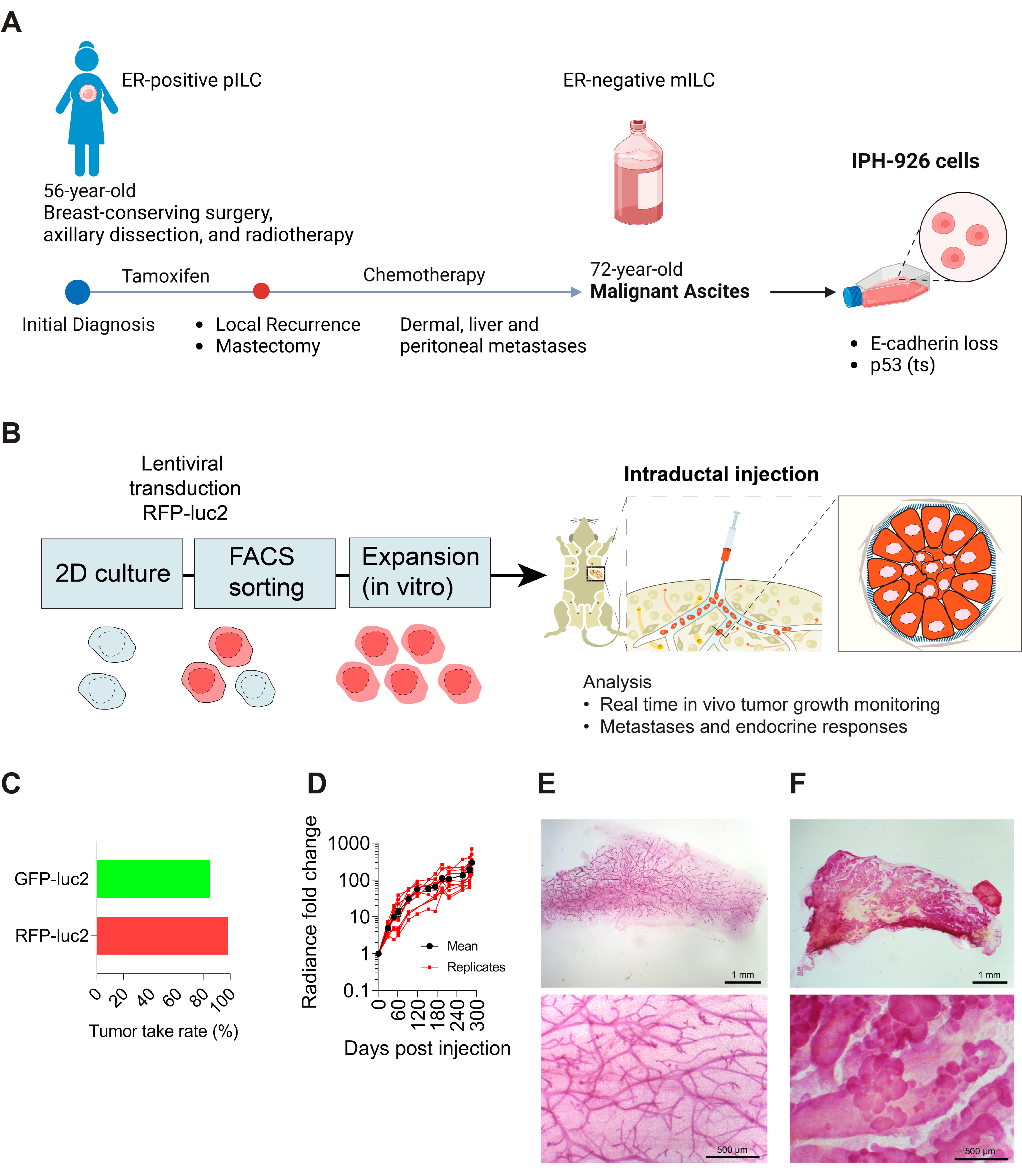
2.1. Animal Experiments
2.2. Cell Culture
2.3. Xenograft Tissue Handling and Digestion
2.4. Intraductal Injections
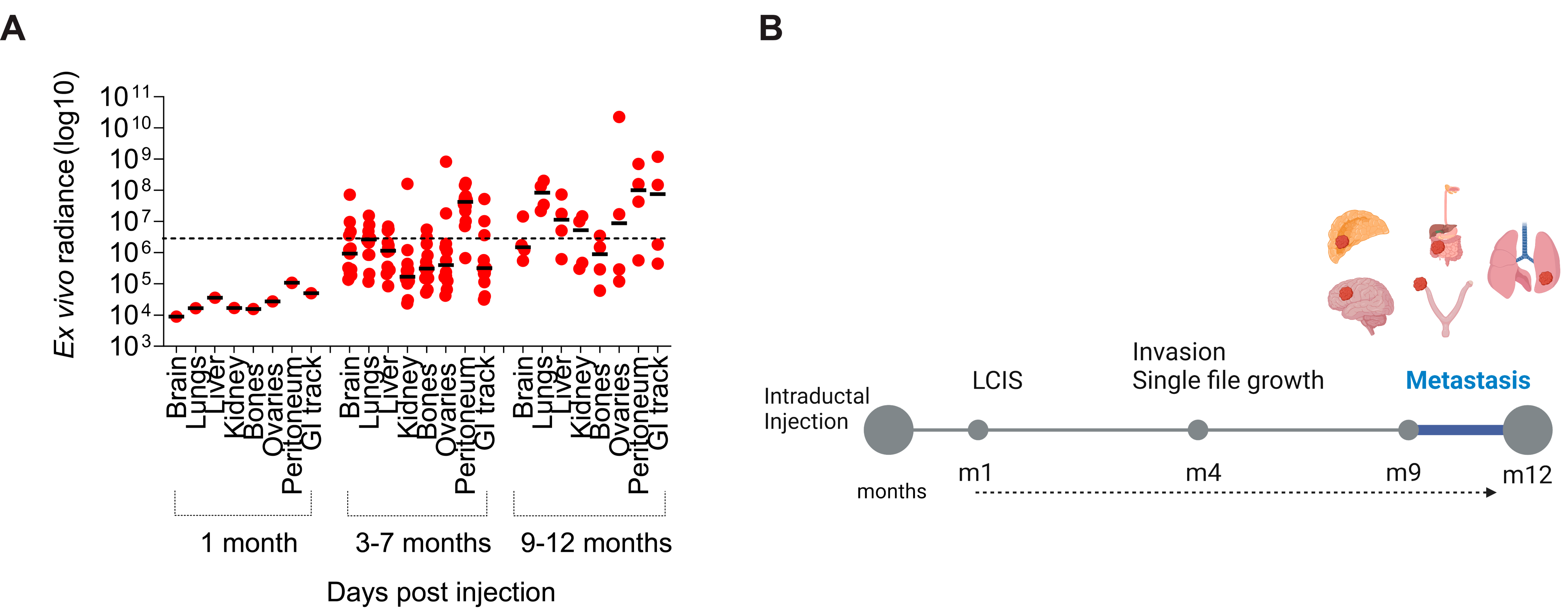
2.5. Tumor Growth and Metastasis Analysis
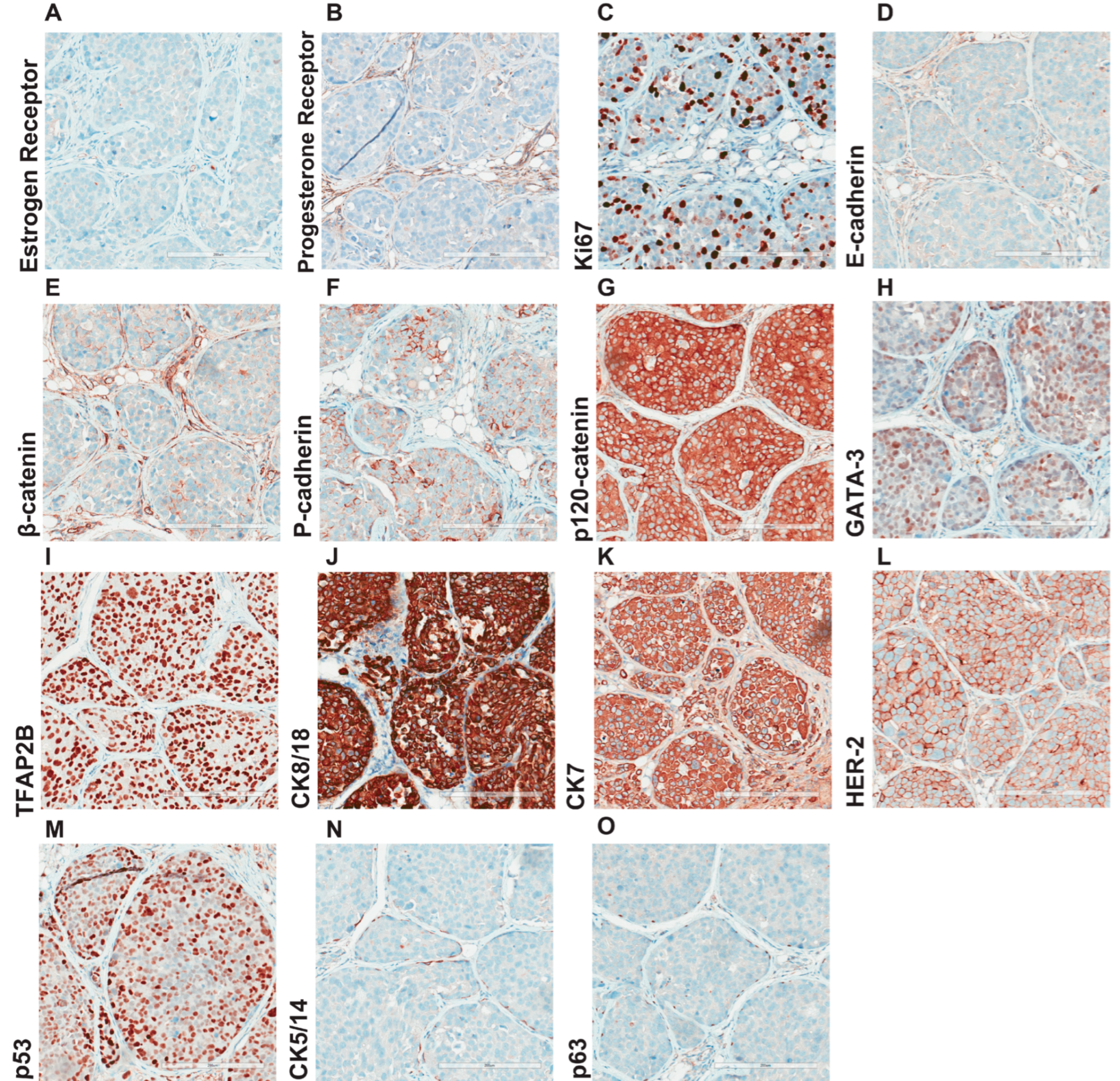
2.6. Immunohistochemistry
2.7. Next-Generation Sequencing
3. Results
3.1. Generation of Triple-Negative Lobular Xenografts
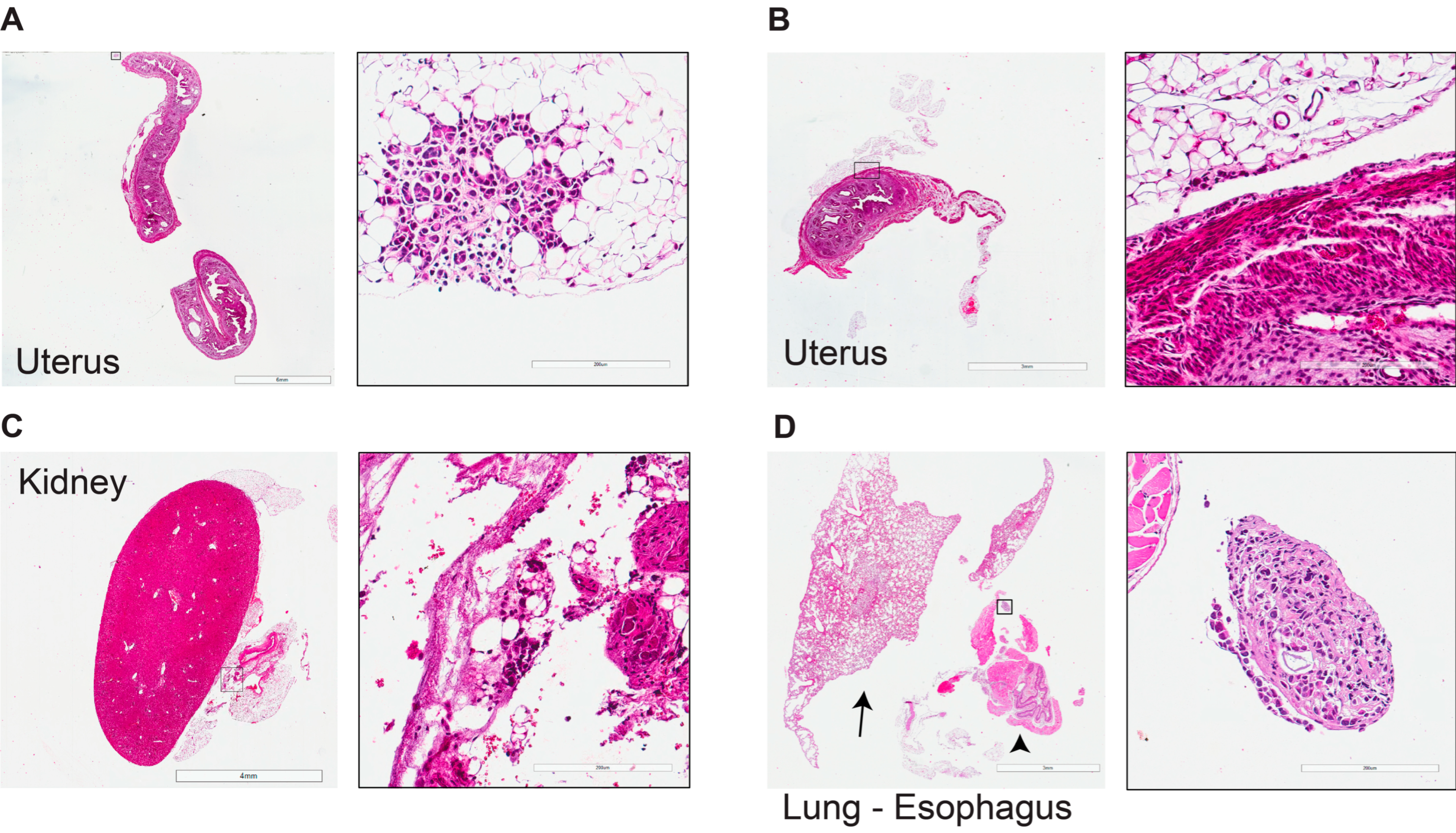
3.2. Tumor Progression and Metastatic Spread
3.3. Mutational Profile of Primary Lobular Lesions
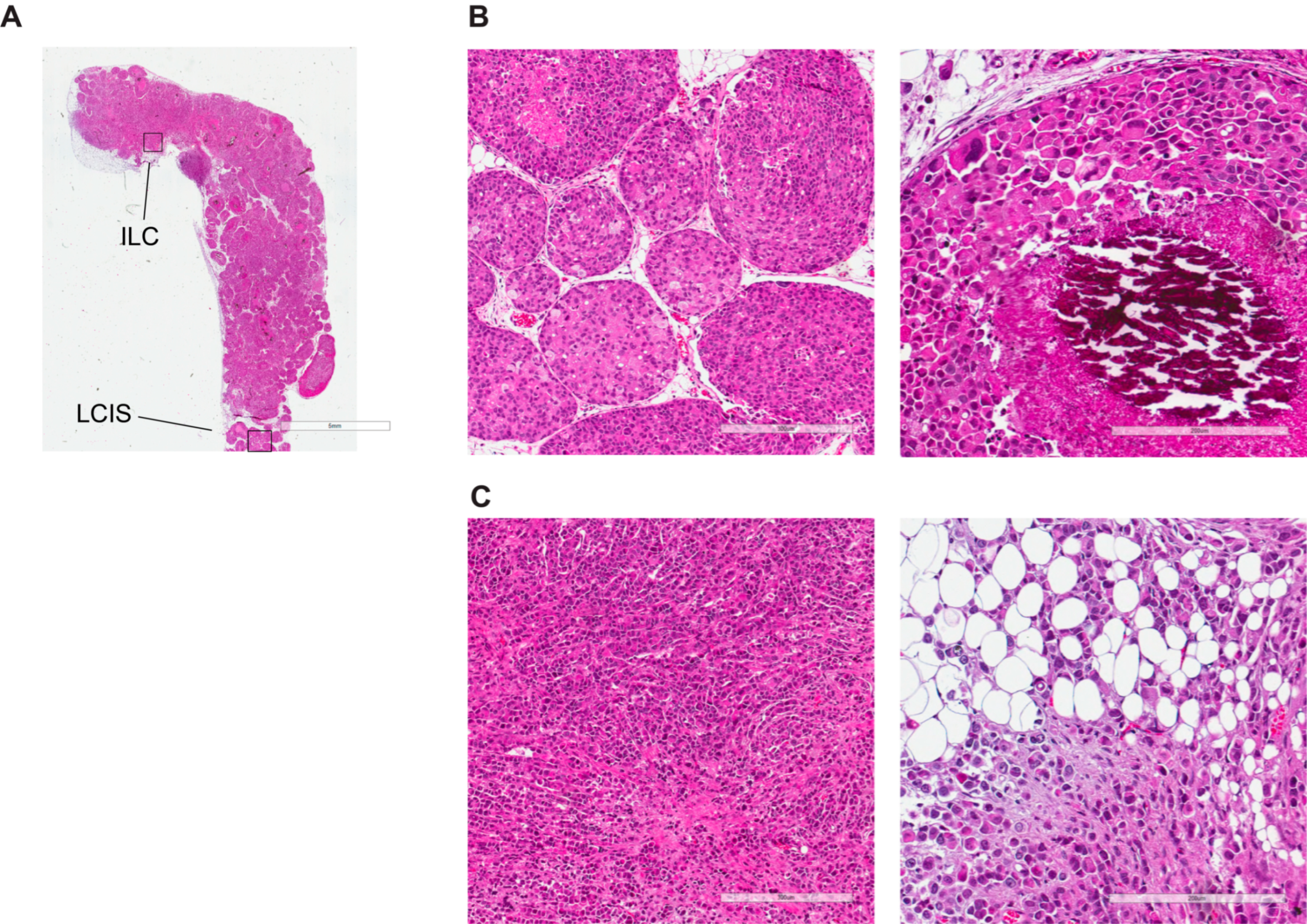
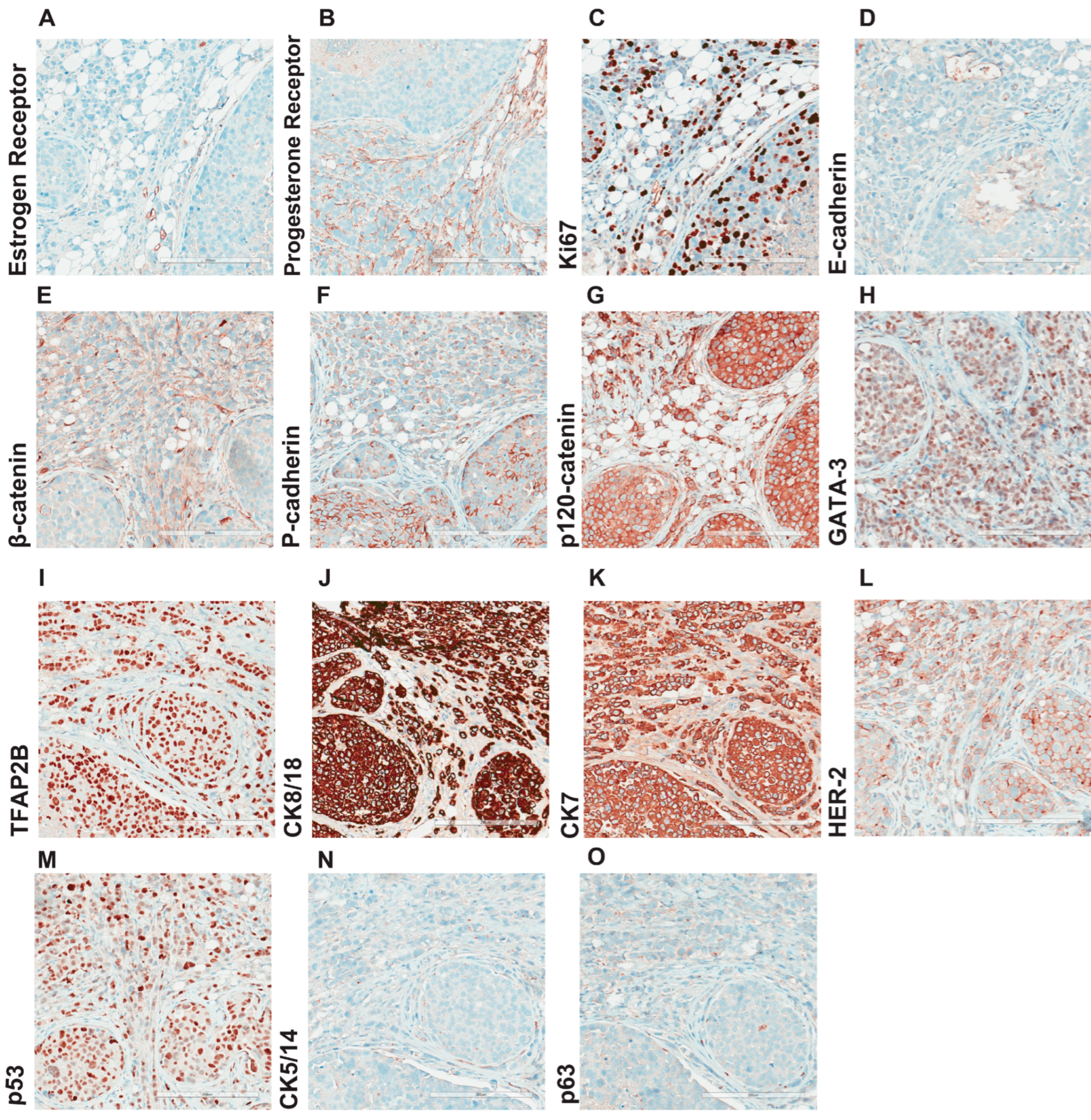
3.4. Morphological and Histological Characterization of Primary Lobular Lesions
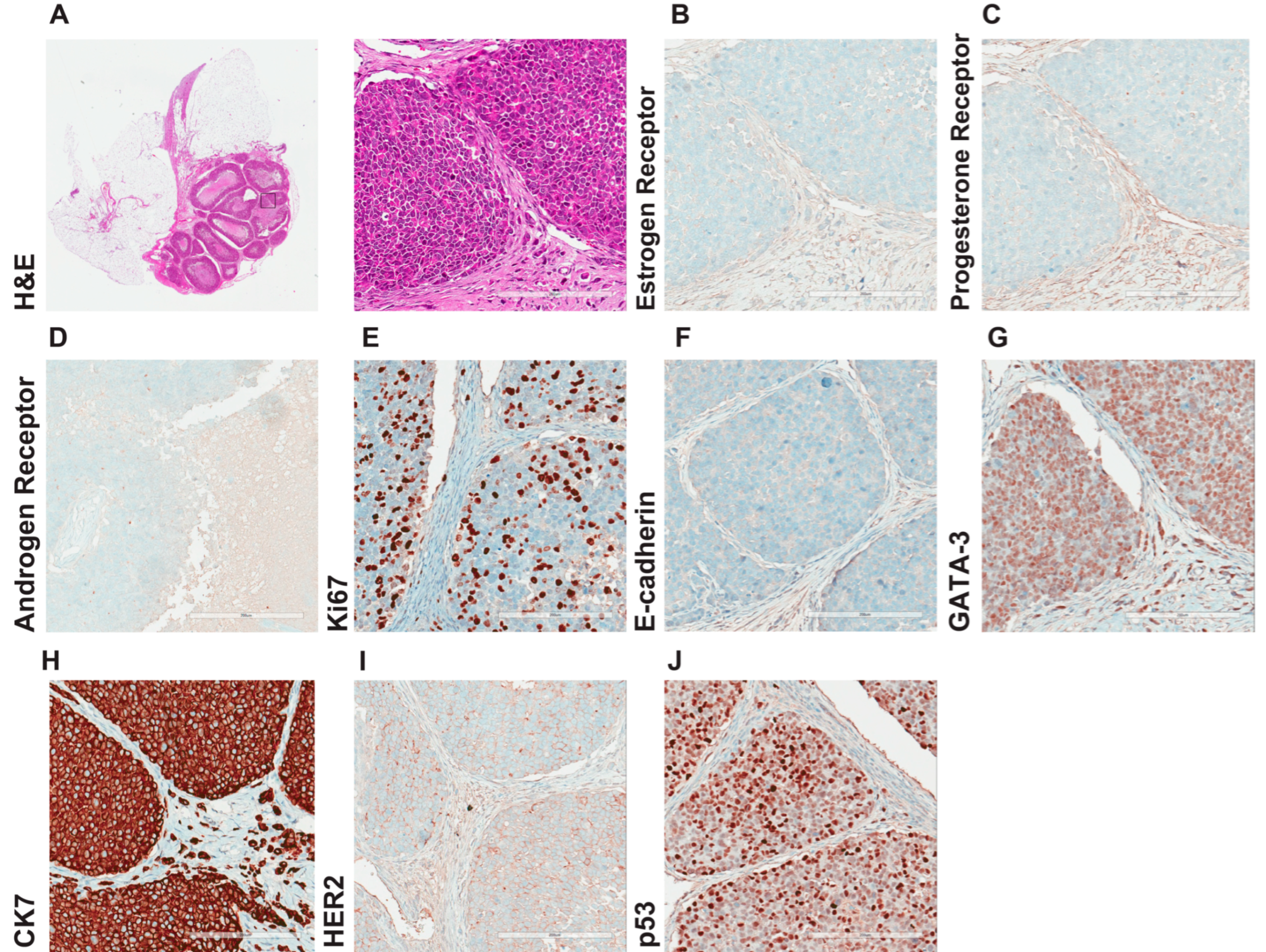
3.5. Comprehensive Histopathological Characterization of Metastatic Lesions
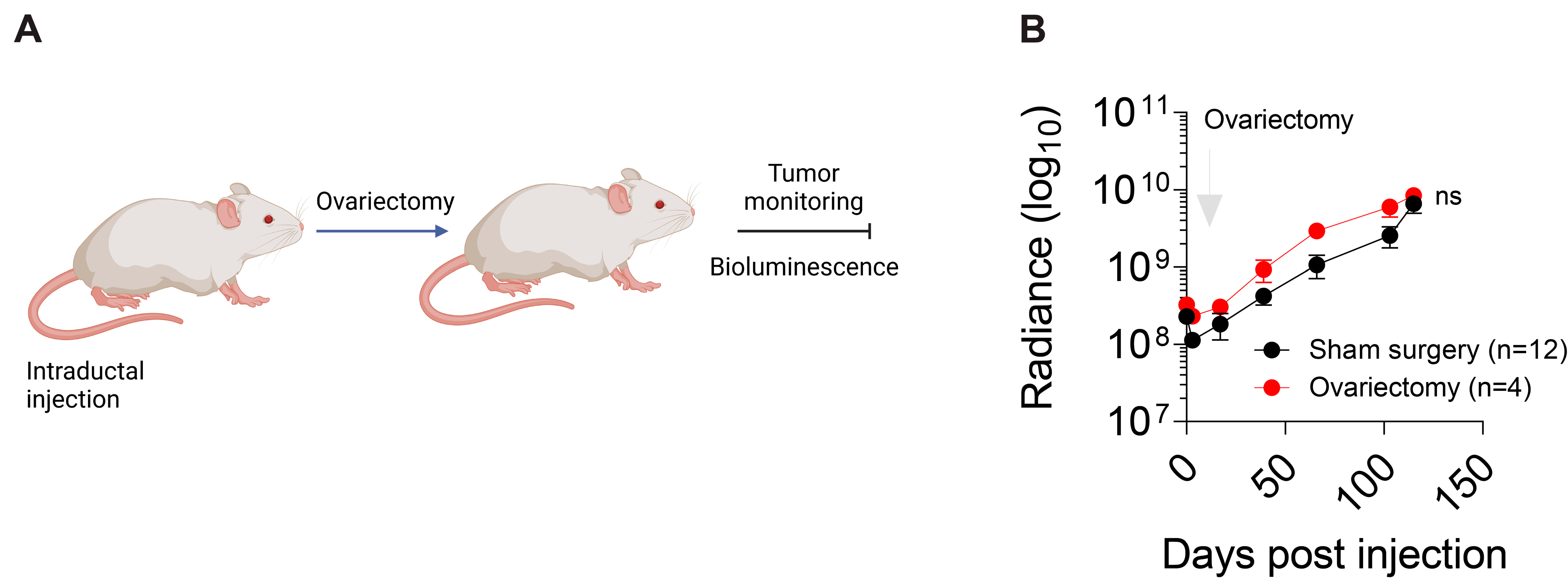
3.6. Hormonal Regulation of IPH-926 Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Baelen, K.; Geukens, T.; Maetens, M.; Tjan-Heijnen, V.; Lord, C.J.; Linn, S.; Bidard, F.-C.; Richard, F.; Yang, W.W.; Steele, R.E.; et al. Current and Future Diagnostic and Treatment Strategies for Patients with Invasive Lobular Breast Cancer. Ann. Oncol. 2022, 33, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhu, L.; Levine, K.M.; Tasdemir, N.; Lee, A.V.; Vignali, D.A.A.; Houten, B.V.; Tseng, G.C.; Oesterreich, S. Invasive Lobular and Ductal Breast Carcinoma Differ in Immune Response, Protein Translation Efficiency and Metabolism. Sci. Rep. 2018, 8, 7205. [Google Scholar] [CrossRef] [PubMed]
- Djerroudi, L.; Cabel, L.; Bidard, F.-C.; Vincent-Salomon, A. Invasive Lobular Carcinoma of the Breast: Toward Tailoring Therapy? J. Natl. Cancer Inst. 2022, 114, 1434–1436. [Google Scholar] [CrossRef]
