18F-Fluoroethyl-L Tyrosine Positron Emission Tomography Radiomics in the Differentiation of Treatment-Related Changes from Disease Progression in Patients with Glioblastoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodological Aspects
2.1. Imaging Acquisition and Analysis
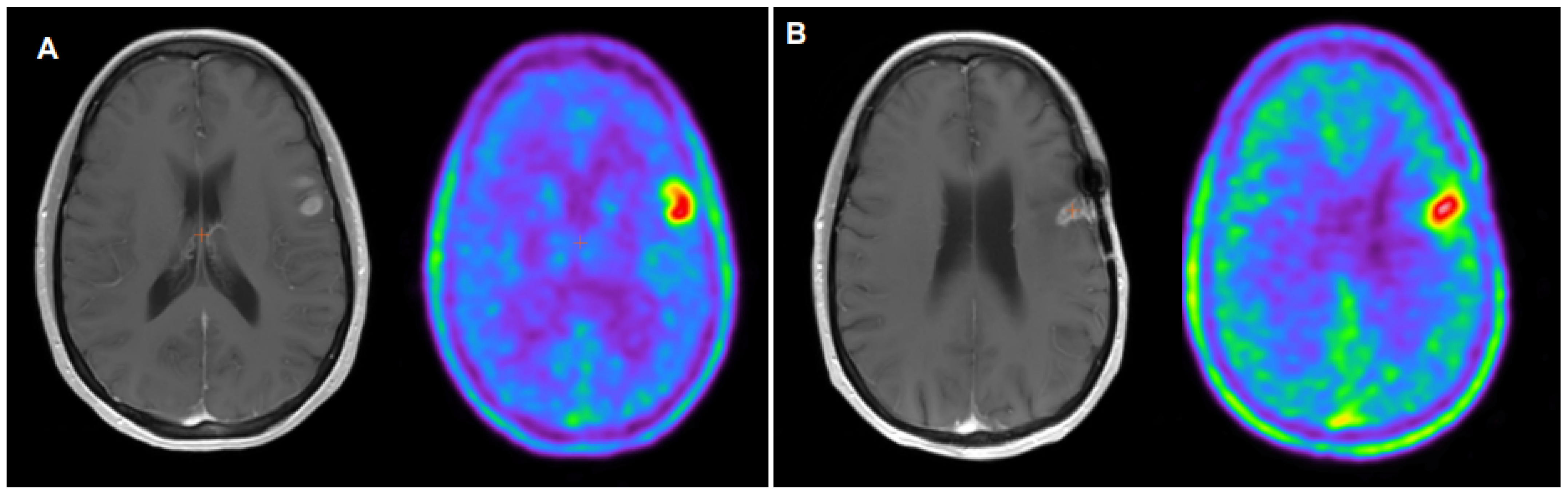
2.2. 18F-FET PET Interpretation
3. Differentiation of Tumor Progression and Therapy-Related Changes
3.1. Pseudoprogression
3.2. Pseudoresponse
3.3. Early Response Evaluation
3.4. Molecular Dependence
4. Comparison with Other PET Radiotracers
4.1. Fluorodeoxyglucose (FDG)
4.2. Choline Analogues
4.3. Other Amino Acid Tracers
4.4. Prostate-Specific Membrane Antigen Ligands
5. Future Directions and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langen, K.J.; Hamacher, K.; Weckesser, M.; Floeth, F.; Stoffels, G.; Bauer, D.; Conen, H.H.; Pauleit, D. O-(2-[18F] fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006, 33, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, W.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standars for imagine of gliomas using PET with radiolabelled amino acids and [18F]FDG. Nucl. Med. Mol. Imaging 2019, 6, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Stockhammer, F.; Plotkin, M.; Amthauer, H.; van Landeghem, F.K.H.; Woiciechowsky, C. Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J. Neurooncol. 2008, 88, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxue, T.; Yinzhong, W.; Meng, Q.; Lu, X.; Junqiang, L. Diagnostic value of PET with different radiotracers and MRI for recurrent glioma: A Bayesian network meta-analysis. BMJ Open 2023, 13, e062555. [Google Scholar] [CrossRef] [PubMed]
- IASOglio Data-Sheet. Available online: https://curium-austria.com/products/iasoglio (accessed on 22 September 2023).
