Metabolic Tumor Volume from 18F-FDG PET/CT in Combination with Radiologic Measurements to Predict Long-Term Survival Following Transplantation for Colorectal Liver Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
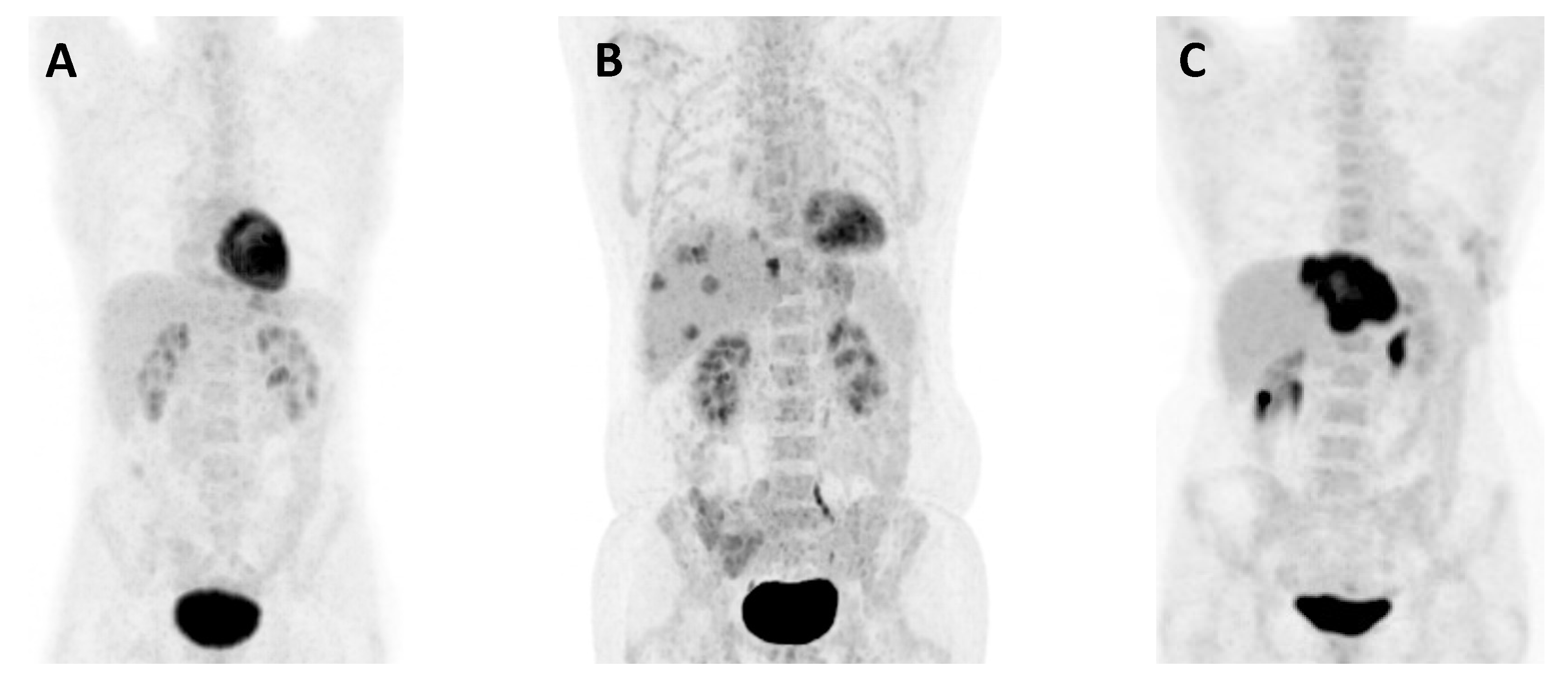
2.2. Imaging Procedure and Image Assessments
2.3. Statistical Analysis
3. Results
3.1. Patient and Baseline Characteristics
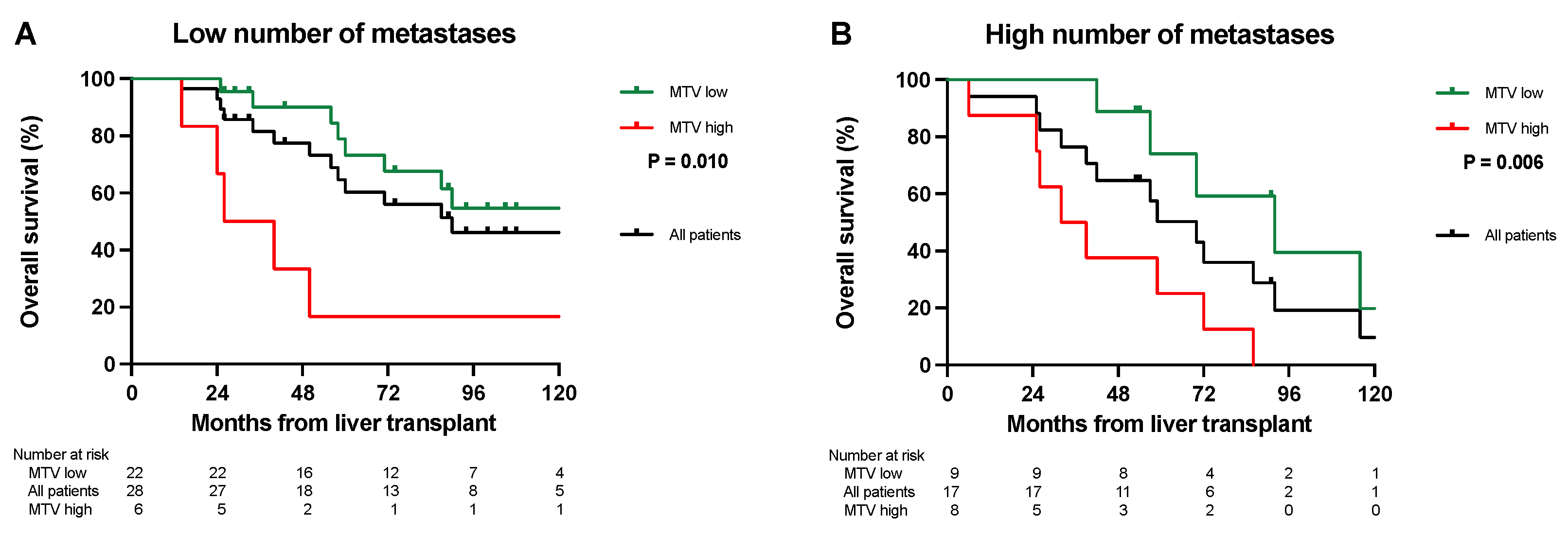
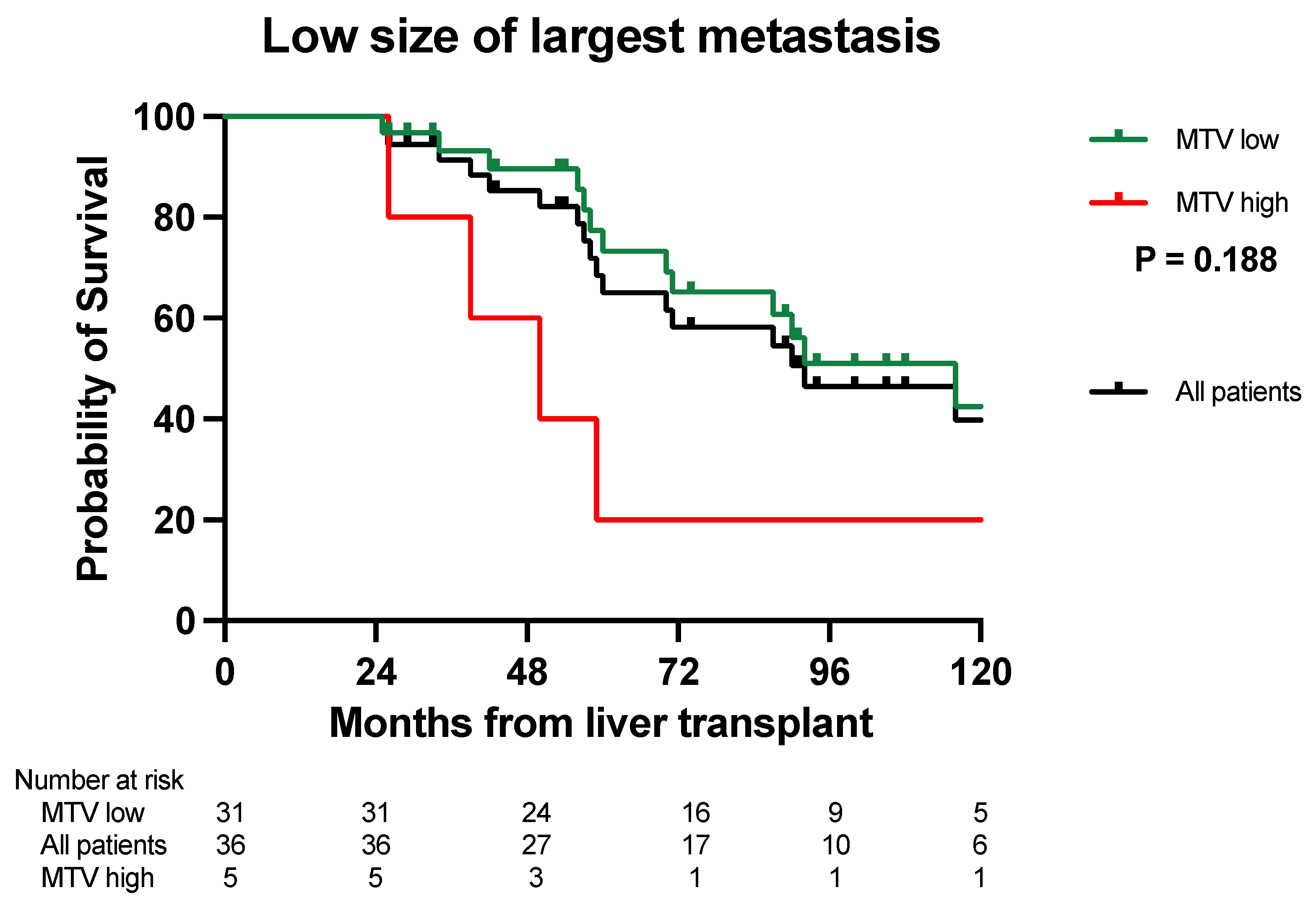
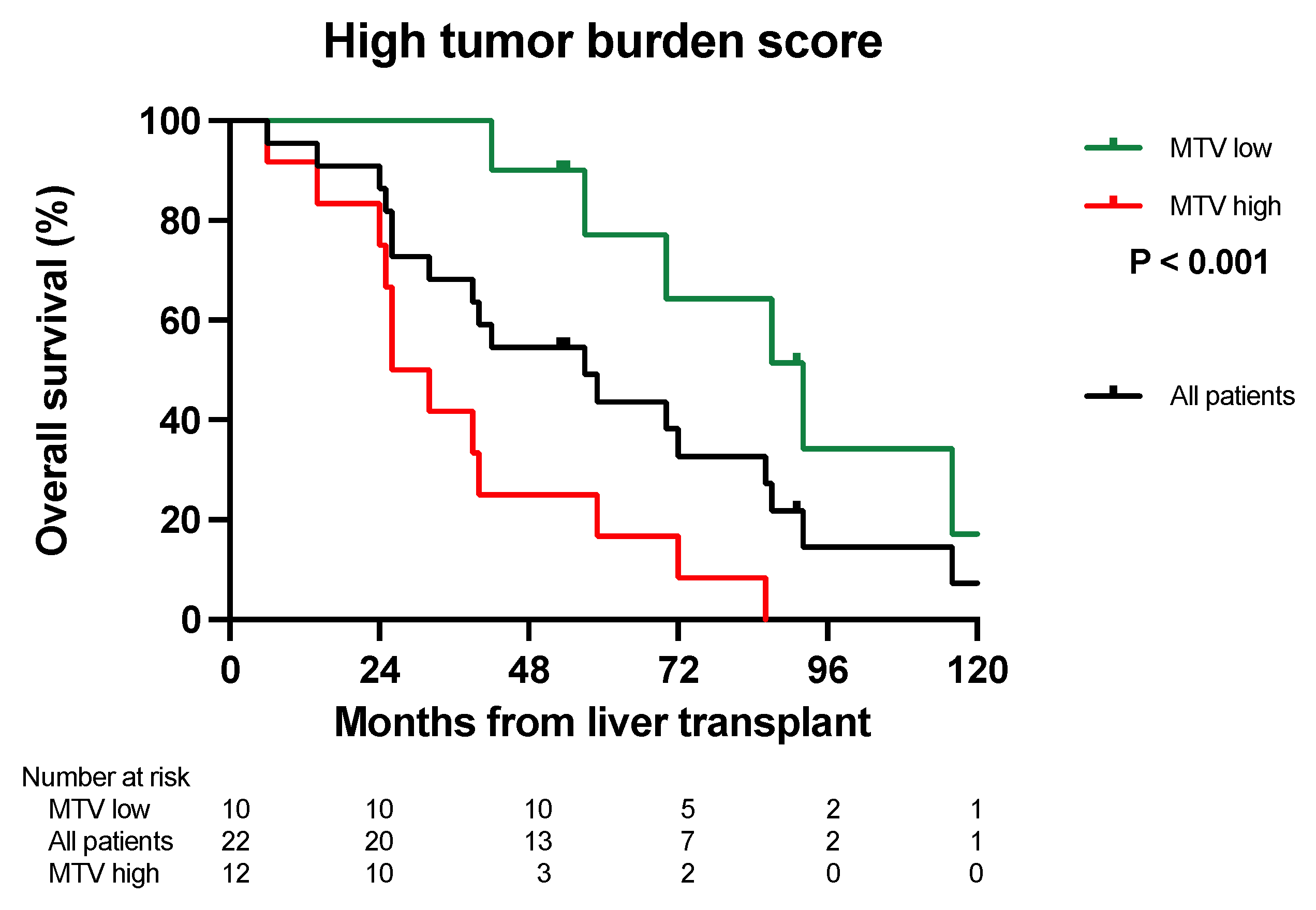
3.2. Overall Survival Analysis
3.3. Disease-Free Survival Analysis