- Onkar, S.; Cui, J.; Zou, J.; Cardello, C.; Cillo, A.R.; Uddin, M.R.; Sagan, A.; Joy, M.; Osmanbeyoglu, H.U.; Pogue-Geile, K.L.; et al. Immune Landscape in Invasive Ductal and Lobular Breast Cancer Reveals a Divergent Macrophage-Driven Microenvironment. Nat. Cancer 2023, 4, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Metzger Filho, O.; Giobbie-Hurder, A.; Mallon, E.; Gusterson, B.; Viale, G.; Winer, E.P.; Thürlimann, B.; Gelber, R.D.; Colleoni, M.; Ejlertsen, B. Relative Effectiveness of Letrozole Compared with Tamoxifen for Patients with Lobular Carcinoma in the BIG 1-98 Trial. J. Clin. Oncol. 2015, 33, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ding, K.; Priedigkeit, N.; Elangovan, A.; Levine, K.M.; Carleton, N.; Savariau, L.; Atkinson, J.M.; Oesterreich, S.; Lee, A.V. Single-Cell Transcriptomic Heterogeneity in Invasive Ductal and Lobular Breast Cancer Cells. Cancer Res. 2021, 81, 268–281. [Google Scholar] [CrossRef]
- Christgen, M.; Cserni, G.; Floris, G.; Marchio, C.; Djerroudi, L.; Kreipe, H.; Derksen, P.W.B.; Vincent-Salomon, A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers 2021, 13, 3695. [Google Scholar] [CrossRef]
- Christgen, M.; Derksen, P.W.B. Lobular Breast Cancer: Molecular Basis, Mouse and Cellular Models. Breast Cancer Res. 2015, 17, 16. [Google Scholar] [CrossRef]
- Sflomos, G.; Schipper, K.; Koorman, T.; Fitzpatrick, A.; Oesterreich, S.; Lee, A.V.; Jonkers, J.; Brunton, V.G.; Christgen, M.; Isacke, C.; et al. Atlas of Lobular Breast Cancer Models: Challenges and Strategic Directions. Cancers 2021, 13, 5396. [Google Scholar] [CrossRef]
- Desmedt, C.; Zoppoli, G.; Sotiriou, C.; Salgado, R. Transcriptomic and Genomic Features of Invasive Lobular Breast Cancer. Semin. Cancer Biol. 2017, 44, 98–105. [Google Scholar] [CrossRef]