- Dai, J.; Wang, H.; Xu, Y.; Chen, X.; Tian, R. Clinical application of AI-based PET images in oncological patients. Semin. Cancer Biol. 2023, 91, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Elahmadaway, M.; Gutsche, R.; Werner, J.M.; Bauer, E.K.; Ceccon, G.; Kocher, M.; Lerche, C.W.; Rapp, M.; Fink, G.R.; et al. FET PET Radiomics for Differentiating Pseudoprogression from Early Tumor Progression in Glioma Patients Post-Chemotherapy. Cancers 2020, 12, 3835. [Google Scholar] [CrossRef]
- Verburg, N.; Koopman, T.; Yaqub, M.; Hoekstra, O.T.; Lammertsma, A.; Schwarte, L.A.; Barkhof, F.; Pouwels, P.J.W.; Heimans, J.J.; Reijneveld, J.C.; et al. Direct comparison of [11C] choline and [18F] FET PET to detect glioma infiltration: A diagnostic accuracy study in eight patients. EJNMMI Res. 2019, 9, 57. [Google Scholar] [CrossRef]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffles, G.; Sabel, M.; Wittsack, H.J.; Kops, E.R.; Galldiks, N.; Fink, G.R.; Shah, N.J.; et al. Comparison of O-(2-18F-fluoroethyl)-l-tyrosine positron emission tomography and perfusion-weighted magnetic resonance imaging in the diagnosis of patients with progressive and recurrent glioma: A hybrid positron emission tomography/magnetic resonance study. World Neurosurg. 2018, 113, e727–e737. [Google Scholar] [CrossRef]
- Rausch, I.; Zitterl, A.; Berroterrán-Infante, N.; Rischka, L.; Prayer, D.; Fenchel, M.; Sareshgi, R.A.; Haug, A.R.; Hacker, M.; Beyer, R.; et al. Dynamic [18F]FET-PET/MRI using standard MRI-based attenuation correction methods. Eur. Radiol. 2019, 29, 4276–4285. [Google Scholar] [CrossRef]
- Barry, N.; Francis, R.J.; Evert, M.A.; Koh, E.-S.; Rowshanfarzad, P.; Hassan, G.M.; Kendrick, J.; Gan, H.K.; Lee, S.T.; Lau, E.; et al. Delineation and agreement of FET PET biological volumes in glioblastoma: Results of the nuclear medicine credentialing program from the prospective, multicentre trial evaluating FET PET In Glioblastoma (FIG) study—TROG 18. Eur. J. Nuc Med. Mol. Imaging 2023, 50, 3970–3981. [Google Scholar] [CrossRef]
- Gutsche, R.; Lowis, C.; Ziemons, K.; Kocher, M.; Ceccon, G.; Brambilla, C.R.; Shah, N.J.; Langen, K.J.; Galldiks, N.; Isensee, F.; et al. Automated Brain Tumor Detection and Segmentation for Treatment Response Assessment Using Amino Acid PET. J. Nucl. Med. 2023, 64, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Zounek, A.J.; Albert, N.L.; Holzgreve, A.; Unterrainer, M.; Brosch-Lenz, J.; Lindner, S.; Bollenbacher, A.; Boening, G.; Rupprecht, R.; Brendel, M.; et al. Feasibility of radiomic feature harmonization for pooling of [18F] FET or [18F]GE-180 PET images of gliomas. Z. Med. Phys. 2023, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, R.; Scheins, J.; Kocher, M.; Bousabarah, K.; Fink, G.R.; Shah, N.J.; Langen, K.-J.; Galldiks, N.; Lohmann, P. Evaluation of FET PET Radiomics Feature Repeatability in Glioma Patients. Cancers 2021, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Thon, N.; Eigenbrod, D.; Hartmann, C.; Egensperger, R.; Hermes, J.; Geisler, J.; la Fougère, C.; Lutz, J.; Linn, J. Hot spots in dynamic 18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011, 13, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Floeth, F.W.; Pauleit, D.; Sabel, M.; Reifenberger, G.; Stoffles, G.; Stummer, W.; Rommel, F.; Hamacher, K.; Langen, K.J. 18F-FET PET differentiation of ring-enhancing brain lesions. J. Nucl. Med. 2006, 47, 776–782. [Google Scholar] [PubMed]