3.4. Survival after Relapse Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dueland, S.; Guren, T.K.; Hagness, M.; Glimelius, B.; Line, P.D.; Pfeiffer, P.; Foss, A.; Tveit, K.M. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann. Surg. 2015, 261, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.A.; Adam, R.; Giuliante, F.; Lapointe, R.; Hubert, C.; Ijzermans, J.N.M.; Mirza, D.F.; Elias, D.; Laurent, C.; Gruenberger, T.; et al. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br. J. Cancer 2017, 117, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.; Gallinger, S.; Nhan, C.; Auer, R.A.; Biagi, J.J.; Fletcher, G.G.; Law, C.H.; Moulton, C.A.; Ruo, L.; Wei, A.C.; et al. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: A systematic review. Surgery 2012, 151, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Le Treut, Y.P.; Gregoire, E.; Klempnauer, J.; Belghiti, J.; Jouve, E.; Lerut, J.; Castaing, D.; Soubrane, O.; Boillot, O.; Mantion, G.; et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: A 213-case European liver transplant registry study. Ann. Surg. 2013, 257, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl. 2011, 17 (Suppl. S2), S44–S57. [Google Scholar] [CrossRef]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjornbeth, B.A.; Hagness, M.; Line, P.D. Survival Following Liver Transplantation for Patients with Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2019, 271, 212–218. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Tsili, A.C.; Alexiou, G.; Naka, C.; Argyropoulou, M.I. Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: A meta-analysis. Acta Radiol. 2021, 62, 302–312. [Google Scholar] [CrossRef]
- Briggs, R.H.; Chowdhury, F.U.; Lodge, J.P.; Scarsbrook, A.F. Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin. Radiol. 2011, 66, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wong, K.; Ng, W.L.; Shon, I.H.; Morgan, M. Positron emission tomography and colorectal cancer. Crit. Rev. Oncol. Hematol. 2011, 77, 30–47. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 6 December 2023).