- Voorwerk, L.; Isaeva, O.I.; Horlings, H.M.; Balduzzi, S.; Chelushkin, M.; Bakker, N.A.M.; Champanhet, E.; Garner, H.; Sikorska, K.; Loo, C.E.; et al. PD-L1 Blockade in Combination with Carboplatin as Immune Induction in Metastatic Lobular Breast Cancer: The GELATO Trial. Nat. Cancer 2023, 4, 535–549. [Google Scholar] [CrossRef]
- Christgen, M.; Bruchhardt, H.; Hadamitzky, C.; Rudolph, C.; Steinemann, D.; Gadzicki, D.; Hasemeier, B.; Römermann, D.; Focken, T.; Krech, T.; et al. Comprehensive Genetic and Functional Characterization of IPH-926: A Novel CDH1-Null Tumour Cell Line from Human Lobular Breast Cancer. J. Pathol. 2009, 217, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Noskowicz, M.; Heil, C.; Schipper, E.; Christgen, H.; Geffers, R.; Kreipe, H.; Lehmann, U. IPH-926 Lobular Breast Cancer Cells Harbor a P53 Mutant with Temperature-Sensitive Functional Activity and Allow for Profiling of P53-Responsive Genes. Lab. Investig. J. Tech. Methods Pathol. 2012, 92, 1635–1647. [Google Scholar] [CrossRef]
- Sflomos, G.; Dormoy, V.; Metsalu, T.; Jeitziner, R.; Battista, L.; Scabia, V.; Raffoul, W.; Delaloye, J.-F.; Treboux, A.; Fiche, M.; et al. A Preclinical Model for ERα-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell 2016, 29, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Sflomos, G.; Battista, L.; Aouad, P.; De Martino, F.; Scabia, V.; Stravodimou, A.; Ayyanan, A.; Ifticene-Treboux, A.; RLS; Bucher, P.; et al. Intraductal Xenografts Show Lobular Carcinoma Cells Rely on Their Own Extracellular Matrix and LOXL1. EMBO Mol. Med. 2021, 13, e13180. [Google Scholar] [CrossRef]
- Christgen, M.; Bartels, S.; van Luttikhuizen, J.L.; Bublitz, J.; Rieger, L.U.; Christgen, H.; Stark, H.; Sander, B.; Lehmann, U.; Steinemann, D.; et al. E-Cadherin to P-Cadherin Switching in Lobular Breast Cancer with Tubular Elements. Mod. Pathol. 2020, 33, 2483–2498. [Google Scholar] [CrossRef]