- Hutterer, M.; Galldiks, N.; Hau, P.; Langen, K.J. Pitfalls in der [18F]FET-PET-Diagnostik von Hirntumoren. Nuklearmediziner 2015, 38, 295–303. [Google Scholar] [CrossRef]
- Suchorska, B.; Jansen, N.L.; Linn, J.; Kretzschmar, H.; Janssen, H.; Eigenbrod, S.; Simon, M.; Pöpperl, G.; Kreth, F.W.; la Fougere, C.; et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 2015, 84, 710–719. [Google Scholar] [CrossRef]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Zhang, Z.; Shi, W.; Holodny, A.I.; Omuro, A.M.P. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 2011, 76, 1918–1924. [Google Scholar] [CrossRef]
- Brandsma, D.; van den Bent, M.J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorila, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfeller, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.W.; Westerlaan, H.E.; Holtman, G.A.; Aden, K.M.; van Laar, P.J.; van der Hoorn, A. Incidence of tumor progression and pseudoprogression in high-grade gilomas: A systematic review and meta-analysis. Clin. Neuroradiol. 2018, 28, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kebir, S.; Fimmers, R.; Galldicks, N.; Schäfer, N.; Mack, F.; Schaub, C.; Stuplich, M.; Niessen, M.; Tzaridis, T.; Simon, M.; et al. Late Pseudoprogression in Glioblastoma: Diagnostic Value of Dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin. Cancer Res. 2016, 22, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008, 25, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar]
- Henssen, D.; Leitjen, L.; Meijer, F.J.A.; van der Kolk, A.; Arens, A.I.J.; ter Laan, M.; Smeenk, R.J.; Gijtenbeek, A.; van de Giessen, E.M.; Tolboom, N.; et al. Head-To-Head Comparison of PET and Perfusion Weighted MRI Techniques to Distinguish Treatment Related Abnormalities from Tumor Progression in Glioma. Cancers 2023, 15, 2631. [Google Scholar] [CrossRef]
- Smith, N.J.; Deaton, T.K.; Territo, W.; Graner, B.; Gauger, A.; Snyder, S.E.; Schulte, M.L.; Green, M.A.; Hutchins, G.D.; Veronesi, M.C. Hybrid 18F-Fluoroethyltyrosine PET and MRI with Perfusion to Distinguish Disease Progression from Treatment-Related Change in Malignant Brain Tumors: The Quest to Beat the Toughest Cases. J. Nucl. Med. 2023, 64, 1087–1092. [Google Scholar] [CrossRef]
- Ouyang, Z.Q.; Zheng, G.R.; Duan, X.R.; Zhang, Z.R.; Ke, T.F.; Bao, S.S.; Yang, J.; He, B.; Liao, C.D. Diagnostic accuracy of glioma pseudoprogression identification with positron emission tomography imaging: A systematic review and meta-analysis. Quant. Imaging Med. Surg. 2023, 13, 4943–4959. [Google Scholar] [CrossRef]
- Singnurkar, A.; Poon, R.; Detsky, J. 18F-FET-PET imaging in high-grade gliomas and brain metastases: A systematic review and meta-analysis. Neuro Oncol. 2023, 161, 1–12. [Google Scholar] [CrossRef]