- Kim, Y.I.; Lee, H.S.; Choi, J.Y. Prognostic Significance of Pretreatment 18F-FDG PET/CT Volumetric Parameters in Patients with Colorectal Liver Metastasis: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2021, 46, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Yamasaki, M.; Tanaka, K.; Masuike, Y.; Tatsumi, M.; Motoori, M.; Kimura, Y.; Hatazawa, J.; Mori, M.; Doki, Y. Metabolic Tumor Volume Change Predicts Long-term Survival and Histological Response to Preoperative Chemotherapy in Locally Advanced Esophageal Cancer. Ann. Surg. 2019, 270, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Fonti, R.; Mazziotti, E.; Piccin, L.; Mozzillo, E.; Damiano, V.; Matano, E.; De Placido, S.; Del Vecchio, S. Total metabolic tumor volume by 18F-FDG PET/CT for the prediction of outcome in patients with non-small cell lung cancer. Ann. Nucl. Med. 2019, 33, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Smedman, T.M.; Syversveen, T.; Grut, H.; Hagness, M.; Line, P.D. Long-Term Survival, Prognostic Factors, and Selection of Patients with Colorectal Cancer for Liver Transplant: A Nonrandomized Controlled Trial. JAMA Surg 2023, 158, e232932. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool for Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Grut, H.; Line, P.D.; Syversveen, T.; Dueland, S. Metabolic tumor volume predicts long-term survival after transplantation for unresectable colorectal liver metastases: 15 years of experience from the SECA study. Ann. Nucl. Med. 2022, 36, 1073–1081. [Google Scholar] [CrossRef]
- Grut, H.; Revheim, M.E.; Line, P.D.; Dueland, S. Importance of 18F-FDG PET/CT to select patients with nonresectable colorectal liver metastases for liver transplantation. Nucl. Med. Commun. 2018, 39, 621–627. [Google Scholar] [CrossRef]
- Jadvar, H.; Colletti, P.M.; Delgado-Bolton, R.; Esposito, G.; Krause, B.J.; Iagaru, A.H.; Nadel, H.; Quinn, D.I.; Rohren, E.; Subramaniam, R.M.; et al. Appropriate Use Criteria for (18)F-FDG PET/CT in Restaging and Treatment Response Assessment of Malignant Disease. J. Nucl. Med. 2017, 58, 2026–2037. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Smedman, T.M.; Grut, H.; Syversveen, T.; Jørgensen, L.H.; Line, P.D. PET-Uptake in Liver Metastases as Method to Predict Tumor Biological Behavior in Patients Transplanted for Colorectal Liver Metastases Developing Lung Recurrence. Cancers 2022, 14, 5042. [Google Scholar] [CrossRef] [PubMed]
- Hagness, M.; Foss, A.; Egge, T.S.; Dueland, S. Patterns of recurrence after liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. Oncol. 2014, 21, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Grut, H.; Solberg, S.; Seierstad, T.; Revheim, M.E.; Egge, T.S.; Larsen, S.G.; Line, P.D.; Dueland, S. Growth rates of pulmonary metastases after liver transplantation for unresectable colorectal liver metastases. Br. J. Surg. 2017, 105, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.L.; Felga, G.E.G.; Diniz, M.A.; Silva, R.F.; Mattos, A.A.; Silva, R.C.M.A.; Boin, I.F.S.F.; Garcia, J.H.P.; Lima, A.S.; Coelho, J.C.U.; et al. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: Clinical profile and prognostic factors of survival. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Hoppe-Lotichius, M.; Vollmar, J.; Marquardt, J.U.; Weinmann, A.; Wörns, M.A.; Otto, G.; Zimmermann, T.; Galle, P.R. Long-term observation of hepatocellular carcinoma recurrence after liver transplantation at a European transplantation centre. United Eur. Gastroenterol. J. 2019, 7, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.; Lu, D.; Wei, L.; Feng, X.; Shen, J.; Xu, W. Predicting tumor recurrence using metabolic indices of (18)F-FDG PET/CT prior to orthotopic liver transplantationfor hepatocellular carcinoma. Oncol. Lett. 2020, 20, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Nie, P.; Yang, G.; Wang, Y.; Yan, L.; Zhao, Y.; Yu, T.; Yu, M.; Wu, F.; Rao, W.; et al. An FDG PET/CT metabolic parameter-based nomogram for predicting the early recurrence of hepatocellular carcinoma after liver transplantation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3656–3665. [Google Scholar] [CrossRef]