- Christgen, M.; Bartels, S.; van Luttikhuizen, J.L.; Schieck, M.; Pertschy, S.; Kundu, S.; Lehmann, U.; Sander, B.; Pelz, E.; Länger, F.; et al. Subclonal Analysis in a Lobular Breast Cancer with Classical and Solid Growth Pattern Mimicking a Solid-Papillary Carcinoma. J. Pathol. Clin. Res. 2017, 3, 191–202. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Rocco, E.G.; Bagnardi, V.; Montagna, E.; Peruzzotti, G.; De Pas, T.; Fumagalli, C.; Pileggi, S.; et al. Biological and Clinical Features of Triple Negative Invasive Lobular Carcinomas of the Breast. Clinical Outcome and Actionable Molecular Alterations. Breast 2021, 59, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Zoppoli, G.; Gundem, G.; Pruneri, G.; Larsimont, D.; Fornili, M.; Fumagalli, D.; Brown, D.; Rothe, F.; Vincent, D.; et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J. Clin. Oncol. 2016, 34, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Kozma, K.J.; Done, S.J.; Egan, S.E. The Tumor Cell-Derived Matrix of Lobular Breast Cancer: A New Vulnerability. EMBO Mol. Med. 2021, 13, e13807. [Google Scholar] [CrossRef]
- Aouad, P.; Zhang, Y.; De Martino, F.; Stibolt, C.; Ali, S.; Ambrosini, G.; Mani, S.A.; Maggs, K.; Quinn, H.M.; Sflomos, G.; et al. Epithelial-Mesenchymal Plasticity Determines Estrogen Receptor Positive Breast Cancer Dormancy and Epithelial Reconversion Drives Recurrence. Nat. Commun. 2022, 13, 4975. [Google Scholar] [CrossRef]
- Scabia, V.; Ayyanan, A.; De Martino, F.; Agnoletto, A.; Battista, L.; Laszlo, C.; Treboux, A.; Zaman, K.; Stravodimou, A.; Jallut, D.; et al. Estrogen Receptor Positive Breast Cancers Have Patient Specific Hormone Sensitivities and Rely on Progesterone Receptor. Nat. Commun. 2022, 13, 3127. [Google Scholar] [CrossRef]