- Heinzel, A.; Müller, D.; Langen, K.J.; Blaum, M.; Verburg, F.A.; Mottaghy, F.M.; Galldiks, N. The use of O-(2-18F-fluoroethyl)-L tyrosine PET for treatment management of bevacizumab and irinotecan in patients with recurrent high-grade glioma: A cost-effectiveness analysis. J. Nucl. Med. 2013, 54, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Pyka, T.; Hiob, D.; Preibisch, C.; Gempt, J.; Wiestler, B.; Schlegel, J.; Straube, C.; Zimmer, C. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 2018, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumor progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Carles, M.; Popp, I.; Stark, M.M.; Mix, M.; Urbach, H.; Schimek-Jasch, T.; Eckert, F.; Niyazi, M.; Baltas, D.; Grosu, A.L. FETPET radiomics in recurrent glioblastoma: Prognostic value for outcome after reirradiation? Radiat. Oncol. 2021, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.J. 18F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kebir, S.; Schmidt, T.; Weber, M.; Lazaridis, L.; Galldiks, N.; Langen, K.J.; Kleinschnitz, C.; Hattingen, E.; Herrlinger, U.; Lohmann, P.; et al. A Preliminary Study on Machine Learning-Based Evaluation of Static and Dynamic FET-PET for the Detection of Pseudoprogression in Patients with IDH-Wildtype Glioblastoma. Cancers 2020, 12, 3080. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.L.; O’Neal, C.M.; Andrews, B.J.; Westrup, A.M.; Battiste, J.D.; Glenn, C.A. A systematic review of the utility of amino acid PET in assessing treatment response to bevacizumab in recurrent high-grade glioma. Neurooncol. Adv. 2021, 3, vdab003. [Google Scholar] [CrossRef]
- George, E.; Kijewski, M.F.; Dubey, S.; Belanger, A.P.; Reardon, D.A.; Wen, P.Y.; Kesari, S.; Horky, L.; Park, M.-A.; Huang, R.Y. Voxel-wise analysis of fluoroethyltyrosine PET and MRI in the assessment of recurrent glioblastoma during antiangiogenic therapy. Am. J. Roentgenol. 2018, 211, 1342–1347. [Google Scholar] [CrossRef]
- Galldiks, N.; Dunkl, V.; Ceccon, G.; Tscherpel, C.; Stoffels, G.; Law, I.; Henriksen, O.M.; Muhic, A.; Poulsen, H.S.; Steger, J.; et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2377–2386. [Google Scholar] [CrossRef]
- Galldiks, N.; Rapp, M.; Stoffels, G.; Fink, G.R.; Shah, N.J.; Coenen, H.H.; Sabel, M.; Langen, K.J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 22–33. [Google Scholar] [CrossRef]
- Hutterer, M.; Nowosielski, M.; Putzer, D.; Waitz, D.; Tinkhauser, G.; Kostron, H.; Muigg, A.; Virgolini, I.J.; Staffen, W.; Trika, E.; et al. O-(2-18F-fluoroethyl)-L tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J. Nucl. Med. 2011, 52, 856–864. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Roelcke, U.; Weller, J.; Hundsberger, T.; Hottinger, A.F.; von Moos, R.; Caparrotti, F.; Conen, K.; Remonda, L.; Roth, P.; et al. MRI and 18FET-PET Predict Survival Benefit from Bevacizumab Plus Radiotherapy in Patients with Isocitrate Dehydrogenase Wild-type Glioblastoma: Results from the Randomized ARTE trial. Clin. Cancer Res. 2021, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, G.; Lohmann, P.; Werner, J.M.; Tscherpel, C.; Dunkl, V.; Stoffels, G.; Rosen, J.; Rapp, M.; Sabel, M.; Herrlinger, U.; et al. Early treatment response assessment using 18F-FET PET compared with contrast-enhanced MRI in glioma patients after adjuvant temozolomide chemotherapy. J. Nucl. Med. 2021, 62, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Wollring, M.M.; Werner, J.M.; Bauer, E.K.; Tscherpel, C.; Ceccon, G.S.; Lohmann, P.; Stoffels, G.; Kabbasch, C.; Goldbrunner, R.; Fink, G.R.; et al. Prediction of response to lomustinebased chemotherapy in glioma patients at recurrence using MRI and FET PET. Neuro Oncol. 