- Gulec, S.A.; Suthar, R.R.; Barot, T.C.; Pennington, K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1289–1295. [Google Scholar] [CrossRef]
- Soydal, C.; Kucuk, O.N.; Gecim, E.I.; Bilgic, S.; Elhan, A.H. The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl. Med. Commun. 2013, 34, 501–506. [Google Scholar] [CrossRef]
| Patients, n | 45 |
|---|---|
| Age at liver transplantation (LT), years + | 58 (29–71) |
| Gender, n (%) | |
| Male | 24 (53) |
| Female | 21 (47) |
| Primary tumor, n (%) | |
| Colon | 27 (60) |
| Rectum | 18 (40) |
| T Stage, n (%) | |
| 0 | 3 (6) |
| 1 | 1 (2) |
| 2 | 5 (10) |
| 3 | 35 (78) |
| 4 | 1 (2) |
| N Stage, n (%) | |
| 0 | 21 (47) |
| 1 | 16 (36) |
| 2 | 8 (18) |
| Progressive disease at LT, n (%) * | |
| Yes | 16 (36) |
| No | 29 (64) |
| Metabolic tumor volume (MTV), cm3 + | 21.3 (0–874.12) |
| Carcinoembryonic antigen (CEA), µg/L + | 5 (1–2002) |
| Number of metastases, n + | 7 (1–53) |
| Largest metastasis (mm) + | 30 (3–130) |
| Group | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| MTV Low | MTV High | |||||||
| 1 Year | 3 Years | 5 Years | 1 Year | 3 Years | 5 Years | p-Value | ||
| Number of metastases | Low (≤8) | 59 | 41% | 34% | 17% | 0% | 0% | 0.010 |
| High (>8) | 44% | 33% | 22% | 25% | 0% | 0% | 0.034 | |
| Size of the largest metastasis | Low (<5.5 cm) | 55% | 39% | 29% | 25% | 0% | 0% | 0.002 |
| High (>5.5 cm) | n = 0 | 22% | 0% | 0% | ||||
| Tumor burden score | Group 2 | 62% | 43% | 36% | 50% | 0% | 0% | 0.086 |
| Group 3 | 40% | 30% | 20% | 17% | 0% | 0% | 0.034 | |
| Group | Survival after Relapse | |||||
|---|---|---|---|---|---|---|
| MTV Low | MTV High | |||||
| 5 Years | 10 Years | 5 Years | 10 Years | p-Value | ||
| Number of metastases | Low (≤8) | 54% | 39% | 17% | 0% | 0.144 |
| High (>8) | 57% | 19% | 13% | 0% | 0.026 | |
| Size of the largest metastasis | Low (<5.5 cm) | 60% | 31% | 25% | 0% | 0.749 |
| High (>5.5 cm) | n = 0 | 11% | 0% | |||
| Tumor burden score | Group 2 | 50% | 42% | n = 2 | 0.604 | |
| Group 3 | 75% | 16% | 8% | 0% | 0.006 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grut, H.; Line, P.-D.; Syversveen, T.; Dueland, S. Metabolic Tumor Volume from 18F-FDG PET/CT in Combination with Radiologic Measurements to Predict Long-Term Survival Following Transplantation for Colorectal Liver Metastases. Cancers 2024, 16, 19. https://doi.org/10.3390/cancers16010019
Grut H, Line P-D, Syversveen T, Dueland S. Metabolic Tumor Volume from 18F-FDG PET/CT in Combination with Radiologic Measurements to Predict Long-Term Survival Following Transplantation for Colorectal Liver Metastases. Cancers. 2024; 16(1):19. https://doi.org/10.3390/cancers16010019
Chicago/Turabian StyleGrut, Harald, Pål-Dag Line, Trygve Syversveen, and Svein Dueland. 2024. "Metabolic Tumor Volume from 18F-FDG PET/CT in Combination with Radiologic Measurements to Predict Long-Term Survival Following Transplantation for Colorectal Liver Metastases" Cancers 16, no. 1: 19. https://doi.org/10.3390/cancers16010019
APA StyleGrut, H., Line, P.-D., Syversveen, T., & Dueland, S. (2024). Metabolic Tumor Volume from 18F-FDG PET/CT in Combination with Radiologic Measurements to Predict Long-Term Survival Following Transplantation for Colorectal Liver Metastases. Cancers, 16(1), 19. https://doi.org/10.3390/cancers16010019