- Raap, M.; Gierendt, L.; Werlein, C.; Kuehnle, E.; Kreipe, H.H.; Christgen, M. Co-Expression of Transcription Factor AP-2beta (TFAP2B) and GATA3 in Human Mammary Epithelial Cells with Intense, Apicobasal Immunoreactivity for CK8/18. J. Mol. Histol. 2021, 52, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Raap, M.; Gronewold, M.; Christgen, H.; Glage, S.; Bentires-Alj, M.; Koren, S.; Derksen, P.W.; Boelens, M.; Jonkers, J.; Lehmann, U.; et al. Lobular Carcinoma in Situ and Invasive Lobular Breast Cancer Are Characterized by Enhanced Expression of Transcription Factor AP-2β. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 117–129. [Google Scholar] [CrossRef]
- Mathew, A.; Rajagopal, P.S.; Villgran, V.; Sandhu, G.S.; Jankowitz, R.C.; Jacob, M.; Rosenzweig, M.; Oesterreich, S.; Brufsky, A. Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast. Geburtshilfe Frauenheilkd. 2017, 77, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Geukens, T.; De Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.-L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Intra-Patient and Inter-Metastasis Heterogeneity of HER2-Low Status in Metastatic Breast Cancer. Eur. J. Cancer 2023, 188, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Trillo, P.; Sandoval, J.; Trapani, D.; Nicolò, E.; Zagami, P.; Giugliano, F.; Tarantino, P.; Vivanet, G.; Ascione, L.; Friedlaender, A.; et al. Evolution of Biological Features of Invasive Lobular Breast Cancer: Comparison between Primary Tumour and Metastases. Eur. J. Cancer 2023, 185, 119–130. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Sflomos, G.; Brisken, C. The Challenges of Modeling Hormone Receptor-Positive Breast Cancer in Mice. Endocr. Relat. Cancer 2018, 25, R319–R330. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Geffers, R.; Kreipe, H.; Lehmann, U. IPH-926 Lobular Breast Cancer Cells Are Triple-Negative but Their Microarray Profile Uncovers a Luminal Subtype. Cancer Sci. 2013, 104, 1726–1730. [Google Scholar] [CrossRef]