2023, 25, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Suchorska, B.; Unterrainer, M.; Biczok, A.; Sosnova, M.; Forbrig, R.; Bartenstein, P.; Tonn, J.C.; Albert, N.L.; Kreth, F.W. 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J. Neurooncol. 2018, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Langen, K.J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057. [Google Scholar] [CrossRef]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular predictors of progressionfree and overall survival in patients with newly diagnosed glioblastoma: A prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.J.; Galldiks, N. Static and dynamic 18F–FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 443–451. [Google Scholar] [CrossRef]
- Amelio, D.; Lorentini, S.; Schwarz, M.; Amichetti, M. Intensity-modulated radiation therapy in newly diagnosed glioblastoma: A systematic review on clinical and technical issues. Radiother. Oncol. 2010, 97, 361–369. [Google Scholar] [CrossRef]
- Niyazi, M.; Schnell, O.; Suchorska, B.; Schwarz, S.B.; Ganswindt, U.; Geisler, J.; Bartenstein, P.; Kreth, F.W.; Tonn, J.C.; Eigenbrod, S.; et al. FET-PET assessed recurrence pattern after radio-chemotherapy in newly diagnosed patients with glioblastoma is influenced by MGMT methylation status. Radiother. Oncol. 2012, 104, 78–82. [Google Scholar] [CrossRef]
- Lohmann, P.; Lerche, C.; Bauer, E.K.; Steger, J.; Stoffels, G.; Blau, T.; Dunkl, V.; Kocher, V.; Viswanathan, S.; Filss, C.P.; et al. Predicting IDH genotype in gliomas using FET PET radiomics. Sci. Rep. 2018, 8, 13328. [Google Scholar] [CrossRef]
- Müller, M.; Winz, O.; Gutsche, R.; Leijenaar, R.T.H.; Kocher, M.; Lerche, C.; Filss, C.P.; Stoffels, F.; Steidl, E.; Hattingen, E.; et al. Static FET PET radiomics for the differentiation of treatment-related changes from glioma progression. J. Neurooncol. 2022, 159, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Ninatti, G.; Pini, C.; Gelardi, F.; Sollini, M.; Chiti, A. The Role of PET Imaging in the Differential Diagnosis between Radiation Necrosis and Recurrent Disease in Irradiated Adult-Type Diffuse Gliomas: A Systematic Review. Cancers 2023, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Shuyan, W.; Hara, T.; Harris, R.B.; Degrado, T.R. Biodisposition and metabolism of [18F]fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, N.; Wyss, M.T.; Weber, B.; Scheidegger, S.; Lutz, A.; Verwey, J.; Radovanovic, I.; Pahnke, J.; Wild, D.; Wester, G.; et al. Uptake of 18Ffluorocholine, 18F-fluoroethyl-L- tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: Implications for separation of radiation necrosis from tumor recurrence. J. Nucl. Med. 2004, 45, 1931–1938. [Google Scholar] [PubMed]
- Pasi, F.; Persico, M.G.; Buroni, F.E.; Aprile, C.; Hodolic, M.; Corbella, F.; Nano, R.; Facoetti, A.; Lodola, L. 18F-FET and 18F-FCH uptake in human glioblastoma T98G cell lines after Irradiation with Photons or Carbon Ions. Contrast Media Mol. Imaging 2017, 2017, 6491674. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Inazu, M.; Saiki, I.; Yara, M.; Hara, N.; Yamanaka, T.; Uchino, H. Functional analysis of [methyl-3H]choline uptake in glioblastoma cells: Influence of anti-cancer and central nervous system drugs. Biochem. Pharmacol. 2014, 88, 303–312. [Google Scholar] [CrossRef]