- Abel, M.K.; Shui, A.M.; Chien, A.J.; Rugo, H.S.; Melisko, M.; Baehner, F.; Mukhtar, R.A. The 21-Gene Recurrence Score in Clinically High-Risk Lobular and Ductal Breast Cancer: A National Cancer Database Study. Ann. Surg. Oncol. 2022, 29, 7739–7747. [Google Scholar] [CrossRef]
- Gruel, N.; Lucchesi, C.; Raynal, V.; Rodrigues, M.J.; Pierron, G.; Goudefroye, R.; Cottu, P.; Reyal, F.; Sastre-Garau, X.; Fourquet, A.; et al. Lobular Invasive Carcinoma of the Breast Is a Molecular Entity Distinct from Luminal Invasive Ductal Carcinoma. Eur. J. Cancer 2010, 46, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Timbres, J.; Moss, C.; Mera, A.; Haire, A.; Gillett, C.; Van Hemelrijck, M.; Sawyer, E. Survival Outcomes in Invasive Lobular Carcinoma Compared to Oestrogen Receptor-Positive Invasive Ductal Carcinoma. Cancers 2021, 13, 3036. [Google Scholar] [CrossRef] [PubMed]
- Fiche, M.; Scabia, V.; Aouad, P.; Battista, L.; Treboux, A.; Stravodimou, A.; Zaman, K.; RLS; Dormoy, V.; Ayyanan, A.; et al. Intraductal Patient-Derived Xenografts of Estrogen Receptor α-Positive Breast Cancer Recapitulate the Histopathological Spectrum and Metastatic Potential of Human Lesions. J. Pathol. 2019, 247, 287–292. [Google Scholar] [CrossRef]
- Dobrolecki, L.E.; Airhart, S.D.; Alferez, D.G.; Aparicio, S.; Behbod, F.; Bentires-Alj, M.; Brisken, C.; Bult, C.J.; Cai, S.; Clarke, R.B.; et al. Patient-Derived Xenograft (PDX) Models in Basic and Translational Breast Cancer Research. Cancer Metastasis Rev. 2016, 35, 547–573. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Grellety, T.; Velasco, V.; MacGrogan, G.; Bonnefoi, H.; Iggo, R. The Mammary Ducts Create a Favourable Microenvironment for Xenografting of Luminal and Molecular Apocrine Breast Tumours. J. Pathol. 2016, 240, 256–261. [Google Scholar] [CrossRef]