- Moulin-Romsée, G.; D’Hondt, E.; de Groot, T.; Goffin, J.; Scott, R.; Mortelmans, L.; Menten, J.; Bormans, G.; Van Laere, K. Non-invasive grading of brain tumors using dynamic amino acid PET imaging: Does it work for 11Cmethionine? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2082–2087. [Google Scholar] [CrossRef]
- Kratochwil, C.; Combs, S.E.; Leotta, K.; Afshar-Oromieh, A.; Rieken, S.; Debus, J.; Haberkorn, U.; Giesel, F.L. Intra-individual comparison of 18F-FET and 18F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014, 16, 434–440. [Google Scholar] [CrossRef]
- Grosu, A.L.; Astner, S.T.; Riedel, E.; Nieder, C.; Wiedenmann, N.; Heinemann, F.; Schwaiger, M.; Molls, M.; Wester, H.J.; Weber, W.A. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1049–1058. [Google Scholar] [CrossRef]
- Lapa, C.; Linsemann, T.; Monoranu, C.M.; Samnick, S.; Buck, A.K.; Bluemel, C.; Chernihiv, J.; Kessler, A.F.; Hmola, G.A.; Ernestus, R.I.; et al. Comparison of the amino acid tracers 18F-FET and 18F-dopa in high-grade glioma patients. J. Nuclear Med. 2014, 55, 1611. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Liu, D.; Yang, D. The Value of 68Ga-PSMA-617 PET/CT in Differential Diagnosis between Low-Grade and High-Grade Gliomas. J. Nucl. Med. 2018, 59, 146. [Google Scholar]
- Brighi, C.; Puttick, S.; Woods, A.; Keall, P.; Tooney, P.A.; Waddington, D.E.J.; Sproule, V.; Rose, S.; Fay, M. Comparison between [68Ga]Ga-PSMA-617 and [18F]FET PET as Imaging Biomarkers in Adult Recurrent Glioblastoma. Int. J. Mol. Sci. 2023, 24, 6208. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-W.; Gan, K.H.; Senko, C.; Francis, R.J.; Ebert, M.; Lee, S.T.; Lau, E.; Khasraw, M.; Nowak, A.K.; Bailey, D.L.; et al. [18F]-fluoroethyl-L-tyrosine (FET) in glioblastoma (FIG) TROG 18.06 study: Protocol for a prospective, multicentre PET/CT trial. BMJ Open 2023, 13, e071327. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzarbeitia-Arroba, B.; Hodolic, M.; Pichler, R.; Osipova, O.; Soriano-Castrejón, Á.M.; García-Vicente, A.M. 18F-Fluoroethyl-L Tyrosine Positron Emission Tomography Radiomics in the Differentiation of Treatment-Related Changes from Disease Progression in Patients with Glioblastoma. Cancers 2024, 16, 195. https://doi.org/10.3390/cancers16010195
Manzarbeitia-Arroba B, Hodolic M, Pichler R, Osipova O, Soriano-Castrejón ÁM, García-Vicente AM. 18F-Fluoroethyl-L Tyrosine Positron Emission Tomography Radiomics in the Differentiation of Treatment-Related Changes from Disease Progression in Patients with Glioblastoma. Cancers. 2024; 16(1):195. https://doi.org/10.3390/cancers16010195
Chicago/Turabian StyleManzarbeitia-Arroba, Begoña, Marina Hodolic, Robert Pichler, Olga Osipova, Ángel Maria Soriano-Castrejón, and Ana María García-Vicente. 2024. "18F-Fluoroethyl-L Tyrosine Positron Emission Tomography Radiomics in the Differentiation of Treatment-Related Changes from Disease Progression in Patients with Glioblastoma" Cancers 16, no. 1: 195. https://doi.org/10.3390/cancers16010195
APA StyleManzarbeitia-Arroba, B., Hodolic, M., Pichler, R., Osipova, O., Soriano-Castrejón, Á. M., & García-Vicente, A. M. (2024). 18F-Fluoroethyl-L Tyrosine Positron Emission Tomography Radiomics in the Differentiation of Treatment-Related Changes from Disease Progression in Patients with Glioblastoma. Cancers, 16(1), 195. https://doi.org/10.3390/cancers16010195