- Behbod, F.; Kittrell, F.S.; LaMarca, H.; Edwards, D.; Kerbawy, S.; Heestand, J.C.; Young, E.; Mukhopadhyay, P.; Yeh, H.W.; Allred, D.C.; et al. An Intraductal Human-in-Mouse Transplantation Model Mimics the Subtypes of Ductal Carcinoma in Situ. Breast Cancer Res. 2009, 11, R66. [Google Scholar] [CrossRef]
- van Agthoven, T.; Dorssers, L.C.J.; Lehmann, U.; Kreipe, H.; Looijenga, L.H.J.; Christgen, M. Breast Cancer Anti-Estrogen Resistance 4 (BCAR4) Drives Proliferation of IPH-926 Lobular Carcinoma Cells. PLoS ONE 2015, 10, e0136845. [Google Scholar] [CrossRef]
| Antigen | Antibody | Source | Dilution | Cutoff |
|---|---|---|---|---|
| ER | clone SP1 | Ventana | Undiluted, RTU | 0–5, neg.; 10–100 pos. |
| PR | clone 1E2 | Ventana | Undiluted, RTU | 0–5, neg.; 10–100 pos. |
| HER2 | clone 4B5 | Ventana | Undiluted, RTU | 0-1, neg.; 2+, equivocal; 3+, positive |
| Ki67 | clone 30-9 | Ventana | Undiluted, RTU | n.a. |
| AR | clone AR441 | Dako | 1:40 | IRS 3 |
| p63 | 4A4 | BioCare Medical | 1:100 | n.a. |
| CK5/14 | XM26+LL002 | Diagnostic BioSystems | 1:200 | n.a. |
| CK7 | clone SP52 | Ventana | Undiluted, RTU | n.a. |
| CK8/18 | B22.1 and B23.1 | Ventana | Undiluted, RTU | n.a. |
| GATA3 | L50-823 | Cell Marque | Undiluted, RTU | n.a. |
| E-cadherin | clone ECH-6 | Zytomed | 1:100 | I.R.S. 0–1, negative; 2–12, positive |
| p120-catenin (expression) | clone 98 | BD Transduction Laboratories | 1:250 | IRS 0–1, negative; 2–12, positive |
| p120-catenin (mislocalization) | clone 98 | BD Transduction Laboratories | 1:250 | n.a. |
| P-cadherin | clone 56 | BD Transduction Laboratories | 1:100 | n.a. |
| p53 | clone DO-7 | Novocastra | 1:100 | n.a. |
| β-catenin | clone 14 | BD Transduction Laboratories | 1:100 | 0 |
| TFAP2B | clone H87 | Santa Cruz | 1:250 | 3 |
| Gene | Variant/Mutation | Frequency | Read depth |
|---|---|---|---|
| TP53 | NM_000546.5:exon8:c.853G>A:p.E285K | 99.43% | 1570 |
| ARID1A | NM_006015.5:exon4:c.1813C>T:p.Q605* | 99.50% | 1995 |
| ARID1A | NM_006015.5:exon20:c.5445delG:p.I1816Sfs*67 | 23.07% | 1981 |
| BRCA2 | NM_000059.3:exon20:c.8524C>T:p.R2842C | 99.90% | 1992 |
| NF1 | NM_001042492.2:exon43:c.6555G>C:p.R2185S | 13.11% | 1998 |
| ERCC2 | NM_000400.3:exon21:c.1982C>G:p.A661G | 24.31% | 983 |
| CDH1 | NM_004360:exon3:c.242_243insTGGG:p.V82Gfs*13 | 99.20% | 4207 |
| ABCA13 | NM_152701:exon17:c.3929C>G:p.S1310* | 25.40% | 808 |
| Gene | Locus | Copy Number | CNV CI |
|---|---|---|---|
| FGFR1 | chr8:38271114 | 4.8 | 3.42–6.66 |
| CDKN2A | chr9:21968186 | 0.64 | 0.36–1.00 |
| CDKN2B | chr9:22005844 | 0.98 | 0.58–1.52 |
| CDKN1B | chr12:12870763 | 0.06 | 0.00–0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sflomos, G.; Schaumann, N.; Christgen, M.; Christgen, H.; Bartels, S.; Kreipe, H.; Battista, L.; Brisken, C. Optimized Modeling of Metastatic Triple-Negative Invasive Lobular Breast Carcinoma. Cancers 2023, 15, 3299. https://doi.org/10.3390/cancers15133299
Sflomos G, Schaumann N, Christgen M, Christgen H, Bartels S, Kreipe H, Battista L, Brisken C. Optimized Modeling of Metastatic Triple-Negative Invasive Lobular Breast Carcinoma. Cancers. 2023; 15(13):3299. https://doi.org/10.3390/cancers15133299
Chicago/Turabian StyleSflomos, George, Nora Schaumann, Matthias Christgen, Henriette Christgen, Stephan Bartels, Hans Kreipe, Laura Battista, and Cathrin Brisken. 2023. "Optimized Modeling of Metastatic Triple-Negative Invasive Lobular Breast Carcinoma" Cancers 15, no. 13: 3299. https://doi.org/10.3390/cancers15133299
APA StyleSflomos, G., Schaumann, N., Christgen, M., Christgen, H., Bartels, S., Kreipe, H., Battista, L., & Brisken, C. (2023). Optimized Modeling of Metastatic Triple-Negative Invasive Lobular Breast Carcinoma. Cancers, 15(13), 3299. https://doi.org/10.3390/cancers15133299






